Abstract
Background
Arterial stiffness leads to left ventricular (LV) mass through non-atherosclerotic pathways in mice. In humans, a high ankle brachial index (ABI) indicates stiff peripheral arteries, and is associated with cardiovascular disease (CVD) events. Whether high ABI is associated with LV mass in humans, and whether this may reflect consequences of arterial stiffness, atherosclerosis, or both is unknown.
Methods
Among 4,972 MESA participants without clinical CVD, we used linear regression to evaluate the association of low (< 0.90) and high (>1.40 or incompressible) ABI with LV mass by cardiac magnetic resonance imaging (MRI). Intermediate ABIs served as the reference category. To determine the effect of subclinical atherosclerosis, models were adjusted for common and internal carotid intima media thickness (cIMT) and log-transformed coronary artery calcification (Ln[CAC+1]).
Results
Compared to subjects with intermediate ABI, LV mass was higher with either low (2.70g/m2 higher, 95% CI 0.65–4.75) or high ABI (6.84 g/m2 higher, 95% CI 3.2–10.47) after adjustment for traditional CVD risk factors, kidney function, and CRP. However, further adjustment for cIMT and CAC substantially attenuated the association of low ABI with LVMI (1.24 g/m2 higher, 95% CI −0.84–3.33), whereas the association of high ABI was minimally altered (6.01 g/m2 higher, 95% CI 2.36–9.67).
Conclusions
High ABI is associated with greater LV mass; an association that is not attenuated with adjustment for subclinical atherosclerosis in non-peripheral arterial beds. High ABI may lead to greater LV mass through non-atherosclerotic pathways.
Keywords: vascular stiffness, medial arterial calcification, left ventricular mass, heart failure, cardiovascular disease
INTRODUCTION
The ankle brachial index (ABI) is a simple non-invasive test, reflecting the ratio of the systolic blood pressure (SBP) in the ankle divided by SBP in the brachial artery. Low ABI measurements (< 0.90) have been studied as a marker of atherosclerotic peripheral arterial disease (PAD) for over 40 years.(1) The cardiovascular disease (CVD) consequences of high ABI measurements are less well studied.
Whereas low ABI measurements are sensitive and specific for flow-limiting atherosclerotic PAD,(2) high ABI measurements occur when the lower limb arteries are stiff, therefore requiring higher cuff pressures to occlude the peripheral arteries. This is widely believed to occur as a consequence of a vascular pathology distinct from atherosclerosis; medial arterial calcification (MAC).(3–5) In autopsy studies, MAC is limited to the arterial media as compared to the intimal location of atherosclerosis, is non-inflammatory, and is not associated with lipid plaque.(6,7) Recently, Resnick and colleagues demonstrated a U-shaped relationship between ABI measurements and CVD events in community-living Native Americans. Subjects with either high or low ABI were at approximately equal risk for CVD events, and either extreme was at approximately 2-fold risk compared to persons with intermediate ABI.(8) This finding has since been confirmed in several other community-based cohorts,(9,10) and has been extended to include incident congestive heart failure (CHF) events.(10,11)
The mechanisms responsible for these associations are unknown. One potential mechanism may be through myocardial remodeling, as a consequence of arterial stiffness. Indeed, experimentally induced MAC in rats led to greater arterial stiffness and left ventricular (LV) mass.(12) Alternatively, the association of high ABI with CVD events may be due to co-existing atherosclerosis, as prior studies have demonstrated that both diseases may co-exist within individuals.(13–15)
Here, we evaluate the association of high ABI measurements with LV mass by cardiac magnetic resonance imaging (MRI) in the Multi-Ethnic Study of Atherosclerosis (MESA). We compare the strength of this association with that of low ABI and LV mass. On the basis of the experimental animal data, we hypothesized that the association of high ABI with LV mass would be independent of traditional CVD risk factors, and would not be significantly attenuated after adjustment for subclinical atherosclerosis in other arterial beds.
METHODS
Participants
MESA was initiated to investigate the prevalence and progression of subclinical CVD. Details about the study design have been published previously.(16) In brief, between July 2000 and August 2002, 6,814 men and women aged 45 to 84 years who identified themselves as Caucasian, African-American, Hispanic, or Chinese and were free of clinically apparent CVD were recruited from six US communities: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN. Individuals were excluded if they had physician-diagnosed heart attack; angina; heart failure; stroke or transient ischemic attack; atrial fibrillation; had undergone coronary artery bypass grafting, angioplasty, or valve replacement. The patients were not screened for claudication. The institutional review boards at each participating center approved the study, and participants provided written informed consent.
