Abstract
The purpose of this study was to determine the safety of tinzaparin for deep vein thrombosis prophylaxis in newly diagnosed grade III–IV malignant glioma patients. Patients were initiated on daily tinzaparin at a fixed dose of 4,500 IU subcutaneously between 48 h and 4 weeks post-operative for planned duration of 12 months. During chemotherapy cycles, blood counts were monitored weekly and tinzaparin was held if the platelet count decreased to <50,000 and was re-initiated at a platelet count >100,000. Forty patients were enrolled into the study, 35 with glioblastoma multiforme and 5 with anaplastic astrocytoma. Possible attributable toxicity was limited to two patients who developed CNS hemorrhages (one grade 1 and one grade 2) and one patient with an increase in liver enzymes (grade 3). There were no patients with a grade 4 or 5 CNS hemorrhages or systemic hemorrhages ≥grade 2. The median time on prophylactic tinzaparin was 161 days (range of 5 to 601 days). One patient developed a deep venous thrombosis while taking tinzaparin, and three patients developed thromboembolic complications while off tinzaparin. Tinzaparin at a fixed prophylactic dose is safe and may decrease the incidence of thromboembolic complications in brain tumor patients.
Keywords: Low-molecular-weight heparin, Tinzaparin, Venous thromboembolism, Brain tumors, Malignant glioma
Introduction
In 2007, there were 20,500 new adult cases of cancer of the brain and central nervous system diagnosed in the United States and 12,740 deaths [1]. For patients with malignant gliomas, the median survival has been estimated at approximately 11–18 months for grade IV [2–4] and approximately 3 years for grade III [5, 6]. In an attempt to improve survival, patients often undergo several treatment modalities including surgery, radiation, and chemotherapy. These treatments, the underlying malignancy, and immobility due to paresis predispose patients to venous thromboembolism (VTE).
Low-molecular-weight heparins (LWMHs) have been shown to be effective and safe in decreasing the incidence of VTE in high-risk settings such as critically ill patients, orthopedic surgery, abdominal and pelvic surgery for cancer, after trauma, spinal cord injury, as well as after neurosurgery [7–19]. Trials have demonstrated that prophylactic LMWH had no increased hemorrhagic complications following neurosurgical procedures and decreased the incidence of VTE after neurosurgery [8, 10–15, 17–19].
Despite the use of postoperative VTE prophylaxis, the risk of VTE in patients with malignant glioma continues to be high with estimates of 20.8% at 12 months [20]. Treatment of VTE with therapeutic anticoagulation in this patient population poses a risk of intracranial hemorrhage which can be fatal [21]. Inferior vena caval (IVC) filters provide a temporary alternative when a patient is bleeding or is in a perioperative setting, however, IVC filters do not prevent formation of thrombosis above or below the filter which can place a patient at risk of pulmonary emboli or painful lower extremity edema. VTE contributes to the morbidity and mortality of brain tumor patients and complicates treatment of their malignancy.
The LMWH, tinzaparin, has been found to be safe as VTE prophylaxis in a large prospective clinical trial (n = 746) of patients undergoing intracranial surgery [10]. Tinzaparin has also been found to have more activity in inhibiting thrombin generation compared to other LMWHs using equivalent anti-FXa activity concentrations which may be explained by its low anti-factor Xa/anti-factor IIa ratio [22]. Given the high rate of VTE and concern for hemorrhagic complications in patients with malignant gliomas, VTE prophylaxis with tinzaparin may offer an advantage for this patient population. We report a phase I trial of prophylactic tinzaparin for patients with newly diagnosed grade III–IV malignant glioma.
