Systemic lupus erythematosus (SLE) is arguably the most clinically and serologically diverse autoimmune disease (1, 2). Currently available information suggests that intricate interactions between environmental factors, hormonal factors, and disease susceptibility genes may predispose an individual to develop aberrant immune responses leading to SLE. Such aberrant responses, characterized by polyclonal activation of autoreactive lymphocytes, autoantibody production, immune complex formation, and complement activation, lead to acute and chronic inflammation in various tissue and organ systems. Owing to its complex etiopathogenesis, heterogeneous presentation, and unpredictable course, SLE remains one of the greatest challenges to both investigators and physicians. Currently, the diagnosis of SLE is primarily based on the presence or absence of American College of Rheumatology (ACR) criteria (3, 4). Disease activity in SLE patients is often assessed using indices such as the Systemic Lupus disease Activity Index (SLEDAI) (5), the Systemic Lupus Activity Measurement (SLAM) (6, 7), and the British Isles Lupus Assessment Group (BILAG) index (8). The lack of easy-to-measure, reliable and specific biomarkers for SLE not only hampers precise assessment of disease activity and accurate evaluation of response to treatment, but it also impedes the development of novel therapeutics targeting key pathogenic factors. Therefore, there is an urgent need for reliable, specific biomarkers in not only lupus patient care but also in research.
Complement and SLE: The Historical Bond
The complement system has been linked more intimately to SLE than to any other human disease (9–11). The involvement of complement proteins in the etiopathogenesis of SLE has been recognized and investigated for decades. In the current era of biomarkers and targeted therapeutic approaches, investigators’ interest in the complement system has naturally been reinvigorated.
The complement system
The complement system comprises a group of plasma and membrane-bound proteins that form three distinct - classical, alternative, and lectin - pathways designed to protect the host against invasion of foreign pathogens. The classical pathway is activated by antibodies or antigen-antibody complexes (immune complexes) (12–14). Distinct from the antibody-dependent classical pathway, the alternative pathway is initiated when spontaneously generated complement components bind to surfaces of invading organisms or self-tissues, whereas the lectin pathway is triggered when mannose-binding lectins attach to polysaccharides uniquely expressed on the surface of microorganisms. Overall, the cascading reaction of complement activation generate proteolytic fragments that are capable of attracting inflammatory cells, inducing production and release of inflammatory mediators, and tagging invading organisms to be promptly phagocytosed by neutrophils and monocytes. Consequently, activation of the complement system may lead to not only physiologic immune responses but also pathologic immune-inflammatory tissue damage. An unfortunate example of the latter is the myriad disease manifestations in SLE.
C3 is the most abundant protein in the complement system and also the indispensable molecule involved in all three activation pathways of the complement system (12–14). C4 is the second most abundant component and is essential for the classical and lectin pathways. C3 and C4 are synthesized predominantly in the liver and undergo post-translational modification to become 3-chain proteins linked by disulfide bonds. Both C3 and C4 contain an internal thioester site that is located within the C3d and C4d region in the chain of each respective parental molecule (15, 16). During activation of the classical pathway, proteolytic cleavage of C4 by C1s generates a small peptide C4a and a major fragment C4b. C4b contains the activated, highly reactive thioester that can readily interact with hydroxyl or amino groups on the receptive surface (e.g., pathogens, host cells, or immune complexes) to form covalent ester or amide linkages. Target-bound C4b then cleaves C2, resulting in the formation of C4bC2a complexes that function as the C3 convertase. Like C4, C3 is proteolytically cleaved during activation of the complement system, yielding a small peptide C3a and a large fragment C3b. Similar to C4b, C3b is capable of binding covalently to acceptor molecules on target surfaces via ester or amide linkages. C4b and C3b may be further cleaved by Factor I, yielding ultimately C4d and C3d.
Once C3b binds covalently to target cells, it recruits C5 and cleaves the latter into C5a and C5b. Binding of C5b to target cells initiates the formation of the C5b-9 membrane attack complexes (MAC). Perhaps in a dose-dependent manner, MAC inserted into the membrane can either activate (at sublytic levels) or cause lysis of target cells. In contrast to their larger, enzymatically active counterparts, the smaller fragments C4a, C3a, and C5a (collectively referred to as “anaphylatoxins”) are potent chemotactic factors that are capable of recruiting leukocytes into inflamed tissues. Moreover, by binding to specific receptors expressed on most infiltrating leukocytes and endothelial cells, these anaphylatoxins can cause activation and release of numerous inflammatory mediators from host cells, thereby aggravating and perpetuating inflammatory tissue damage.
