Abstract
IL-4 promotes the development of Th2 cells and allergic inflammation. In Atopic Dermatitis (AD) lesions, IL-4 decreases the expression of multiple genes associated with innate defense including genes in the epidermal differentiation complex (EDC) that regulate epidermal barrier function. However, it is not clear whether IL-4 also contributes to homeostatic control of EDC genes. In this report we demonstrate that expression of EDC genes and barrier function is increased in the absence of endogenous IL-4. Mice that express a constitutively active Stat6 (Stat6VT) are prone to the development of allergic skin inflammation, and have decreased expression of EDC genes. IL-4-deficiency protects Stat6VT transgenic mice from the development of allergic skin inflammation and decreased recovery time in barrier function following skin irritation, with a concomitant increase in EDC gene expression. These data suggest that IL-4 plays an important role in regulating epidermal homeostasis and innate barrier function.
Introduction
The development of allergic responses relies upon the coordinated production of cytokines to promote effector T cell development and the recruitment of inflammatory cells to the target organ. In the development of allergic inflammation, the current paradigm is that IL-4 promotes the development of Th2 cells, while IL-13 is critical in promoting tissue inflammation. This model predicts that these cytokines would only be present and have effects on the target organ during the occurrence of disease. However, it is possible that nominal amounts of cytokines in vivo have an effect on tissues even in the absence of pathology.
Allergic inflammation in the skin results in Atopic Dermatitis (AD)3, a chronic disease characterized by intense pruritis, dryness of the skin and erythema in localized lesions (1-3). AD lesions have eosinophil and mononuclear cell infiltrates and Th2 cytokines are observed in lesional tissue. IL-4 plays multiple roles in promoting AD. In isolated keratinocytes, IL-4 decreases the expression of genes in the Epidermal Differentiation Complex (EDC) that contribute to barrier function and innate immune defense (4-6). Expression of transgenic IL-4 in the skin results in allergic inflammation that resembles AD (7) while systemic expression results in remodeling of the skin (8). Moreover, mice transgenic for the human IL4-IL-13-RAD50-IL5 loci develop pathologies similar to AD (9). Together, these studies show that IL-4 is an important contributing factor to the development of allergic inflammation in the skin.
In this report, we show that in the absence of endogenous IL-4, mice have increased expression of genes that contribute to skin barrier integrity resulting in altered function, compared to wild type mice. Mice that express a constitutively active Stat6 develop AD-like lesions and have decreased expression of EDC genes. Constitutively active Stat6 transgenic mice that lack endogenous IL-4 are protected from skin inflammation and recover barrier function faster following skin irritation than mice expressing active Stat6 on a wild type background. Thus, IL-4 regulates barrier function homeostasis.
Materials and Methods
Generation of Stat6VT transgenic mice
The generation of Stat6VT transgenic mice was previously described (10). Transgene positive founders (CD2:Stat6VT (78) line), where the human Stat6 gene with V625 and T626 mutated to alanine is under transcriptional control of the CD2 locus control region, were backcrossed to C57BL/6 mice (Harlan Bioproducts for Science). IL-4-deficient mice were purchased from The Jackson Laboratory and mated to Stat6VT transgenic mice. All mice were maintained in specific pathogen-free conditions and experiments were approved by the Indiana University Institutional Animal Care and Use Committee.
Selectin ligand expression
P- and E-selectin ligand expression was assessed directly ex vivo from wild type and transgenic T cells on CD4+ T cells as described previously (11).
Isolation of RNA from skin and real time PCR
For real time PCR measurements, involved or uninvolved skin was homogenized in a tissue lyser (Qiagen), and RNA isolated with RNeasy fibrous tissue kit (Qiagen) was used to synthesize cDNA with First-Strand Cloned AMV kit (Invitrogen, Rockville, MD). Message levels of cytokines and barrier function genes were determined by Taqman assay (Applied Biosystems, Foster City, CA). Cycle number of duplicate samples was normalized to the expression of an endogenous control, either β2-microglobulin or GAPDH.