Patients and methods
Patient selection
Adult patients (age ≥ 18 years) with newly diagnosed, histologically confirmed grade III–IV glioma were eligible for the study. Conditions required for entry into the study included the following: (a) patient is at least 48 h after craniotomy or stereotactic biopsy but no later than 4 weeks from the last surgical procedure; (b) Karnofsky performance status ≥60%; and (c) life expectancy of at least 6 months. Eligibility also required showing acceptable hematologic and coagulation variables within 14 days of enrollment (platelet count >100,000/μl; prothrombin time, PT ≤ 1.2× control; activated partial thromboplastine time, aPTT ≤ 1.2× control). Exclusion criteria included the following: (a) symptomatic intracranial bleeding, which includes inter- or intratumor bleeding; (b) presence of acute or chronic deep venous thrombosis demonstrated by ultrasonography or venography; (c) active systemic bleeding, such as gastrointestinal bleeding or gross hematuria; (d) excessive risk of bleeding as defined by stroke within the prior 6 months, history of CNS or intraocular bleed, or septic endocarditis; (e) prior history of documented deep vein thrombosis (DVT) or pulmonary embolus (PE); (f) history of heparin induced thrombocytopenia; (g) contraindication to tinzaparin or other heparins, including allergy or hypersensitivity to heparin or pork products, sulfite allergy, benzyl alcohol allergy or have or had had an epidural catheter or traumatic spinal puncture within 7 days prior to screening; (h) serum creatinine >3.0 mg/dl; (i) medical condition requiring long-term anticoagulants such as atrial fibrillation or a mechanical heart valve; and (j) females who were pregnant or nursing. Agreement to practice adequate birth control methods was required for fertile patients. The Duke University Medical Center Institutional Review Board approved this study and each patient signed a written informed consent form.
Patient evaluation
Evaluations done within 14 days of initiating therapy included a medical history, physical and neurologic examinations, performance status determination, complete blood count, PT, and aPTT. This evaluation was repeated at follow-up visits (month 2, 4, 6, 9, 12) and the end of study visit. CBCs were performed weekly and increased to three times a week if the platelet count dropped to <100,000/μl. If PTs or PTTs increased to 1.2 times control, then these tests were repeated monthly. The National Cancer Institution Common Toxicity Criteria (CTC; version 3.0) was used to grade toxicities. CTC definitions for grades of toxicity due to intracranial hemorrhage are as follows: (1) grade 1—asymptomatic, radiographic findings only; (2) grade 2—medical intervention indicted; (3) grade 3—ventriculostomy, ICP monitoring, intraventricular thrombolysis, or operative intervention indicated; (4) grade 4—life-threatening consequences, neurologic deficit or disability; and (5) grade 5—death.
Deep vein thrombosis and pulmonary embolus evaluation
For patients with clinically suspected DVT, an ipsilateral extremity duplex ultrasonography (DUS) was performed immediately. If negative for DVT, but the calf was not evaluated, then a repeat DUS was obtained 5–7 days later. For a non-diagnostic DUS or if the DUS shows calf vein thrombosis, a contrast venogram was obtained and the interpretation based on accepted venographic criteria. Given that baseline exams are not routinely performed, patients were only evaluated for clinically suspected DVT.
For patients with clinically suspected PE, a ventilation/perfusion scan or spiral CT was performed. If non-diagnostic, then bilateral DUS or venography of the lower extremities was performed. If these tests are negative for DVT, but there was high clinical suspicion for PE, then pulmonary angiography was performed.
Patients with negative evaluations were continued on tinzaparin, whereas patients diagnosed with a DVT or PE had tinzaparin discontinued and were managed according to local practice.
Drug administration
This was a single arm, open-labeled pilot trial. Tinzaparin was supplied by Pharmion, Inc. Patients received a single daily subcutaneous injection of tinzaparin at 4500 IU, which was initiated at least 48 h after craniotomy or stereotactic biopsy and within 4 weeks after the most recent surgery. Outpatient administration of tinzaparin was performed by patient self administered or by a family member as would be in usual clinical practice. Tinzaparin was continued daily for a planned duration of 12 months. Patients were allowed to continue on tinzaparin after the 12 months as long as there were no adverse reactions. These patients continued to have the same labs and clinical follow-up.