Soluble complement proteins as lupus biomarkers
Because antibody/immune complex-triggered activation of the complement system is thought to play an important role in the pathogenesis of SLE, one might expect complement proteins to be consumed to an extent proportional to the disease activity. Thus, measures of complement C3 and C4 have historically been viewed as “gold standard” laboratory tests for SLE. Many physicians consider decreases in serum C3 and C4 levels as indications of increased inflammation and SLE disease activity. However, there are several drawbacks to this approach. First, there is a wide range of variation in serum C3 and C4 levels among healthy individuals, and this range overlaps with the range observed in SLE patients. Second, standard laboratory tests measure the concentration of parental C3 and C4 molecules rather than products of activation. Third, acute phase response during inflammation may lead to an increase in C4 and C3 synthesis, which can balance the activation and increased consumption of these proteins. Fourth, partial deficiencies of C4, which are commonly present in both the general population and SLE patients, may result in lower than normal serum C4 levels because of decreased synthesis rather than increased complement activation and/or active SLE. As a result of these confounding factors, there have been conflicting conclusions regarding the value of serial measurement of serum C4 in monitoring disease activity in SLE patients (17–21) (see also (22, 23) for recent reviews). Some studies have found serum C4 and C3 levels valuable in this regard, while others have found C4 and C3 levels to remain normal during SLE flares. These conflicting results suggest that current standard tests, based on serum levels of the native form of complement proteins, are inadequate to accurately and promptly detect SLE disease flares. During the past several years, other investigators have explored the potential for measurement of soluble complement activation products such as C3a, C5a, and C4d to serve as biomarkers in SLE (24–29). Despite some intriguing observations, serum levels of complement activation products have not replaced measurement of native C3 and C4 as gold standards.
Cell-Bound Complement Biomarkers: Complement Measures and Lupus Revisited
Given the less-than-satisfactory performance of soluble complement components as lupus biomarkers, there is strong incentive for developing alternative complement-based biomarkers.
Rationale for cell-bound complement biomarkers
Complement proteins are abundant in the circulation and in tissues. Besides floating freely as soluble proteins, both the parental molecules and their activation derivatives can readily interact with cells circulating in the blood (e.g., erythrocytes and lymphocytes) or tissues (e.g., endothelial cells). Conceivably, complement activation products generated during SLE flares may attach to various circulating and tissue cells and alter physiological functions of those cells. The rationale for exploring cell-bound complement biomarkers is as follows. First, most soluble complement activation products are easily subjected to hydrolysis in circulation or in tissue fluids and thus are short-lived. Second, activation products derived from C3 and C4 contain thioester bonds capable of covalently attaching to circulating cells and may decorate the surfaces for the lifespan of those cells (16). Third, many hematopoietic cells express receptors for proteolytic fragments generated upon complement activation. Fourth, products of C4 activation are known to be present on surfaces of erythrocytes of healthy individuals (30, 31). Therefore, cell-bound complement components have the potential to be long-lived and may perform more reliably than soluble complement proteins as biomarkers for SLE.
Experimental studies of cell-bound complement activation products
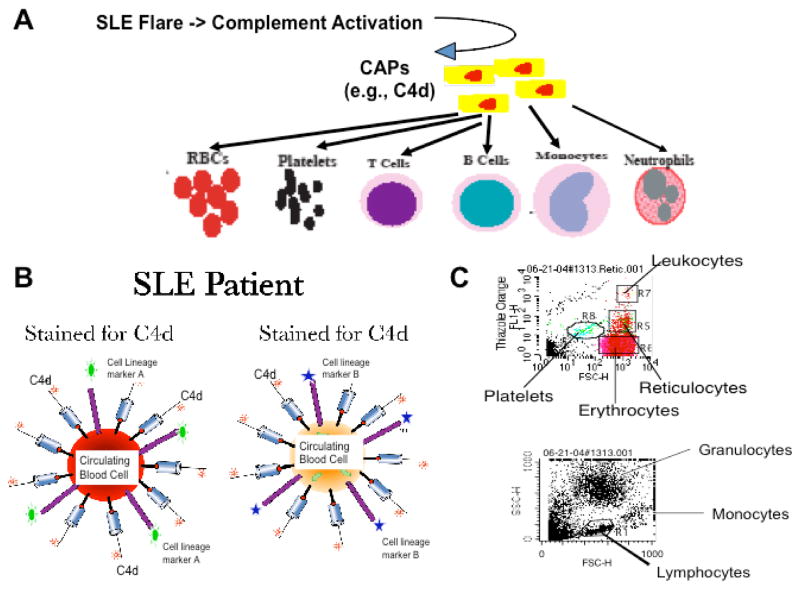
Recent studies in our laboratory have been focused on discovery and validation of cell-bound complement activation products (CB-CAP) as potential lupus biomarkers. Using flow cytometry assays, a unique CB-CAP phenotype of circulating blood cells that is highly specific for SLE has been identified (32–35) (Fig. 1).