Western analysis of EDC proteins
Shaved dorsal skin from wild type, Il4-/- or Stat6VT mice was stretched and frozen in liquid nitrogen. Epidermis was mechanically separated from the dermis by scratching. Epidermis was then homogenized in ice-cold lysis buffer containing 1% TritonX-100, 0.5% deoxycholate, 0.1% SDS, 2mM EDTA, 10% Glycerol, 150mM NaCl, 50mM Tris, pH 7.4, and protein inhibitors (1mM phenylmethanesulphonylfluoride, aprotinin at 1 mg/ml, and leupeptin at 1 mg/ml). Western blot analysis was performed as previously described (12) using specific antibodies against filaggrin (Abcam Inc. Cambridge, MA) and involucrin (Covance, IN), or control antibody GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). For densitometric analysis the scanned images of immunoblots were quantified with Image J software.
Antibody treatments
The dorsal side of ears from Stat6VT transgenic mice was injected with isotype control antibody (Rat IgG1 isotype control, eBioscience) in the left ear and either anti-IL-4 (rat anti-mouse IL-4, clone 11B11) or anti-IL-13 antibody (rat anti-mouse IL-13 antibody, eBioscience) in the right ear on days 0 and 1 respectively. On day 2, the ear skin corresponding to the injection site was excised 18h later and RNA was isolated from the skin for real time PCR measurements.
Transepidermal water loss (TEWL) measurements
Female wild type C57BL/6, Stat6VT, Il4-/- Stat6VT mice (4-6 months old) were used for TEWL measurements. Briefly, the mice were anesthetized with ketamine/xylazine (100/10 μg/kg body weight) before the backs of mice were shaved. Twenty-four hours later, baseline TEWL measurements were taken with an evaporimeter and 0.1 ml 0.1% retinoic acid (Sigma, Aldrich) was applied topically to irritate skin. The following day TEWL was measured. The process of application of retinoic acid and TEWL measurements were repeated for 5 days until the levels for TEWL reached 100-150 g/m2 per h. The recovery of TEWL was determined at the time points indicated.
Epicutaneous sensitization and skin DC migration to draining lymph nodes
Wild type and Il4-/- mice were epicutaneously sensitized with OVA-Alexafluor 647 (Invitrogen). Briefly, the back skin of anesthetized mice was shaven, and tape stripped 3× before painting with 500μg OVA-Alexafluor 647. Twenty-four hours later, mice were sacrificed before draining lymph nodes were harvested. Cells were first incubated with anti-CD16/CD32 mAb (2.4G2; BD Biosciences) and stained with FITC anti-mouse MHC class II and PE anti-mouse CD11c (BD Biosciences). The proportion of OVA-Alexafluor 647+ cells was quantified by gating on 7-aminoactinomycin-negative, MHC II+ CD11c+ cells on a FACSCalibur.
Results
Altered barrier function in the absence of endogenous IL-4
While IL-4 regulates the expression of genes involved in skin barrier integrity during AD, it is not clear if nominal levels of IL-4 contribute to homeostatic barrier integrity. To test this, we isolated RNA from ear tissue of wild type and Il4-/- C57BL/6 mice and tested for the expression of genes associated with skin barrier integrity. While the expression of keratin 14 (Krt14) was not different between wild type and Il4-/- tissue, we observed 2-3-fold increases in Loricrin (Lor), Spink5, Kalikrein 7 (Klk7) and Transglutaminase 3 (Tsg3) (Fig. 1A and data not shown). We observed 5-15-fold increases in the expression of filaggrin (Flg) and involucrin (Ivl). To confirm that changes in mRNA levels resulted in altered protein expression, we examined protein levels of filaggrin and involucrin in epidermal protein extracts from wild type and Il4-/- mice. Expression assessed by immunoblot was averaged from 3-5 mice and showed a 40% increase in the expression of both proteins (Fig. 1B).