Tinzaparin was temporarily held for the following reasons: (a) platelet count <50,000/μl and was restarted when the platelet count increased to ≥100,000/μl; (b) minor bleeding and restarted when bleeding resolved; (c) 24–48 h prior to surgery and was restarted 48 h post-operatively. Tinzaparin was discontinued permanently if patients developed any of the following: (a) ≥grade 2 non-CNS hemorrhage or a new grade 3 CNS hemorrhage or a grade 4 CNS hemorrhage occurred; (b) heparin induced thrombocytopenia; (c) a thromboembolic event or cardiovascular event that required anticoagulation or anti-platelet therapy; (d) patient withdrawal of consent or non-compliance; and (e) an interruption in tinzaparin for >14 days.
Statistical considerations
The primary objective of this study was to determine the safety of DVT/PE prophylaxis with tinzaparin in newly diagnosed patients with grade III–IV malignant glioma. Of specific interest was the proportion of patients who experience grade 2 non-CNS hemorrhage, new grade 3 CNS hemorrhage or grade 4 CNS hemorrhage during the time on treatment. A 3-stage study design was used to differentiate between an unacceptable toxicity rate of 20 and 5%. Forty patients were accrued into this study as follows: (a) 10 patients in stage #1; (b) 15 patients in stage #2; and (c) 15 patients in stage #3. Stopping rules for an unacceptable toxicity rate was defined as 3 patients in stage #1 or ≥4 patients in stage #2 or stage #3. Unacceptable toxicity rates of 5% or less were considered desirable, whereas rates of 20% or greater were considered undesirable. The type I and type II error rates associated with this testing are 0.03 and 0.14, respectively.
Results
Patient characteristics
The study population included 40 newly diagnosed grade III–IV malignant glioma patients enrolled between June 2004 and January 2007 (Table 1). The median age was 52 years and the majority of patients were male (67%) and were diagnosed with a grade IV malignant glioma (87%). Median time to initiate tinzaparin was 23 days postoperative and median time on tinzaparin was 161 days.
Table 1.
Patient characteristics
| Characteristic | Number (N = 40) |
|---|---|
| Median age (range) | 52 (30–67) |
| Female | 13 (33%) |
| Histology | |
| Glioblastoma multiforme | 35 (87%) |
| Anaplastic astrocytoma | 5 (13%) |
| Median days from surgery to initiation of tinzaparin | 23 (2–29) |
| Median days on tinzaparin | 161 (5–601) |
Toxicity/events
Two patients developed CNS hemorrhage, one with grade 1 and the other with grade 2 (Table 2). The patient who developed a grade 1 CNS hemorrhage was initiated on tinzaparin 19 days after biopsy which revealed glioblastoma multiforme and he remained on tinzaparin for 50 days. He was clinically stable after two cycles of irinotecan and temozolomide and platelet count was within the normal range. His MRI showed stable contrast enhancement and increased blood around the tumor cavity, therefore tinzaparin was discontinued (Fig. 1). The patient who developed a grade 2 CNS hemorrhage was initiated on tinzaparin 7 days after a partial resection which revealed anaplastic astrocytoma and he remained on tinzaparin for 47 days. He had received his second cycle of temozolomide and his platelet count was within the normal range 3 days prior to being admitted for lethargy and hemiparesis. He improved on high dose decadron. MRI showed hemorrhage within the tumor. The patient was felt to have progressive disease; however, tinzaparin was discontinued due to the hemorrhage. Three days after discontinuing tinzaparin, he developed a DVT. Two other patients developed a DVT while tinzaparin was held due to thrombocytopenia. One patient had been off for just 10 days and the other for 30 days. One patient developed elevated liver enzymes which resolved after 10 days of being off of tinzaparin. Only one patient developed a DVT while on tinzaparin. Only one patient had documentation of an aPTT to increase to >1.2 of control with prolongation documented to 40.7 s (normal range 24.1–32.3 s).