Figure 1. Schematic summary of the rationale and methodology of the CB-CAP biomarkers.
(A) Rationale: During SLE flares, considerable amounts of complement activation products may be generated. These CAPs may bind stably to various circulating cells in proportion to the extent of complement activation. (B) Schematic illustration of the multicolor staining of circulating cells for cell type-specific surface markers and surface-bound CAPs (e.g., C4d). (C) Representative dotplots demonstrate the identification of erythrocytes, reticulocytes, platelets, lymphocytes, monocytes, and granulocytes. These cell types can also be differentiated using cell lineage-specific mAbs added to the cell suspension (e.g., anti-CD3, anti-CD19, etc).
Considering the physiological abundance and localization of erythrocytes, we have hypothesized that erythrocytes, circulating throughout the body and hence having easy assess to products derived from systemic as well as local activation of the complement system, may serve as biological beacons of the inflammatory condition in vivo (and hence the disease activity) in patients with SLE or other inflammatory diseases. To verify this hypothesis, the first CB-CAP study was a cross-sectional investigation examining erythrocyte-bound C4d (E-C4d) levels in patients with SLE (n=100), patients with other inflammatory and immune-mediated diseases (n=133), and healthy controls (n=84) (32). In light of the previous reported association of low E-CR1 levels in SLE, erythrocyte-CR1 (E-CR1) was determined simultaneously. This study demonstrated unambiguously for the first time that patients with SLE have significantly higher levels of E-C4d (specific mean fluorescence intensity [SMFI]=24.6±28.5) as compared to patients with other diseases (SMFI=9.3±6.5; p<0.001) and healthy individuals (SMFI=6.7±5.3; p<0.001).
A subsequent study took advantage of the knowledge that erythrocytes develop from hematopoietic stem cells in the bone marrow and emerge as reticulocytes, which then maintain distinct phenotypic features for 1–2 days before fully maturing into erythrocytes. Reticulocytes, if released into the peripheral circulation during an active disease state, may immediately be exposed to and bind C4-derived fragments generated from activation of the complement system. Therefore, it was hypothesized that the levels of C4d bound on reticulocytes (R-C4d) may effectively and precisely reflect the current disease activity in a given SLE patient at a specific point in time. The results of a cross-sectional study involving 156 patients with SLE, 140 patients with other autoimmune and inflammatory diseases, and 159 healthy controls showed that: 1) R-C4d levels of patients with SLE (SM(median)FI=5.5±9.0; range: 0.0–66.8) were significantly higher than those of patients with other diseases (SMFI=1.8±2.0; range: 0.0–17.6) or healthy controls (SMFI=1.4±0.7; range; 0.0–4.7), and 2) R-C4d levels fluctuated over time in patients with SLE and correlated with clinical disease activity as measured by the SLEDAI and SLAM indices (33).
Additional studies explored the possibility that CAP may also bind to non-erythroid lineages of circulating cells such as platelets and lymphocytes. A cross-sectional comparison of platelet-bound C4d (P-C4d) in patients with SLE (n=105), patients with other inflammatory and immune-mediated diseases (n=115), and healthy controls (n=100) showed that abnormal levels of C4d were present on platelets in 18% of SLE patients, 1.7% of patients with other diseases, and 0% of healthy controls (34). More recently, we tested our hypothesis by flow cytometric analysis of C4d on T and B lymphocytes (referred to as T-C4d and B-C4d, respectively) from patients with SLE (n=224), patients with other diseases (n=179), and healthy controls (n=114). Remarkably, both T-C4d and B-C4d levels were significantly and specifically elevated in SLE patients (SM(median)FI=12.1±20.5 (T-C4d) and 49.0±73.2 (B-C4d)), as compared with healthy controls (SMFI=1.7±1.0 (T-C4d) and 8.8±8.5 (B-C4d); both p<0.001) and patients with other diseases (SMFI=2.5±3.0 (T-C4d) and 14.7±26.8 (B-C4d); both p<0.001) (35).
Collectively, these studies strongly suggest a CB-CAP phenotype that is highly specific for patients with SLE. Moreover, we have noticed that high levels of C4d are not necessarily concurrently present on erythrocytes, reticulocyte, platelets, and lymphocytes of a given SLE patient at a particular time (Liu et al, manuscript in preparation). These findings suggest that binding of CAP to circulating blood cells does not merely reflect complement activation occurring during SLE disease flares, but may also reflect specific cellular and molecular mechanisms in lupus pathogenesis.