Figure 1.
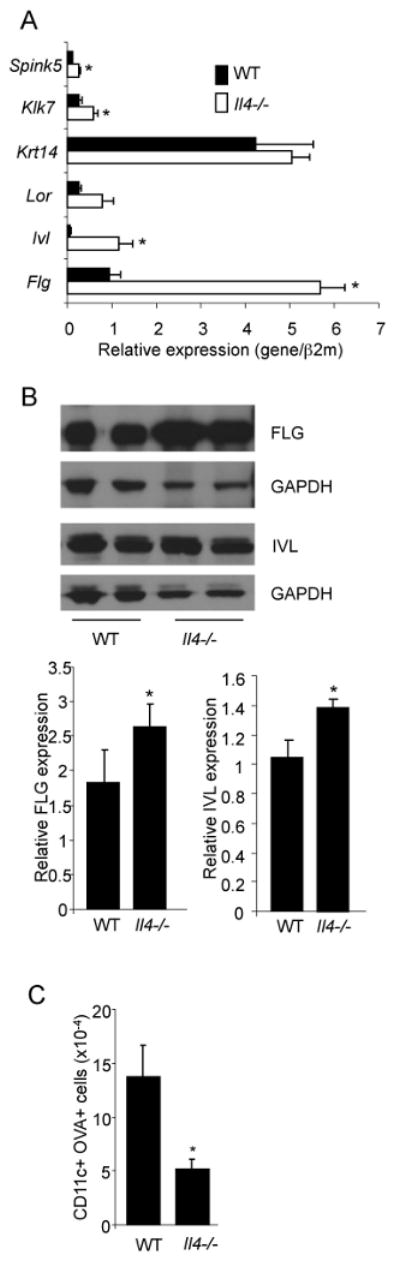
IL-4-deficiency increases skin barrier function. A, RNA was isolated from wild type and Il4-/- ear tissue before analysis of gene expression by qPCR. Results are the average ± SEM from samples of 4-6 mice. Results are presented as expression of each gene relative to the control (β2-microglobulin) using the 2-ΔCt method. B, Immunoblots of protein extracts from epidermis of WT and Il4-/- mice for filaggrin (FLG) and involucrin (IVL) with GAPDH as a loading control. Bar graphs represent the average densitometry of 3-5 samples normalized to expression of GAPDH. C, The backs of wild type and Il4-/- mice were shaved and applied with OVA-Alexa647. Twenty-four hours later draining lymph nodes were isolated and dendritic cells (CD11c+MHCIIhi) were examined for Alexa647 fluorescence. Numbers are the average ± SEM of 4 mice, calculated as percent Alexa647+ DC multiplied by cell number, and is representative of 3 experiments. *, significantly different from WT, p<0.05.
To determine if increased expression of these genes had functional consequences, and knowing that dendritic cell function is normal in the absence of IL-4 (13), we tested the ability of protein antigen to cross the skin and be taken up by dendritic cells. The shaved backs of wild type and Il4-/- mice were painted with Alexa647-labelled ovalbumin and 24 hours later Alexa647 fluorescence was assessed in CD11c+ cells present in the draining lymph nodes. Significantly fewer Alexa647+ CD11c+ cells were observed in Il4-/- mice than in wild type mice (Fig 1C). These results suggest that in the absence of endogenous IL-4, there is increased barrier function.