All participants underwent a day-long study visit where they provided medical history, venous blood specimens after an overnight (8-hour) fast, spot urine specimens, and physical examination. In addition, participants underwent a detailed evaluation for subclinical atherosclerosis, which included ABI, thoracic CT for CAC, carotid ultrasound for common and internal cIMT, and cardiac MRI for evaluation of LV mass and function.
For this study, we excluded subjects who did not undergo cardiac MRI (n=1716), had technically inadequate cardiac MRI data (n=94), or did not provide ABI measurements (n=32), resulting in a final analytic sample of 4,972 (73% of MESA participants).
Measurements
Ankle Brachial Index
After the participants had rested supine for 5 minutes, SBPwas measured in both arms. For each leg, the SBP in the posterior tibial (PT) and dorsalis pedis (DP) artery was measured using continuous-wave Doppler ultrasound probes. The leg-specific ABI was calculated as the higher SBP in the PT or DP divided by the higher of the two arm SBPs. The higher arm SBP was used because of the strong association between PAD and subclavian artery stenosis.(17) Subject specific ABIs were defined by the lower of their two leg-specific ABI measurements. If leg blood pressure could not be abolished with the cuff inflated to > 300mmHg, subjects were deemed to have incompressible peripheral arteries.
Cardiac MRI Measurements
Consenting participants underwent cardiac MRI scans a median of 16 days after the baseline examination, and 95% were completed by 11 weeks after the baseline visit. MRI scans were performed with 1.5-T magnets with determination of LV mass and volumes as previously described.(18) The epicardial and endocardial myocardial borders were contoured using a semi-automated method (MASS 4.2, Medis, Leiden, the Netherlands). The difference between the epicardial and endocardial areas were multiplied by the slice thickness and section gap, then multiplied by the specific gravity of the myocardium (1.05 g/ml) to determine LV mass. LV mass was indexed to body surface area, calculated by the DuBois and DuBois formula.(19) In similar fashion, LV end diastolic volume (LVEDV) and end systolic volume (LVESV) were calculated by summation of the areas of separate slices, multiplied by the sum of slice thickness and image gap. Stroke volume was calculated as LVEDV-LVESV. LV ejection fraction (LVEF) was calculated as stroke volume divided by LVEDV multiplied by 100. We evaluated these parameters in addition to LV mass / LVEDV (M/V ratio); a measure of concentric cardiac remodeling.(20)
Seventy-nine participants had repeat cardiac MRI measurements 3 to 6 months after the initial measurement. Analysis showed the technical error (expressed as % of the mean value) was 6% and 4% for LV mass and LVEDV, respectively. The intraclass correlation coefficients were 0.98 and 0.98, respectively.(18)
Subclinical Atherosclerosis Measures
Coronary artery calcification (CAC) was assessed by thoracic computed tomography (CT) using either a cardiac-gated electron-beam computed tomography scanner (Chicago, Los Angeles, and New York field centers) or a multidetector computed tomography system (Baltimore, Forsyth County, and St Paul field centers). All participants were scanned twice. A phantom of known physical calcium concentration was included in the field of view. A radiologist or cardiologist read all CT scans at a central reading center at Harbor–UCLA Medical Center. CAC was identified and quantified from images calibrated according to the readings of the calcium phantom. The Agatston score was determined from plaque densities in all coronary arteries.(21) The mean phantom-adjusted Agatston score was used for the 2 scans in all analyses. Carr et al have reported the details of the MESA CT scanning and interpretation methods.(22) Trained technicians performed B-mode ultrasonography of the near and far walls of the internal and common carotid arteries bilaterally using a Logiq 700 ultrasound (General Electric, Waukesha, WI).(23,24) Images were read centrally at Tufts-New England Medical Center. Maximal cIMT at each site was determined as previously described.(24)
Other Measurements
Standardized questionnaires were used to obtain information on medical history, race/ethnicity, and medication use. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medication. Diabetes mellitus was defined as a fasting glucose ≥ 126mg/dL or use of hypoglycemic medications.(25) Smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes, and body mass index (BMI) was calculated (in kg/m2). Resting blood pressure was measured three times with participants in the seated position with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon). The average of the last two measurements was used in analyses. Total, high density lipoprotein (HDL) cholesterol, and triglyceride concentrations were measured from fasting venous blood samples. Low density lipoprotein (LDL) concentration was calculated from the Friedewald equation.(26) C-reactive protein (CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL). Serum creatinine was measured using a colorimetric method by a Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY) and was standardized to the Cleveland Clinic Foundation reference standard for direct calibration. Creatinine, age, sex, and race were used to calculate eGFR using the abbreviated MDRD formula.(27) Urine albumin and creatinine were measured by nephelometry and the rate Jaffe reaction, respectively. Spot urine albumin to creatinine ratios were calculated.