Clinical Applications of CB-CAP as Lupus Biomarkers
Despite extensive research and numerous trials of potential new therapeutics, SLE remains one of the greatest challenges for physicians. This is a critical situation for several reasons. First, SLE is commonly misdiagnosed, even by experienced rheumatologists. As no single test is sufficiently sensitive and specific to be diagnostic, the diagnosis requires interpretation of complex ACR criteria, many of which are subject to interpretation and may require years to evolve. Second, the course of SLE in a given patient is characterized by unpredictable flares and remissions. Again, the monitoring of SLE disease activity relies on sophisticated indices such as the SLEDAI and BILAG index, since there is no laboratory test with reliable capacity to identify or predict a disease flare. Third, lack of biomarkers has impeded efforts to evaluate the response to treatment. Consequently, it is of importance to develop accurate and easy-to-use biomarkers to improve daily management of SLE patients and facilitate the development of new SLE therapeutics. CB-CAP appear to have the potential to serve as clinically practical biomarkers for SLE (Table 1).
Table 1.
Potential Clinical Applications of CB-CAP as Lupus Biomarkers
| Cell Type | CB-CAP | Clinical Application | Reference |
|---|---|---|---|
| Erythrocyte | E-C4d, E-C3d | diagnosis; monitoring | (32, 38) |
| Reticulocyte | R-C4d (R-C3d) | monitoring | (33) |
| Platelet | P-C4d (P-C3d) | diagnosis; stratification | (34) |
| T Lymphocyte | T-C4d, T-C3d | diagnosis; others (under investigation) | (35) |
| B Lymphocyte | B-C4d, B-C3d | diagnosis; others (under investigation) | (35) |
| Monocyte | M-C4d | under investigation | |
| Granulocyte | G-C4d | under investigation | |
| Circulating endothelial cell | CEC-C4d | under investigation |
CB-CAP as diagnostic biomarkers for SLE
The diagnostic utility of CB-CAP has been demonstrated for E-C4d. P-C4d, T-C4d, and B-C4d. In the inaugural CB-CAP study, an abnormally high level of E-C4d in combination with an abnormally low level of E-CR1 was shown to be 72% sensitive and 79% specific in differentiating SLE from other inflammatory or immune-mediated diseases, and 81% sensitive and 91% specific in differentiating SLE from healthy conditions, with an overall negative predictive value of 92% (32). Similarly, T-C4d and B-C4d levels, as diagnostic tools, were 56% sensitive/80% specific and 60% sensitive/82% specific in differentiating SLE from other diseases and healthy controls, respectively (35). Remarkably, despite being present in only a subset of SLE patients evaluated in a cross-sectional study, an abnormal P-C4d test has high diagnostic specificity, being 100% specific for a diagnosis of SLE compared to healthy controls and 98% specific for SLE compared with patients with other diseases (34).
Until recently, the single most useful laboratory test for confirming a diagnosis of SLE has been determination of anti-double stranded DNA (dsDNA) antibodies. This test is highly specific for SLE, detected in less than 5% of patients with other diseases. However, the mean sensitivity of anti-dsDNA testing for SLE among published studies is only 57% (36). In contrast, the commonly used anti-nuclear antibody (ANA) test is sensitive (>95%) but highly non-specific for a diagnosis of SLE, with a positive predictive value as low as 11% in some studies (37). Therefore, the reported diagnostic specificity and sensitivity of E-C4d, P-C4d, T-C4d, and B-C4d tests indicate that a single CB-CAP assay is in general may be more sensitive than the anti-dsDNA test and more specific than the ANA test. It remains to be investigated if combinations of CB-CAP assays of different cell types will provide greater diagnostic utility than individual CB-CAP assays of a particular cell type.