Table 2.
Reasons for coming off study
| 1–6 months (N = 40) | 6–12b months (N = 18) | |
|---|---|---|
| Completed | 18 (45%) | 7 (39%) |
| Patient withdrew | 6 (15%) | 3 (17%) |
| Progressive disease | 4 (10%) | – |
| Avastin was initiated | 4 (10%) | 6 (33%) |
| Thrombocytopenia | 1 (2.5%) | – |
| Elevated liver enzymes (grade 3) | 1 (2.5%) | – |
| CNS hemorrhage (grade 1 & 2) | 2 (5.0%) | – |
| DVTa | 3 (7.5%) | – |
| Non-complaint | 0 (0%) | 2 (11%) |
| Lost to follow up | 1 (2.5%) | – |
DVT occurred in 2 patients while tinzaparin was held due to thrombocytopenia
Two patients remained on tinzaparin longer than 12 months
Fig. 1.
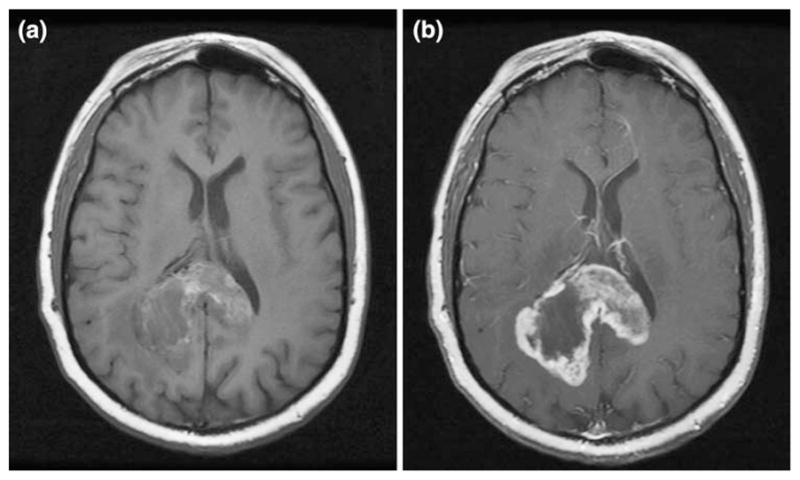
a Patient with grade 1 CNS hemorrhage. Axial T1 without contrast. b Patient with grade 1 CNS hemorrhage. Axial T1 with contrast
Reasons for coming off study
Eighteen of the 40 patients (45%) completed 6 months of prophylactic tinzaparin and 7 patients (17.5%) completed ≥12 months of tinzaparin without toxicity or developing VTE (Table 2). Patients taken off study due to safety concerns included the following: (1) two patients with CNS hemorrhage (5%); (2) one patient with elevated liver enzymes (2.5%); and (3) one with severe thrombocytopenia (2.5%). Over the 12 month period, 25% of patients came off study so that treatment with Avastin could be initiated. This was due to eligibility criteria of protocols with anti-VEGF agents which required patients were not on anticoagulation. Another 30% of patients came off study due to one of the following: (1) 2 patients were non-compliance; (2) 1 patient was lost to follow up; and (3) 9 patients withdrew from the study. The major reason patients withdrew consent was centered on concern for a hemorrhagic event. The remaining 17.5% of patients were taken off study due to either progressive disease (4 patients) or due to developing a DVT (3 patients).
Discussion
Since the mid-nineteenth century, we have known the basic risk factors for thrombosis and that thrombosis is a complication of cancer. Rudolf Virchow and his colleagues developed the concept of Virchow’s triad consisting of stasis, altered blood vessel wall, and hypercoagulability [23] and Amand Trousseau described the association of thrombosis and cancer [24]. Over the past 150 years, we have developed a greater understanding of how cancer and its therapies can affect all three parts of Virchow’s triad and thereby cause thrombosis [25].