Statistical Analyses
Previous studies have consistently demonstrated that subjects with ABI <0.90 and ≥1.40 or incompressible arteries are at greater risk for CVD events and mortality than subjects with intermediate ABI measurements.(8,10) We categorized subjects according to these cut-points a priori. Baseline differences in demographic and clinical variables were compared across ABI groups using analysis of variance (ANOVA) for continuous variables, and Chi-squared test for continuous variables. For continuous variables, when Bartlett’s test for equal variance was violated (P < 0.05) the Kruskal-Wallis test was used in place of ANOVA, and when the expected number in any cell for categorical variables was < 10, the Fisher’s exact test was used in place of the Chi-squared test. Linear regression analysis was used to evaluate the association of ABI categories with LV mass / body surface area. Sequential models were developed. Model 1 was adjusted for age, sex, race/ethnicity, and field center site. Candidate covariates for model 2 were all those listed in table 1, except for the subclinical atherosclerosis measures. Among these, variables were retained in model 2 if statistically significant differences were observed across ABI categories in bivariate analysis (P < 0.05). Finally, model 3 included all model 2 variables plus the 3 markers of subclinical atherosclerosis (common cIMT, internal cIMT, and ln[CAC + 1]). 48% of participants in this study had some CAC (score >0), and among these, CAC scores were strongly right-skewed. We adjusted for CAC on a continuous scale by adding 1 to CAC scores, and naturally log-transformed the resulting variable. Both common and internal cIMT scores were approximately normally distributed, and were modeled as continuous (un-transformed) covariates. To evaluate possible co-linearity, we evaluated the Pearson correlation between common cIMT and internal cIMT (r=0.41), thus both variables were included as covariates simultaneously.
Table 1.
Characteristics of MESA Participants by Ankle Brachial Index Categories
| < 0.90 | 0.90 – 1.40 | > 1.40 / Incompressible |
|
|---|---|---|---|
| n=171 | n=4,748 | n=53 | |
| Demographics | |||
| Age (yrs) ± SD | 70 ± 9 ‡ | 61 ± 10 | 66 ± 10 ‡ |
| Female (%) | 54% | 52% | 34% ‡ |
| Race / Ethnicity ** | |||
| White (%) | 35% ‡ | 39% | 47% |
| Black (%) | 45% ‡ | 25% | 17% |
| Hispanic (%) | 13% ‡ | 22% | 30% |
| Chinese (%) | 7% ‡ | 13% | 6% |
| Medical History | |||
| Hypertension (%) | 70% ‡ | 41% | 53% |
| Diabetes (%) | 27% ‡ | 12% | 28% ‡ |
| Smoking (%) ** | 26% ‡ | 12% | 6% |
| Traditional and Novel Cardiovascular Risk Factors | |||
| Body mass index (kg/m2) ± SD | 27.4 ± 5.1 | 27.7 ± 4.9 | 28.7 ± 5.6 |
| Systolic blood pressure (mmHg) ± SD | 139 ± 29 ‡ | 125 ± 21 | 132 ± 20 † |
| Diastolic blood pressure (mmHg) ± SD | 71 ± 12 | 72 ± 10 | 70 ± 10 |
| Total Cholesterol (mg/dl) ± SD | 197 ± 36 | 194 ± 35 | 182 ± 36 † |
| LDL Cholesterol (mg/dL) ± SD | 120 ± 34 | 117 ± 31 | 107 ± 28 † |
| HDL Cholesterol (mg/dL) ± SD | 50 ± 15 | 51 ± 15 | 49 ± 17 |
| Triglycerides (mg/dL) * | 117 (82, 169) | 111 (77, 161) | 104 (86, 144) |
| C-reactive protein (mg/L) * | 2.9 (1.0, 5.9) ‡ | 1.8 (0.8, 4.0) | 2.9 (1.2, 6.2) ‡ |
| eGFR (ml/min/1.73m2) ± SD | 74 ± 21 ‡ | 80 ± 16 | 77 ± 19 † |
| Urine Albumin/Creatinine (mg/g) * | 9 (4, 21) ‡ | 5 (3, 10) | 7 (4, 18) † |
| Markers of Subclinical Atherosclerosis | |||
| Common Carotid Intima-Media Thickness (mm) ± SD |
1.02 ± 0.23 ‡ | 0.85 ± 0.18 | 0.96 ± 0.24 ‡ |
| Internal Carotid Intima-Media Thickness (mm) ± SD |
1.63 ± 0.80 ‡ | 1.02 ± 0.55 | 1.31 ± 0.76 † |
| CAC Prevalence | 82% ‡ | 47% | 68% ‡ |
| CAC Severity § * | 196 (68, 258) ‡ | 74 (19, 278) | 269 (82, 1027) ‡ |
| Left Ventricular Mass (g/m2) | 84 ± 21 ‡ | 78 ± 16 | 90 ± 22 ‡ |
Median (Interquartile Range) - Evaluated by Kruskal-Wallis test
Evaluated by Fisher’s Exact test.