CB-CAP as biomarkers for SLE disease activity
Initial studies demonstrated that E-C4d levels in the same SLE patient examined on different days varied considerably, suggesting that changes in E-C4d levels in SLE patients might reflect fluctuations in disease activity (32). The utility of E-C4d as a biomarker for monitoring SLE disease activity was subsequently investigated through a longitudinal study (38). This study was conducted with 157 patients with SLE, 290 patients with other diseases, and 256 healthy individuals who were followed prospectively over a 5-year period (2001–2005), encompassing 1005 patient-visits (SLE patients), 660 patient-visits (patients with other diseases), and 395 subject-visits (healthy individuals). The disease activity in SLE patients was measured using the SLAM and the Safety of Estrogen in Lupus Erythematosus: National Assessment version of the SLEDAI index (SELENA-SLEDAI). Consistent with the initial cross-sectional study, the results showed that SLE patients had higher levels of E-C4d and E-C3d than did the healthy controls and patients with other diseases. The variances of E-C4d and E-C3d were high, not only within the same SLE patient, but also between different SLE patients, suggesting again the possibility that levels of these biomarkers may track with changes in disease activity over time. This possibility was verified by a regression formulation in which each patient’s evolving clinical status was regressed on each of the biomarkers, using both univariate and multivariate analyses. While the univariate analysis demonstrated that E-C4d and E-C3d, as well as the gold standards anti-dsDNA and serum C3 levels, were significantly associated with disease activity in SLE patients, the multivariate analysis showed that E-C4d and E-C3d remained significant predictors of SLE disease activity measured by SELENA-SLEDAI (E-C4d) and/or SLAM (E-C4d and E-C3d), even after adjusting for serum C3, C4, and anti-dsDNA antibody. These observations suggest that erythrocyte-bound CAP can serve as informative measures of SLE disease activity as compared with anti-dsDNA and serum complement levels and should be considered for monitoring disease activity in patients with SLE.
To devise a laboratory test that can differentiate ongoing active disease from cumulative past disease activity in an SLE patient, we have embarked on a series of studies focused on analyzing C4d levels on reticulocytes. The rationale underlying these studies is that the level of CAP bound to reticulocytes (e.g., R-C4d), which are short-lived (0–2-day) intermediates transiting into mature erythrocytes, should reflect precisely and promptly the extent of complement activation (and disease activity) at the time of blood sample procurement. During longitudinal follow-up of 156 patients with SLE, it was noted that the R-C4d levels in a significant fraction of SLE patients varied considerably over time (33), suggesting that fluctuations in R-C4d levels coincide with changes in disease activity. Indeed, initial studies showed that, within the SLE patient population, the level of R-C4d appeared proportionate to the clinical disease activity in a given SLE patient, i.e., patients with higher R-C4d levels have higher disease activity as measured using the SLAM and SELENA-SLEDAI (33). In cross-sectional comparison, patients with R-C4d levels in the highest quartile, compared to those in the lowest quartile, had significantly higher SELENA-SLEDAI (p<0.001) and SLAM (p=0.02) scores. Moreover, longitudinal observations showed that the R-C4d levels appeared to change promptly in relation to the clinical course in individual SLE patients. Taken together, these results suggest that R-C4d levels, compared with C4d levels on the 120 day-lived erythocytes, may reflect more precisely ongoing disease activity in an SLE patient, supporting a potential role for CAP-bearing reticulocytes as “instant messengers” of SLE disease activity.
CB-CAP as biomarkers for identifying clinical subsets of SLE patients
The various studies outlined above indicate that the paradigm CB-CAP as lupus biomarkers is not limited to a particular lineage of circulating cells. Observations to date also suggest that CB-CAP associated with a particular cell type may provide clues to clinical stratification or subsetting of SLE patients. In view of the biological role of platelets in hemostasis and coagulation, we postulated that the presence of abnormal levels of CAPs on platelets may serve as a useful biomarker for SLE patients who are at increased risk of cardiovascular and cerebrovascular events. Indeed, our previous cross-sectional study of 100 SLE patients showed that P-C4d correlated with a history of neurological event (seizure and psychosis; p=0.006) and positive anti-phospholipid antibody tests (p=0.013), a clinical manifestation and a known risk factor for thrombotic complications of SLE, respectively (34). Recently, a longitudinal study of 341 SLE patients who had at least 3 consecutive office visits identified 57 patients (17%) with abnormal P-C4d levels. Moreover, the P-C4d-positive SLE patients, compared to the P-C4d-negative patients, were found to be more likely to have a history of seizure disorder (16% vs. 7%, p=0.04) and positive anti-phospholipid antibody tests (46% vs. 26%, p=0.003). Furthermore, P-C4d-positive patients had a significantly higher frequency of cardiovascular events associated with acute thrombosis than did P-C4d-negative patients (p=0.03) (Kao et al, manuscript in preparation). The results of the cross-sectional and longitudinal studies together suggest that SLE patients with abnormal P-C4d levels may represent a subset of patients with increased thrombotic tendency and risk of cardiovascular and cerebrovascular complications.
Associations between the CB-CAP phenotypes of other circulating cells (e.g., lymphocytes) and clinical manifestations of SLE warrant further investigation.