Atopic Dermatitis in mice expressing a constitutively active Stat6 in T cells
We next wanted to test if IL-4-defciency would affect allergic skin inflammation. We have previously described mice that express constitutively active Stat6 in T cells (10). These mice are prone to allergic inflammation, including blepharitis and recruitment of eosinophils and lymphocytes to the lung (14). These mice are also prone towards the development of skin inflammation that appears similar to atopic dermatitis. Stat6VT transgenic mice display increased scratching behavior, resulting in dermatitic plaques with alopecia on the face, back or other areas of the body (Fig. 2A). Lesions can become chronically irritated and infected resulting in more severe inflammation (Fig. 2A). Examination of ear tissue by histology, even in mice that do not have obvious lesions, shows considerable thickening of the dermis and epidermis with cellular infiltration of eosinophils and lymphocytes (Fig. 2B). In 3-6 month old mice that do not have AD lesions, there is a significant increase in Il4 mRNA in ear tissue isolated from Stat6VT transgenic mice, compared to wild type mice (Fig 2C). In contrast, expression of other Th2 cytokines including Il13, Th17 cytokines Il17 and Il17f, and the Th1 cytokine Ifng, were not significantly increased in non-lesional skin (data not shown). However, in mice with AD lesions, Il4 and Il13 mRNA were increased in both lesional and non-lesional tissue (Fig. 2D). Peripheral T cells from Stat6VT transgenic mice had increased percentages of selectin-ligand-positive CD4+ T cells, suggesting an increased propensity for migration to the skin (Fig. 2E).
Figure 2.
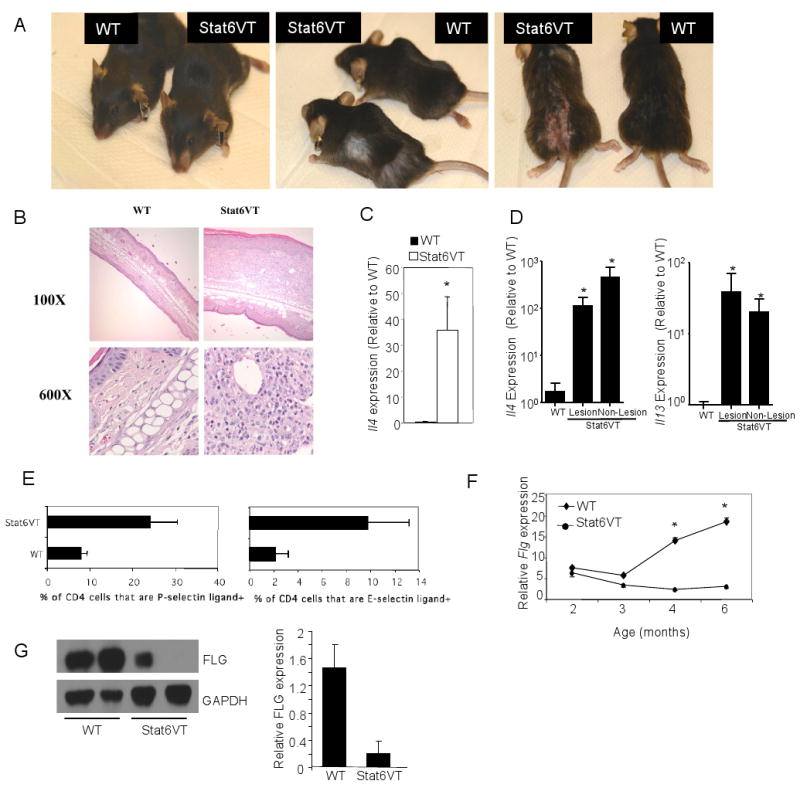
Mice expressing constitutively active Stat6 in T cells develop allergic skin inflammation. A, Photographs of Stat6VT transgenic mice and littermate controls showing areas affected by skin inflammation. B, Histological analysis of ear tissue from wild type and Stat6VT transgenic mice. Samples were fixed and stained with hematoxylin/eosin. C, RNA was isolated from skin of wild type and Stat6VT transgenic mice that did not have lesions before analysis of cytokine mRNA using qPCR. D, RNA was isolated from skin of wild type and lesional or non-lesional skin of Stat6VT transgenic mice that had active lesions before analysis of cytokine mRNA using qPCR. Results are the average ± SEM of 4-5 mice. E, Selectin ligand expression was examined on CD4+ splenic T cells using flow cytometry. Results are the average ± SD of 2-4 mice and are representative of two experiments. F, RNA was isolated from skin of wild type and Stat6VT transgenic mice that did not have lesions before analysis of Flg mRNA using qPCR. G, Immunoblots of protein extracts from epidermis of WT and Stat6VT transgenic mice for filaggrin (FLG) with GAPDH as a loading control. Bar graphs represent the average densitometry of 3-5 samples normalized to expression of GAPDH. Results in panels C, D and F are the average ± SEM of 5-9 mice. *, significantly different from WT, p<0.05.