< 0.05 compared to the ABI 0.90 – 1.40 category
< 0.01 compared to the ABI 0.90 – 1.40 category
Limited to subjects with CAC > 0
In similar fashion, we evaluated the association of ABI categories with LVEDV, M/V ratio, and LVEF using linear regression. Models were adjusted as described for LV mass. In all cases, results were similar in models 1, 2, and 3, we therefore present data only for model 1.
In a sensitivity analysis, we evaluated ankle SBP as the predictor variable of interest in place of ABI, and adjusted for the identical covariates as described above, while forcing brachial blood pressure into the model. This was done because previous studies have evaluated the test characteristics of ankle SBP for MAC, and to our knowledge, similar data are not available for a high ABI.(5) In addition, we performed sensitivity analysis wherein LV mass was adjusted for height and weight rather than body surface area. In all cases, results were similar, so data presentation is limited to LV mass indexed to body surface area.
All statistical analyses were conducted with STATA version 9.2 for Windows (Stata Co, College Station, TX).
RESULTS
Among the 4,972 study participants, the mean age was 61 ± 10 years and 52% were female. Thirty-nine percent were Caucasian, 26% were African-American, 22% were Hispanic, and 13% were Chinese. One hundred and seventy-one subjects (3%) had ABI measurements < 0.90, and 53 (1%) had ABI measurements >1.40 or incompressible lower limb arteries. Among those in the high ABI category, 17 subjects (32%) were classified as such on the basis of incompressible lower limb arteries. The mean LV mass was 78 ± 16 g/m2.
Compared to subjects with intermediate ABI scores (0.90 – 1.40), those with ABI scores < 0.90 had higher prevalence of traditional CVD risk factors. Exceptions were BMI and serum lipid levels (Table 1). In contrast, subjects with high ABI were older, more frequently male and diabetic, had higher SBP, CRP levels, and more advanced kidney disease, yet also had lower total and LDL cholesterol levels, on average. Both low and high ABI groups had more subclinical atherosclerosis by common and internal cIMT and CAC scores compared to subjects with intermediate ABI scores. Men were more likely to have high ABI, and had greater prevalence of subclinical atherosclerosis by each measure. Both extreme ABI groups had higher LV mass in unadjusted analysis.
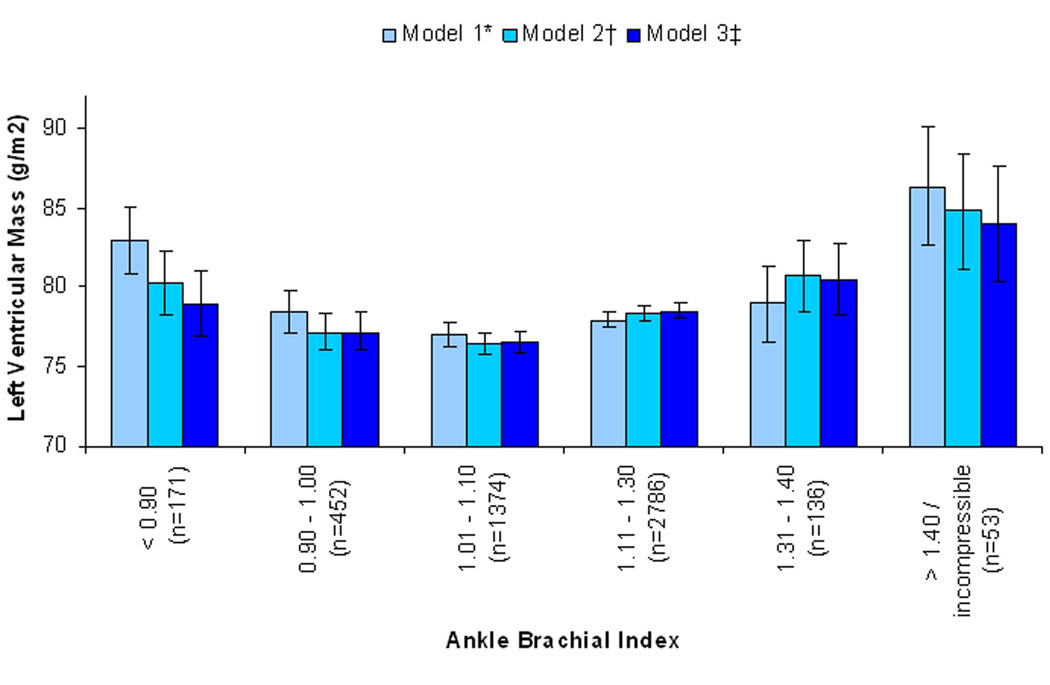
In models adjusted for age, sex, race / ethnicity, and field center site, subjects with either low or high ABI had a significantly higher LV mass compared to subjects with intermediate ABI scores (Table 2). When adjusted for traditional CVD risk factors, kidney function, and CRP, the association of low ABI with LV mass was attenuated more so than the association of high ABI with LV mass, yet both extreme ABI groups remained statistically significantly associated with higher LV mass. When models were further adjusted for common and internal cIMT and CAC score, the association of low ABI with LV mass was further attenuated and was rendered no longer statistically significant. In contrast, adjustment of these variables had only a minor effect on the association of high ABI with LV mass, such that subjects with high ABI had 6 g/m2 higher LV mass compared to the intermediate ABI group despite adjustment for traditional CVD risk factors, kidney function, CRP, and subclinical atherosclerosis (Figure 1).