Exploratory Studies of CB-CAP as Biomarkers of Disease Course in SLE
CAP-bearing erythrocytes as “time capsules” of SLE disease activity
Studies of E-C4d as a specific biomarker for lupus diagnosis led to the hypothesis that EC4d may also be useful to monitor lupus disease activity. This hypothesis was based on the following rationale. Because the life span of human erythrocytes is approximately 120 days, erythrocytes ranging from 1-day to 120-day-old are circulating at any given time. The natural course of SLE consists of intermittent episodes of disease flares and remission, which are thought to correlate with the extent of complement-involved inflammatory reactions. Together, these physiological facts suggest that a disease flare in an SLE patient may lead to an increased level of C4 binding to erythrocytes circulating at that time. Once the flare subsides and the patient enters remission, erythrocytes that emerge from the bone marrow may have a lower (“remission”) level of surface C4. Thus, detection of erythrocyte subpopulations expressing distinct levels of C4d at a specific time point should theoretically reveal, much like “time capsules,” SLE disease activity during the preceding 120 days (Fig. 2).
Figure 2. The “Erythrocyte Time Capsules” model.
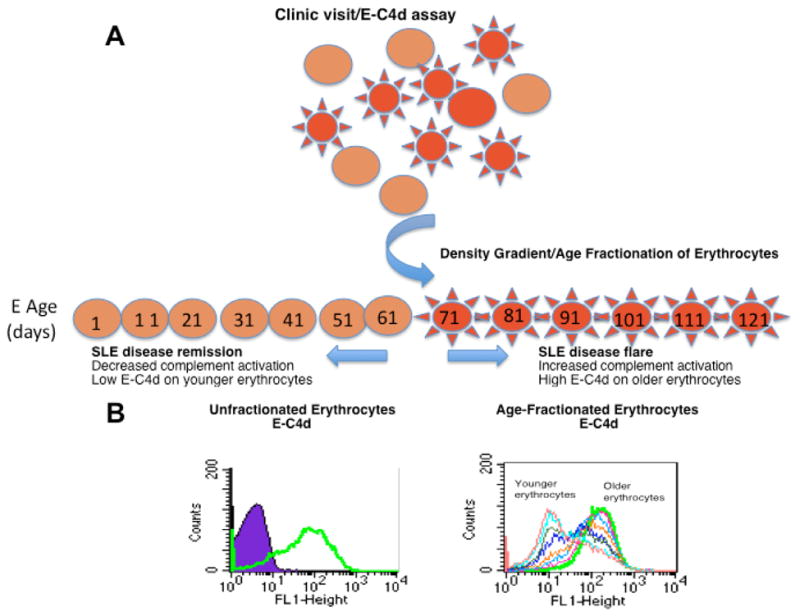
(A) Schematic illustration of the hypothetical model; (B) Histograms of E-C4d levels on age-fractionated erythrocytes prepared from a representative SLE patient. The open histograms represent the C4d staining on the entire population (left panel) or density/age-fractionated erythroctyes (right panel). The purple closed peak depicts the background staining of erythrocytes using an istotype mouse IgG control.
To explore this hypothesis, erythrocytes from patients with SLE were fractionated using density gradient centrifugation, a widely accepted technique for separating erythrocytes of different ages (39, 40), and the C4d levels of different fractions of erythrocytes were determined by flow cytometry. These studies demonstrated that in some SLE patients, the levels of C4d on older erythrocytes were distinctively higher than levels of C4d on younger erythrocytes (Fig. 2, panel B), suggesting there may have been an event such as a disease flare, that had occurred at a distinct time point prior to the day of examination. To date, pilot studies have identified three patterns of E-C4d on age-fractionated erythrocytes: 1) high E-C4d levels on old erythrocytes, 2) constant E-C4d levels on all fractions regardless of the age of erythrocytes, and 3) elevated E-C4 levels associated with young erythrocytes (unpublished results). It is conceivable that these different E-C4d patterns may reflect a previously active, a stable (or chronically active), and a recently activated disease status, respectively. These findings have provided support for the “time capsule” hypothesis that CAP-bearing erythrocytes can contribute informative clues to the course of disease activity in a given SLE patient.
Perspective
The studies summarized above have not only identified a unique CB-CAP phenotype that appears to be highly specific for SLE, but they also have demonstrated the potential for CB-CAP to serve as diagnostic and monitoring biomarkers for SLE. Preliminary data have further suggested that complement activation products can bind to different combinations of circulating cell types in individual SLE patients. Therefore, it is plausible that a composite profile of CB-CAP biomarkers on different cell types may reflect a more complete picture of the underlying pathogenic process and correlate with specific clinical features/outcomes (e.g., organ involvement) of individual patients, thereby serving as a novel class of “personalized” biomarkers for diagnosis and monitoring disease activity, assessment/prediction of clinical features, and stratification of SLE “subtypes”. Precise classification of SLE, in turn, may not only help customize therapeutic approaches suiting specific patients, but also assist in judicious trials of novel therapeutics.