Since IL-4-deficiency increased the expression of Flg we tested whether expression of this gene was altered in skin from Stat6VT transgenic mice. At early time points (2-3 months) Flg expression was not different between wild type and Stat6VT skin (Fig. 2F). Expression of Flg increased over time in wild type mice, but not in Stat6VT transgenic mice resulting in significant Flg expression differences between transgenic and non-transgenic mice (Fig. 2F). We observed a similar decrease in filaggrin protein expression (Fig. 2G). The time frame where we observed a difference in Flg expression (4-6 months) was also the time frame where symptoms of skin inflammation became apparent.
IL-4-deficiency protects from the onset of allergic skin inflammation in Stat6VT transgenic mice
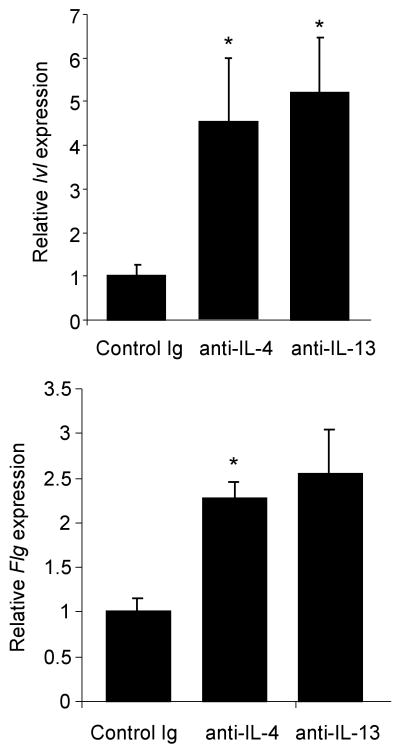
We next wanted to determine if blocking cytokines that promote allergic inflammation had an effect on EDC gene expression. To test this, we performed intra-dermal injections of control Ab, anti-IL-4 or anti-IL-13 into the dorsal side of ears from Stat6VT transgenic mice (age 4-5 months). Mice received two daily injections and skin was harvested for analysis of mRNA using qPCR. We observed that either anti-IL-4 or anti-IL-13 treatment led to increased Ivl expression (Fig. 3) while only anti-IL-4 significantly increased Flg expression (Fig. 3).
Figure 3.
Antibodies to Th2 cytokines increase EDC gene expression in Stat6VT transgenic mice. Antibodies (control, anti-IL-4, anti-IL-13; all IgG1) were injected intradermally into the dorsal side of the ears of Stat6VT transgenic mice daily for two days. Eighteen hours after the last injection RNA was isolated from ear tissue and used for qPCR of Ivl and Flg expression. Results are the average ± SEM of 3-5 mice. *, significantly different from control Ig treated mice, p<0.05.
Ideally, mating Stat6VT transgenic mice to Il4-/- and Il13-/- mice would define the relative role of each cytokine in allergic skin inflammation. However, Il13-/- mice also have deficiencies in IL-4 production (15) that would complicate the interpretation of that analysis. Thus, to determine if IL-4-deficiency would protect from the development of allergic skin inflammation, we generated Il4-/- Stat6VT transgenic mice. We have previously shown that in the absence of endogenous IL-4, Stat6VT-expressing T cells are still capable of developing into Th2-like cells expressing IL-5 and IL-13 (14). While AD-like symptoms appear in about 40% of Stat6VT transgenic mice, there was no observed skin inflammation in wild type or Il4-/- Stat6VT transgenic mice. Histology indicated that the dermis of Il4-/- Stat6VT transgenic mice was thickened compared to wild type skin, though still less than that observed in Stat6VT transgenic mice on a wild type background, and lacked eosinophilic infiltration (Fig. 4A). Skin from Stat6VT transgenic mice had decreased expression of several EDC genes, including Lor, Ivl, and most prominently, Flg (Fig. 4B). However, IL-4-deficiency was able to increase the expression level of these genes in Stat6VT transgenic mice above wild type levels, similar to the pattern observed in non-transgenic Il4-/- skin (Fig. 1A and 4B), suggesting that eliminating IL-4 increased the barrier function of skin in Stat6VT transgenic mice and attenuated the propensity for the development of allergic inflammation.