Table 2.
Associations of Ankle Brachial Index Categories with Left Ventricular Mass
| Ankle Brachial Index |
|||
|---|---|---|---|
| < 0.90 | 0.90 – 1.40 | > 1.40 / incompressible | |
| n=171 | n=4748 | n=53 | |
| β (g/m2); 95% CI; P-value | β (g/m2); 95% CI; P-value | β (g/m2); 95% CI; P-value | |
| Model 1 * | 5.33; (3.16 – 7.50); < 0.001 | Reference | 8.52; (4.72 – 12.32); < 0.001 |
| Model 2 † | 2.70; (0.65 – 4.75); 0.010 | Reference | 6.84; (3.22 – 10.47); < 0.001 |
| Model 3 ‡ | 1.24; (−0.84 – 3.33); 0.24 | Reference | 6.01; (2.36 – 9.67); 0.001 |
Adjusted for age, sex, race, and field center site
Adjusted for model 1 variables plus hypertension, diabetes, smoking, systolic blood pressure, total cholesterol, LDL cholesterol, ln(C-reactive protein), eGFR, ln(urine albumin/creatinine)
Adjusted for model 2 variables plus common cIMT, internal cIMT, and ln(CAC+1).
Figure 1. Association of the Spectrum of Ankle Brachial Index with Left Ventricular Mass, with Adjustment for Demographics*, Traditional CVD Risk Factors†, and Subclinical Atherosclerosis‡.
* Model 1: Adjusted for age, sex, race / ethnicity, and field center site.
† Model 2: Adjusted for Model 1 variables and hypertension, diabetes, smoking, systolic blood pressure, total cholesterol, LDL cholesterol, ln(C-reactive protein), eGFR, Ln(urine albumin/creatinine).
‡ Model 3: Adjusted for Model 2 variables and common cIMT, internal cIMT, and ln(CAC+1).
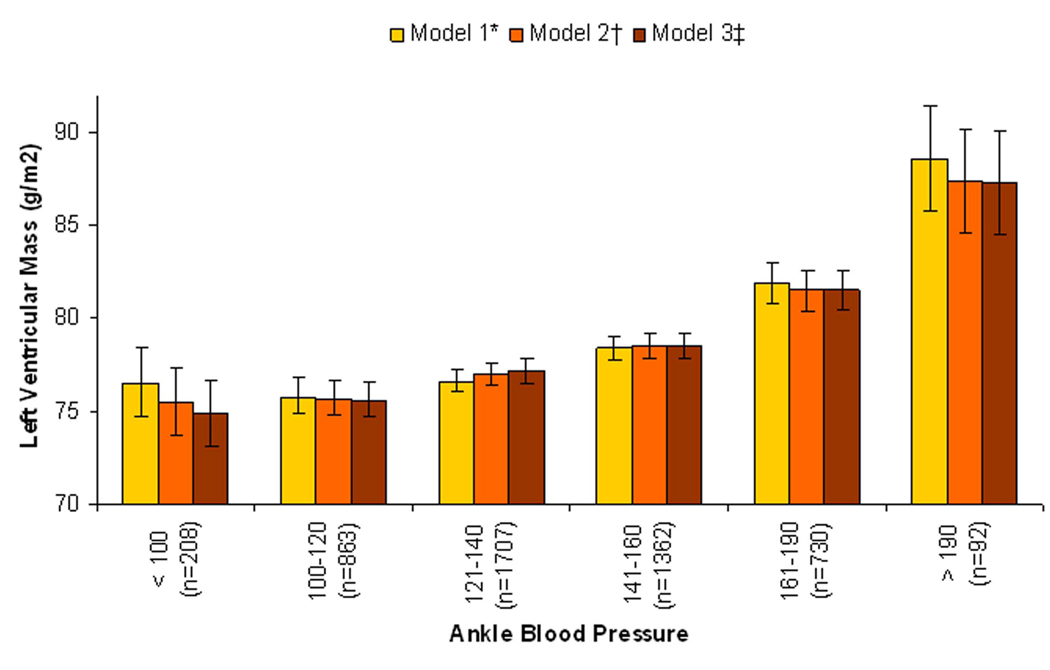
Results were similar in sensitivity analyses in which ankle SBP was evaluated as the predictor variable of interest instead of ABI (Figure 2). Results were also qualitatively similar when a cut-point < 1.00 was used to define low ABI, and a cut-point of >1.30/incompressible was used to define high ABI. In this sensitivity analysis, in the final multivariable model adjusting for CVD risk factors, kidney function, CRP, and subclinical atherosclerosis markers (Model 3), there was no association of ABI < 1.00 with LV mass (β = −0.13; 95% CI −1.29 to 1.03), while the ABI >1.30 group had significantly higher LV mass (β= 3.41; 95% CI 1.50 to 5.33).
Figure 2. Association of the Spectrum of Ankle Blood Pressure with Left Ventricular Mass, with Adjustment for Brachial Blood Pressure and Demographics*, Traditional CVD Risk Factors†, and Subclinical Atherosclerosis‡.