More recently, greater understanding of the best treatment and prevention of recurrent VTE in patients with cancer has evolved. The randomized comparison of low-molecular-weight heparin versus oral anticoagulant therapy for the prevention of recurrent venous thromboembolism in patients with cancer (CLOT) investigators [26] performed a multi-center randomized trial of dalteparin versus coumadin derivative for newly diagnosed VTE in cancer patients. After 6 months of anticoagulation, the probability of recurrent thrombosis was 9% in the dalteparin group and 17% in the coumadin derivative group. The incidence of major bleeding was not significantly different at 6% in the dalteparin group and 4% in the coumadin derivative group. Of interest, there were 14 patients in the dalteparin group and 13 patients in the coumadin derivative group with brain tumor as the primary tumor site. One patient with a primary brain tumor in the dalteparin group (1/14, 7%) had an intracranial bleed.
With an estimated 20% of patients with malignant glioma developing VTE within the first year of diagnosis, many patients will have interruptions and delays in their therapy. Also, concern for intracranial bleeding in brain tumor patients with VTE has lead to the use of IVC filters. In cases when anticoagulation for acute VTE is contraindicated, IVC filters offer an immediate option in an attempt to prevent a PE from a known DVT. However, IVC filters should be thought of as a temporary step until anticoagulation can be initiated. In a retrospective study, Levin et al. [27] identified complications in 62% of patients with brain tumors after IVC filter placement. Thrombotic complications included 12% with recurrent PE, 26% with IVC or filter thrombosis, and 10% with postphlebitic syndrome.
Others have evaluated LMWH in patients with malignant glioma and VTE. Schmidt et al. [28] treated 11 patients with tinzaparin at 175 IU/kg for 10 days and then 100 IU/kg for 3 months. There were no hemorrhagic complications reported. During the 3 months of tinzaparin at 100 IU/kg, one patient had progressive VTE with resolution upon increasing tinzaparin back to 175 IU/kg. In a retrospective study, Nghiemphu et al. [29] evaluated 21 patients who received anticoagulation for VTE and were receiving bevacizumab 5 mg/kg every 2 weeks in combination with chemotherapy. Median time patients received anticoagulation and bevacizumab was 172 days, with 9 patients receiving enoxaparin and 12 patients receiving warfarin. There were no lobar hemorrhages. MRI findings revealed 2 patients with minor increase in signal on non-contrast T1-weighted sequence and 3 patients with small intraparenchymal hemorrhages with only one patient presenting with symptoms. From the PRODIGE study [30], an international phase III randomized clinical trial of dalteparin for primary prophylaxis of VTE in brain tumor patients, Perry et al. reported that although there was a trend toward reducing VTE for patients receiving dalteparin 5,000 anti-Xa units daily for 6 months at 11% versus 17% for the patients in the placebo group, there was a trend toward increase in intracranial bleeds at 5.1% versus 1.2% in the placebo group.
The primary objective of our study was to determine the safety of thromboprophylaxis using tinzaparin in newly diagnosed patients with grade III–IV malignant glioma. Two patients (5%) experienced CNS hemorrhage (grade 1 and 2) which was within the acceptable toxicity rate. Lieu et al. [31] found the incidence of brain tumor hemorrhage in 632 cases of primary brain tumors to be 2.4%. In the patient with grade 1 CNS hemorrhage (Fig. 1), the tumor was large and the patient was asymptomatic. In the patient with a grade 2 CNS hemorrhage, the tumor was found to be progressive and the patient improved with decadron. With the advent of new therapies such as bevacizumab which can cause hemorrhagic and thrombotic events, it is important to continue to evaluate anticoagulant agents for therapeutic and thromboprophylaxis use in malignant glioma patients. Studies to predict which patients may benefit from thromboprophylaxis and dose intensity, timing, and duration in relation to certain treatments as well as any survival benefit [32–35] still need to be addressed.