Acknowledgments
We thank our colleagues in the Lupus Center of Excellence and Division of Rheumatology and Clinical Immunology for providing clinical samples, helpful discussion, and skilled technical as well as administrative support. Investigations in the authors’ laboratory were supported by grants from the National Institutes of Health (RO1HL074335, RO1AI077995, RO1 AR-46588, K23 AR-051044, and K24 AR-02213), the Department of Defense (W81XWH-06-2-0038), the Alliance for Lupus Research, and the Lupus Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. [see comment] Seminars in Arthritis & Rheumatism. 2004;34(2):501–37. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM, Cohen AS, Fries JF. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C, Gladman DD, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis & Rheumatism. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 6.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis & Rheumatism. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 7.Bae SC, Koh HK, Chang DK, Kim MH, Park JK, Kim SY. Reliability and validity of systemic lupus activity measure-revised (SLAM-R) for measuring clinical disease activity in systemic lupus erythematosus. Lupus. 2001;10(6):405–9. doi: 10.1191/096120301678646146. [DOI] [PubMed] [Google Scholar]
- 8.Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. QJM. 1993;86(7):447–58. [PubMed] [Google Scholar]
- 9.Vaughan JH, Bayles TB, Favour CB. The response of serum gamma globulin level and complement titer to adrenocorticotropic hormone (ACTH) therapy in lupus erythematosus disseminatus. J Lab Clin Med. 1951;37:698–702. [PubMed] [Google Scholar]
- 10.Schur PH, Sandson J. Immunological factors and clinical activity in systemic lupus erythematosus. N Engl J Med. 1968;278:533–538. doi: 10.1056/NEJM196803072781004. [DOI] [PubMed] [Google Scholar]
- 11.Cook HT, Botto M. Mechanisms of Disease: the complement system and the pathogenesis of systemic lupus erythematosus. Nature Clinical Practice Rheumatology. 2006;2(6):330–7. doi: 10.1038/ncprheum0191. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 13.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Advances in Immunology. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 14.Walport MJ. Complement. First of two parts N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 15.Law SK, Levine RP. Interaction between the third complement component and cell surface macromolecules. Proc Natl Aca SciUSA. 1977;74:2701. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law SKA. The covalent binding reaction of C3 and C4. Annals of New York Academy of Sciences. 1983:246–258. doi: 10.1111/j.1749-6632.1983.tb18113.x. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd W, Schur PH. Immune complexes, complement, and anti-DNA in exacerbations of systemic lupus erythematosus (SLE) Medicine. 1981;60:208–217. doi: 10.1097/00005792-198105000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Valentijn RM, van Overhagen H, Hazevoet HM, Hermans J, Cats A, Daha MR, et al. The value of complement and immune complex determinations in monitoring disease activity in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 1985;28:904–913. doi: 10.1002/art.1780280810. [DOI] [PubMed] [Google Scholar]
- 19.Ricker DM, Hebert LA, Rohde R, Sedmak DD, Lewis EJ, Clough JD, et al. Serum C3 levels are diagnostically more sensitive and specific for systemic lupus erythematosus activity than are serum C4 slevels. American Journal of Kidney Diseases. 1991;19:678–685. doi: 10.1016/s0272-6386(12)80609-3. [DOI] [PubMed] [Google Scholar]
- 20.Swaak AJ, Groenwold J, Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Annals of Rheumatic Diseases. 1986;45:359–366. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrass CK, Nies KM, Louie JS, Border WA, Glassock RJ. Correlation and predictive accuracy of circulating immune complexes with disease activity in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 1980;23:273–282. doi: 10.1002/art.1780230302. [DOI] [PubMed] [Google Scholar]
- 22.Liu C-C, Ahearn JM, Manzi S. Complement as a source of biomarkers in systemic lupus erythematosus: past, present, and future. Current Rheumatology Reports. 2004;6:85–88. doi: 10.1007/s11926-004-0046-8. [DOI] [PubMed] [Google Scholar]