Figure 4.
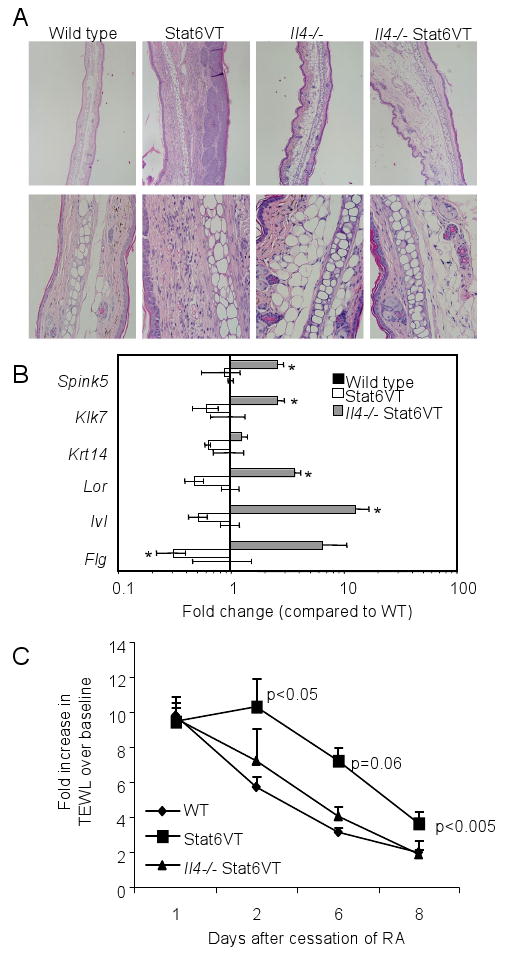
IL-4-deficiency protects from the development of allergic skin inflammation and promotes recovery from injury. A, Histology of ear tissue from mice of the indicated genotypes. Samples were fixed and stained with hematoxylin/eosin. B, RNA was isolated from ear tissue of wild type, Stat6VT transgenic and Il4-/- Stat6VT transgenic mice before analysis of gene expression by qPCR. Results are the average ± SEM from samples of 4-6 mice. Wild type values are equal to one, and are not above the axis. C, Fold-increase in pre-treatment TEWL following retinoic acid application was examined at the indicated time points. Results are the average ± SEM of 4-6 mice. *, significantly different from WT, p<0.05.
To directly test if IL-4-deficiency affects skin homeostasis, we irritated shaved skin by applying topical retinoic acid for one week and examined recovery of transepidermal water loss (TEWL) over the following week. Application of the retinoic acid resulted in a 10-fold increase in TEWL from baseline levels that recovered to near baseline levels after 8 days without treatment (Fig. 4C). Stat6VT transgenic mice had a slower recovery, still having a 4-fold elevated TEWL after 8 days. In contrast, Il4-/- Stat6VT transgenic mice had recovery in TEWL similar to wild type mice (Fig. 4C).