* Model 1: Adjusted for brachial systolic blood pressure, age, sex, race / ethnicity, and field center site.
† Model 2: Adjusted for Model 1 variables and hypertension, diabetes, smoking, total cholesterol, LDL cholesterol, ln(C-reactive protein), eGFR, Ln(urine albumin/creatinine).
‡ Model 3: Adjusted for Model 2 variables and common cIMT, internal cIMT, and ln(CAC+1).
We compared the relative strength of the association of high ABI with other established risk factors for LV mass by comparing beta coefficients in linear regression models. The association of high ABI with LV mass was of similar magnitude to that of prevalent hypertension, approximately 2.5 times stronger than that of prevalent diabetes, and 2 to 3 times stronger than that of cIMT and CAC in models adjusted for age, sex, race / ethnicity, and field center site (Table 3).
Table 3.
Relative Strength of Association of Risk Factors for Left Ventricular Mass *
| Risk Factor | Left Ventricular Mass (g/m2) |
|---|---|
| β; (95% CI); P-value | |
| ABI > 1.40/incompressible | 8.52; (4.72 – 12.32); < 0.001 |
| ABI < 0.90 | 5.33; (3.16 – 7.50); < 0.001 |
| CAC‡ | |
| 1–100 | 1.80; (0.82 – 2.78); < 0.001 |
| 101–300 | 2.53; (1.13 – 3.94); < 0.001 |
| > 300 | 4.51; (3.08 – 5.94); < 0.001 |
| Common cIMT (per 0.19mm increase) † | 3.02; (2.58 – 3.46); < 0.001 |
| Internal cIMT (per 0.58mm increase) † | 1.83; (1.40 – 2.25); < 0.001 |
| Age (10.1-year increase)† | 0.42; (0.02 – 0.81); 0.04 |
| Systolic blood pressure (per 16.5 mmHg increase) † | 4.97; (4.56 – 2.37); < 0.001 |
| Prevalent Hypertension | 7.65; (6.83 – 8.47); < 0.001 |
| Prevalent Diabetes | 3.36; (2.18 – 4.55); < 0.001 |
Adjusted for age, sex, race, and field center site.
Reference category was CAC score=0.
One standard deviation increase.
Next, we investigated the association of high ABI with other parameters of LV morphology and function. High ABI was associated with higher LVEDV to a similar degree as to LV mass (Table 4). Thus, the association of high ABI with LV mass / volume ratio (a marker of concentric left ventricular remodeling) was near unity. Similarly, we observed no significant association of high ABI with LVEF. For each comparison, results were similar when models were adjusted for all traditional CVD risk factors, and further adjusted for the measures of subclinical atherosclerosis (data not shown).
Table 4.