- 23.Liu CC, Manzi S, Danchenko N, Ahearn JM. New advances in measurement of complement activation: lessons of systemic lupus erythematosus. Current Rheumatology Reports. 2004;6(5):375–81. doi: 10.1007/s11926-004-0012-5. [DOI] [PubMed] [Google Scholar]
- 24.Sturfelt G, Jonasson L, Sjoholm AG. Sequential studies of complement activation in systemic lupus erythematosus. Scandinavian Journal of Rheumatology. 1985;14:184–196. doi: 10.3109/03009748509165503. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins P, Belmont HM, Buyon JP, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis & Rheumatism. 1988;31:632–641. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- 26.Wild G, Watkins J, Ward AM, Hughes P, Hume A, Rowell NR. C4a anaphylatoxin levels as an indicator of disease activity in systemic lupus erythematosus. Clinical and Experimental Immunology. 1990;80:167–170. doi: 10.1111/j.1365-2249.1990.tb05227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porcel JM, Ordi J, Castro-Salomo A, Vilardell M, Rodrigo MJ, Gene T, et al. The value of complement activation products in the assessment of systmeic lupus erythematosus flares. Clinical Immunology and Immunopathology. 1995;74:283–288. doi: 10.1006/clin.1995.1040. [DOI] [PubMed] [Google Scholar]
- 28.Manzi S, Rairie JE, Carpenter AB, Kelly RH, Jagarlapudi SP, Sereika SM, et al. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis & Rheumatism. 1996;39:1178–1188. doi: 10.1002/art.1780390716. [DOI] [PubMed] [Google Scholar]
- 29.Buyon JP, Tamerius J, Belmont HM, Abramson SB. Assessment of disease activity and impending flare in patients with systemic lupus erythematosus: comparison of the use of complement split products and conventional measurements of complement. Arthritis & Rheumatism. 1992;35:1028–1037. doi: 10.1002/art.1780350907. [DOI] [PubMed] [Google Scholar]
- 30.Tieley CA, Romans DG, Crookston MC. Localization of Chido and Rodgers determinants to the C4d fragment of human C4. Nature. 1978;276:713–715. doi: 10.1038/276713a0. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson JP, Chan AC, Karp DR, Killion CC, Brown R, Spinella D, et al. Origin of the fourth component of complement related Chido and Rodgers blood group antigens. Complement. 1988;5:65–76. doi: 10.1159/000463037. [DOI] [PubMed] [Google Scholar]
- 32.Manzi S, Navratil JS, Ruffing MJ, Liu C-C, Danchenko N, Nilson SE, et al. Measurement of erythrocyte C4d and complement receptor 1 in the diagnosis of systemic lupus erythematosus. Arthritis & Rheumatism. 2004;50:3596–3604. doi: 10.1002/art.20561. [DOI] [PubMed] [Google Scholar]
- 33.Liu CC, Manzi S, Kao AH, Ruffing MJ, Navratil JS, Ahearn JM. Reticulocytes bearing C4d as biomarkers of disease activity for systemic lupus eryhematosus. Arthritis & Rheumatism. 2005;52:3087–3099. doi: 10.1002/art.21305. [DOI] [PubMed] [Google Scholar]
- 34.Navratil JS, Manzi S, Kao AH, Krishnaswami S, Liu CC, Ruffing MJ, et al. Platelet C4d is highly specific for systemic lupus erythematosus. Arthritis & Rheumatism. 2006;54(2):670–4. doi: 10.1002/art.21627. [DOI] [PubMed] [Google Scholar]
- 35.Liu CC, Kao AH, Hawkins DM, Manzi S, Sattar A, Wilson N, et al. Lymphocyte-bound complement activation products as biomarkers for diagnosis of systemic lupus erythematosus. Clinical and Translational Science. 2009;2(4):300–308. doi: 10.1111/j.1752-8062.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon DH, Kavanaugh AJ, Schur PH American College of Rheumatology Ad Hoc Committee on Immunologic Testing G. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. [see comment] Arthritis & Rheumatism. 2002;47(4):434–44. doi: 10.1002/art.10561. [DOI] [PubMed] [Google Scholar]
- 37.Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Archives of Internal Medicine. 1996;156(13):1421–5. [PubMed] [Google Scholar]
- 38.Kao AH, Navratil JS, Ruffing MJ, Liu CC, Hawkins DM, McKinnon KM, et al. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis & Rheumatism. 2009 doi: 10.1002/art.27267. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennie CM, thompson s, Parker AC, Maddy A. Human erythrocyte fractionation in “Percoll” density gradients. Clinca Chimica Acta. 1979;98:119–125. doi: 10.1016/0009-8981(79)90172-4. [DOI] [PubMed] [Google Scholar]
- 40.Piomelli S, Seaman C. Mechanism of red blood cell aging: Relationship of cell density and cell age. American Journal of Hematology. 1992;42:46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]