Discussion
Allergic inflammation results from a complex interaction between environment, immune system and target organ that has not been well defined. It is still unclear whether a primary defect lies in a target organ that provides insufficient barrier function or in an inappropriate Th2 immune response that develops to innocuous antigen. While polymorphisms in genes required for skin barrier function are associated with AD (16-18), it is unclear if these result in altered gene expression, or perhaps altered sensitivity to cytokines produced during an allergic response. In this report, we used a model of atopic dermatitis arising from T cell-specific expression of an active Stat6 to test whether predisposition to a Th2 response would result in specific changes in target organ gene expression. We demonstrated increased EDC gene expression and barrier function in the absence of endogenous IL-4, contrasting with decreased EDC gene expression and allergic skin inflammation when the immune system is predisposed towards a Th2 response. Importantly, IL-4-deficiency protects from the development of allergic skin inflammation and hastens the recovery of barrier function following skin irritation.
The ability of IL-4 to regulate basal expression of EDC genes could result from several mechanisms. Effects could be direct resulting from homeostatic levels of IL-4 present in the circulation or the tissue that act directly on the keratinocytes (5). It has not been demonstrated whether IL-4-induced changes in keratinocyte gene expression are a result of STAT6 binding to EDC gene promoters, or interference with other transcription factors that positively regulate mRNA levels. Effects could also be indirect, and in the absence of IL-4 the production of Th1 or Th17 cytokines that affect expression of EDC genes could be altered (5, 19). However, we did not observe increases in mRNA levels of Ifng or Il22 in skin from Il4-/- mice (data not shown). That expression of Ivl was increased most by the absence of IL-4, while Flg was decreased the most in the presence of a high Th2 environment (Stat6VT transgenic mice), suggests that EDC gene regulation is likely more complex than simply the presence or absence of IL-4.
The model of AD in Stat6VT transgenic mice is unique in that disease results from a perturbation of T cell function, not of ectopic gene expression within the skin, and is dependent on IL-4 for development. This is important as allergic skin inflammation in other mouse models of AD may appear as Th1-mediated inflammation in mice that are genetically-deficient in the ability to develop Th2 responses (3). Thus, the dependence on IL-4 for disease development in this model is significant. The requirement for a specific effector cytokine suggests that while genetic predisposition to changes in barrier function including mutations in FLG is an important risk factor in AD development (17, 18), a strong skewing of immune function can have similar effects. The increased Th2 cytokine production in Stat6VT transgenic mice decreases expression of multiple genes in the EDC complex resulting in decreased barrier function, which at least partially contributes to the development of allergic skin inflammation. Our results also provide a mechanism for the AD-like disease that develops in IL-4 transgenic models (9, 20).
While IL-4 is clearly required for the development of allergic skin inflammation in Stat6VT transgenic mice, the relative roles of IL-4 and IL-13 are not fully defined. We demonstrate that acute treatment of skin from Stat6VT transgenic mice with either anti-IL-4 or anti-IL-13 increased the level of Ivl and Flg mRNA. Other reports have shown that treatment of keratinocytes with IL-4 and IL-13 can decrease EDC gene expression (4, 6). But while transgenic mice expressing IL-4, or IL-4 and IL-13, develop allergic skin inflammation (7, 9), the ability of IL-13 alone to promote allergic skin inflammation has not been determined. The decreases in IL-4 production caused by targeting the Il13 locus prevent a precise determination of the role of IL-13 in this model (15). Importantly, the ability of IL-4-deficiency to prevent allergic skin inflammation in Stat6VT transgenic mice, and that IL-13-secreting cells develop in Il4-/- Stat6VT transgenic mice (14), suggests that while both cytokines can induce regulation of EDC genes, they are not functionally redundant in the development of allergic skin inflammation.
The development of AD in patients relies on multiple factors. As mentioned above, mutations in FLG are a significant risk factor for AD (17, 18), and barrier function, as assessed by increased TEWL, is decreased (21-24). Similarly, a family history of allergic disease, which likely reflects an increased propensity for development of allergic responses, is a significant risk factor (25, 26). Importantly our studies show that the effect of IL-4 on keratinocyte gene expression is not restricted to active lesions (4-6), but that it also contributes to homeostatic expression of genes involved in skin barrier function. Thus, increases in systemic or local IL-4, through the ability to regulate barrier function, may be a predisposing factor for the development of AD. Modulating the levels of IL-4 in patients might be an effective method for not only treating lesions, but also limiting future exacerbations.