Associations of High ABI with Left Ventricular Morphology and Function*
| β; (95% CI); P-value | |
|---|---|
| LV Mass Index | 8.52; (4.72 – 12.32); < 0.001 |
| LV End Diastolic Volume | 8.41; (1.16 – 15.66); 0.03 |
| LV Mass / Volume Ratio | 0.02; (−0.02 – 0.05); 0.29 |
| LV Ejection Fraction | 1.30; (−0.55 – 3.15); 0.12 |
Compares 53 subject with ABI > 1.40 / incompressible to 4748 subjects with ABI 0.90 – 1.40. Models are adjusted for age, sex, race / ethnicity, field center site, and low ABI.
DISCUSSION
We demonstrate a strong cross-sectional association of high ABI measurements with greater LV mass in an ethnically diverse community-living population without clinical CVD. We observed a graded relationship between higher ABI scores and greater LV mass which was not materially affected when adjusted for traditional CVD risk factors, kidney function, and CRP, nor when adjusted for measures of subclinical atherosclerosis in other non-peripheral arterial beds. We observed a similar association of low ABI with LV mass in unadjusted analysis, however this association was substantially attenuated and rendered no longer statistically significant when adjusted for measures of subclinical atherosclerosis. In the context of prior studies in experimental animals, these data suggest that high ABI may be associated with LV mass through pathways distinct from atherosclerosis. Moreover, previous studies in humans have demonstrated that high ABI identifies individuals at increased risk for all-cause and CVD-mortality, non-fatal CVD events, and incident CHF.(8–11) Higher LV mass has also been associated with these same outcomes in community-living populations.(28) Future studies are required to determine if higher LV mass may represent a causal intermediary between high ABI and CVD events.
In rodent studies, experimentally induced MAC resulted in greater arterial stiffness and LV mass. The change in LV mass was strongly correlated to the amount of vascular calcium content, and this relationship was observed despite an absence of change in mean arterial pressure or arterial diameter, suggesting that MAC may lead to LV mass through mechanisms distinct from atherosclerosis.(12) These data are relevant here because high ABI measurements are widely regarded as a marker of MAC.(3–5) Indeed, ankle SBP measurements > 190mmHg have 90% specificity for characteristic x-ray patterns of MAC in humans, albeit with poor sensitivity (~50%).(5) Thus, it is possible that the association of high ABI with LV mass demonstrated here reflect similar biology to that observed in rodent models of MAC.
However, in referral populations of patients with symptomatic PAD, prior studies have demonstrated that 24–75% of subjects found to have high ABI also had concomitant atherosclerotic PAD when interrogated by other confirmatory tests.(13–15) While the coexistence of high ABI measurements and atherosclerotic PAD in community-living, largely asymptomatic populations is unknown, it remains possible that the associations of high ABI and LV mass may reflect the consequences of undetected atherosclerotic PAD. To provide insights into this competing hypothesis, we conducted our study in a cohort without clinically apparent CVD, and took advantage of several measurements of subclinical atherosclerosis in non-peripheral arterial beds. Statistical adjustment for these measurements substantially attenuated the association of low ABI with LV mass, perhaps because both low ABI and these other measures are markers of the same systemic disease; atherosclerosis. In contrast, adjustment for markers of subclinical atherosclerosis had relatively little effect on the association of high ABI with LV mass. These data and prior experimental animal data are consistent with the hypothesis that high ABI may identify a pathologic entity distinct from atherosclerosis, and may provide insights into novel non-atherosclerotic pathways leading to higher LV mass. Whereas the mechanisms responsible for this association are uncertain, we hypothesize that MAC may lead to arterial stiffness, and may contribute to more rapid reflection in the arterial pulse-wave during diastole, contributing to chronic increased left ventricular after-load.
It remains possible, however, that subjects with high ABI in our study had undetected atherosclerotic PAD despite adjustment for subclinical atherosclerosis in other arterial beds. Moreover, despite the reported high specificity of high ankle SBP measurements for MAC, our study did not include plain radiographs of the lower limbs to confirm the characteristic x-ray pattern of this disease. Future studies that simultaneously provide x-ray characterization of patterns of calcification and secondary confirmatory tests for atherosclerotic PAD are required to confirm and extend our findings.