Acknowledgments
The authors thank M Al-Hassani and Q Yi for technical assistance, M Southall for guidance in TEWL studies, and R Tepper and B Zhou for review of this manuscript.
Footnotes
Abbreviations used: AD, atopic dermatitis; EDC, epidermal differentiation complex, TEWL, transepidermal water loss
This work was supported by grants from the NIH (U19AI070448 to MHK and JBT; RO1AR41256 to DYML) and a Veteran's Administration Merit Award (JBT).
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehra S, Tuana FM, Holbreich M, Mousdicas N, Tepper RS, Chang CH, Travers JB, Kaplan MH. Scratching the surface: towards understanding the pathogenesis of atopic dermatitis. Crit Rev Immunol. 2008;28:15–43. doi: 10.1615/critrevimmunol.v28.i1.20. [DOI] [PubMed] [Google Scholar]
- 4.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–983. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 8.Elbe-Burger A, Egyed A, Olt S, Klubal R, Mann U, Rappersberger K, Rot A, Stingl G. Overexpression of IL-4 alters the homeostasis in the skin. J Invest Dermatol. 2002;118:767–778. doi: 10.1046/j.1523-1747.2002.01753.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee GR, Flavell RA. Transgenic mice which overproduce Th2 cytokines develop spontaneous atopic dermatitis and asthma. Int Immunol. 2004;16:1155–1160. doi: 10.1093/intimm/dxh117. [DOI] [PubMed] [Google Scholar]
- 10.Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003;170:3478–3487. doi: 10.4049/jimmunol.170.7.3478. [DOI] [PubMed] [Google Scholar]
- 11.White SJ, Underhill GH, Kaplan MH, Kansas GS. Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J Immunol. 2001;167:628–631. doi: 10.4049/jimmunol.167.2.628. [DOI] [PubMed] [Google Scholar]
- 12.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JM, Yang J, Ronchese F. IL-4 deficiency does not impair the ability of dendritic cells to initiate CD4+ and CD8+ T cell responses in vivo. Int Immunol. 2004;16:1451–1458. doi: 10.1093/intimm/dxh145. [DOI] [PubMed] [Google Scholar]
- 14.Sehra S, Bruns HA, Ahyi AN, Nguyen ET, Schmidt NW, Michels EG, von Bulow GU, Kaplan MH. IL-4 Is a Critical Determinant in the Generation of Allergic Inflammation Initiated by a Constitutively Active Stat6. J Immunol. 2008;180:3551–3559. doi: 10.4049/jimmunol.180.5.3551. [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Hu-Li J, Zhu J, Pannetier C, Watson C, McKenzie GJ, McKenzie AN, Paul WE. Disrupting Il13 impairs production of IL-4 specified by the linked allele. Nat Immunol. 2001;2:461–466. doi: 10.1038/87778. [DOI] [PubMed] [Google Scholar]
- 16.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–988. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 17.Baurecht H, Irvine AD, Novak N, Illig T, Buhler B, Ring J, Wagenpfeil S, Weidinger S. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120:1406–1412. doi: 10.1016/j.jaci.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 18.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 20.Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 21.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa'ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730 e722. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 22.Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682–689. doi: 10.1038/jid.2008.280. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai K, Sugiura H, Matsumoto M, Uehara M. Occurrence of patchy parakeratosis in normal-appearing skin in patients with active atopic dermatitis and in patients with healed atopic dermatitis: a cause of impaired barrier function of the atopic skin. J Dermatol Sci. 2002;30:37–42. doi: 10.1016/s0923-1811(02)00047-6. [DOI] [PubMed] [Google Scholar]
- 24.Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1985;65:102–105. [PubMed] [Google Scholar]
- 25.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–725. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 26.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–1190. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]