Strengths of this study include its evaluation in a large, ethnically and geographically diverse population without clinical CVD, and the simultaneous availability of cardiac MRI and subclinical atherosclerosis measurements. The study also has important limitations. First, while we observed a dose-response relation between increasing levels of ABI and LV mass, few subjects had ABI > 1.40 (n=53, 1%); the level at which risk for CVD events was increased in prior studies. Second, as stated above, we lack x-ray data of the lower limbs to confirm that subjects with high ABI had MAC, thus non-calcific pathology may have contributed to lower limb arterial stiffness, high ABI, and LV mass. Third, while extensive statistical adjustment was undertaken to account for atherosclerosis in non-peripheral arterial beds, it remains possible that subjects with high ABI had concomitant atherosclerotic PAD. Future studies with confirmatory test for atherosclerotic PAD are required. This study is cross-sectional, and cannot evaluate temporality of associations. We used cut-points to define low ABI and high ABI that were used in prior studies to facilitate comparison, and a high ABI cut-point that was consistently associated with CVD events in prior studies.(8–10) Our analysis demonstrated a U-shaped relationship, and a more narrow ABI range for the intermediate group would have captured individuals with the lowest LV mass. However, results were similar in sensitivity analyses where the intermediate group was limited to individuals with ABI between 1.00 and 1.30. Finally, we observed commensurate increases in LV mass and LVEDV in subjects with high ABI. Results may differ in older cohorts or among persons with established CVD, where the spectrum of disease and time of exposure to high ABI and / or other associated risk factors may have been more prolonged or severe.
In conclusion, high ABI measurements are strongly associated with greater LV mass in community-living persons without clinical CVD. This association was not materially altered when adjusted for subclinical atherosclerosis in non-peripheral arterial beds. Future studies are required to determine if high ABI may lead to greater LV mass through non-atherosclerotic pathways, and whether or not greater LV mass may be a causal intermediary between high ABI measurements and CVD events and heart failure.
ACKNOWLEDGEMENTS
The authors thank the invaluable contributions of the MESA study participants and staff to this manuscript, and Ms. Clydene Nee for assistance in manuscript review.
Support:
This study was supported by the National Heart Lung and Blood Institute (NHLBI) grant R21HL091217 (JHI), an American Heart Association Fellow to Faculty Transition Award, and contracts N01-HC-95159 through N01-HC-95169 from the NHLBI.
List of Abbreviations
- ABI
Ankle Brachial Index
- BMI
Body Mass Index
- CVD
Cardiovascular Disease
- DP
Dorsalis Pedis
- PT
Posterior Tibial
- CHF
Congestive Heart Failure
- CAC
Coronary Artery Calcification
- cIMT
Carotid Intima Media Thickness
- CRP
C-Reactive Protein
- eGFR
Estimated Glomerular Filtration Rate
- LV
Left Ventricular
- MRI
Magnetic Resonance Imaging
- MAC
Medial Arterial Calcification
- PAD
Peripheral Arterial Disease
- LVEDV
Left Ventricular End Diastolic Volume
- LVESV
Left Ventricular End Systolic Volume
- M/V Ratio
Mass Volume Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Statement of Conflict of Interest:
There are no financial conflicts of interest to disclose.
REFERENCES
- 1.Quigley FG, Faris IB, Duncan HJ. A comparison of Doppler ankle pressures and skin perfusion pressure in subjects with and without diabetes. Clin Physiol. 1991;11:21–25. doi: 10.1111/j.1475-097x.1991.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 2.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Strandness DE., Jr Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association September 18–20, 1992 New Orleans, Louisiana. Circulation. 1993;88:819–828. doi: 10.1161/01.cir.88.2.819. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36:615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- 6.Micheletti RG, Fishbein GA, Currier JS, Fishbein MC. Monckeberg sclerosis revisited: a clarification of the histologic definition of Monckeberg sclerosis. Arch Pathol Lab Med. 2008;132:43–47. doi: 10.5858/2008-132-43-MSRACO. [DOI] [PubMed] [Google Scholar]
- 7.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 8.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 9.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 10.Sutton-Tyrrell K, Venkitachalam L, Kanaya AM, et al. Relationship of ankle blood pressures to cardiovascular events in older adults. Stroke. 2008;39:863–869. doi: 10.1161/STROKEAHA.107.487439. [DOI] [PubMed] [Google Scholar]
- 11.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292–1298. doi: 10.1016/j.jacc.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 12.Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension. 1997;29:999–1006. doi: 10.1161/01.hyp.29.4.999. [DOI] [PubMed] [Google Scholar]
- 13.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008 doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Suominen V, Rantanen T, Venermo M, Saarinen J, Salenius J. Prevalence and Risk Factors of PAD among Patients with Elevated ABI. Eur J Vasc Endovasc Surg. 2008;35:709–714. doi: 10.1016/j.ejvs.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med. 2006;11:29–33. doi: 10.1191/1358863x06vm663oa. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 19.DuBois D, DuBios EF. A formula to estimate the approximate surface area if height and weight are known. Archives of Internal Medicine. 1916;17:863–871. [Google Scholar]
- 20.Rosen BD, Edvardsen T, Lai S, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003:S5–S20. doi: 10.2337/diacare.26.2007.s5. 26 Suppl 1. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2001;11:A0828. [Google Scholar]
- 28.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]