Acknowledgments
Stephanie Perry was supported by grant K23-HL084233-02.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin . 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Akman F, Cooper RA, Sen M, Tanriver Y, Kentli S. Validation of the Medical Research Council and a newly developed prognostic index in patients with malignant glioma: how useful are prognostic indices in routine clinical practice? J Neurooncol . 2002;59:39–47. doi: 10.1023/A:1016353614525. [DOI] [PubMed] [Google Scholar]
- 3.Mastronardi L, Guiducci A, Puzzilli F. Proposal of a clinico-histological “glioma score” as a prognostic index in high-grade glioma patients. Preliminary observations in a series of 80 cases. J Neurosurg Sci. 2001;45:195–201. (discussion 201) [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med . 2005;352:987–996. doi: 10.1056/NEJMoa 043330. [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Theodosopoulos P, Sneed P. Multidisciplinary management of adult anaplastic astrocytomas. Semin Radiat Oncol . 2001;11:163–169. doi: 10.1053/srao.2001.21428. [DOI] [PubMed] [Google Scholar]
- 6.Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: a retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17:3389–3395. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- 7.ENOXACAN Study Group. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]
- 8.Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D’Angelo A, Beltrametti C, Damiani M, Andrioli GC, Pugliese R, Iorio A, Brambilla G. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med . 1998;339:80–85. doi: 10.1056/NEJM1998070933 90204. [DOI] [PubMed] [Google Scholar]
- 9.Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med . 2001;161:1268–1279. doi: 10.1001/archinte. 161.10.1268. [DOI] [PubMed] [Google Scholar]
- 10.Chibbaro S, Tacconi L. Safety of deep venous thrombosis prophylaxis with low-molecular-weight heparin in brain surgery. Surg Neurol . 2008;70:117–121. doi: 10.1016/j.surneu.2007.06.081 (discussion 121). [DOI] [PubMed] [Google Scholar]
- 11.Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, Jr, Wheeler HB. Prevention of venous thromboembolism. Chest. 2001;119:132S–175S. doi: 10.1378/chest.119.1_suppl.132S. [DOI] [PubMed] [Google Scholar]
- 12.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, Hamilton PA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med . 1996;335:701–707. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 13.Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122:1933–1937. doi: 10.1378/chest.122.6.1933. [DOI] [PubMed] [Google Scholar]
- 14.Green D, Lee MY, Ito VY, Cohn T, Press J, Filbrandt PR, VandenBerg WC, Yarkony GM, Meyer PR., Jr Fixed- vs adjusted-dose heparin in the prophylaxis of thromboembolism in spinal cord injury. JAMA . 1988;260:1255–1258. doi: 10.1001/jama.260.9.1255. [DOI] [PubMed] [Google Scholar]
- 15.Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med . 2000;160:2327–2332. doi: 10.1001/archinte.160.15.2327. [DOI] [PubMed] [Google Scholar]
- 16.Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, Vandenbroucke JP, Briet E. Low-molecular-weight heparin versus standard heparin in general and orthopaedic surgery: a meta-analysis. Lancet . 1992;340:152–156. doi: 10.1016/0140-6736(92)93223-A. [DOI] [PubMed] [Google Scholar]
- 17.Nurmohamed MT, van Riel AM, Henkens CM, Koopman MM, Que GT, d’Azemar P, Buller HR, ten Cate JW, Hoek JA, van der Meer J, van der Heul C, Turpie AG, Haley S, Sicurella A, Gent M. Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost. 1996;75:233–238. [PubMed] [Google Scholar]
- 18.Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol . 1983;13:334–336. doi: 10.1002/ana.410130320. [DOI] [PubMed] [Google Scholar]
- 19.Weitz JI. Low-molecular-weight heparins. N Engl J Med . 1997;337:688–698. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- 20.Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, Zampieri P, Baiocchi C, Fiorentino MV. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer . 1997;33:1592–1596. doi: 10.1016/S0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 21.Quevedo JF, Buckner JC, Schmidt JL, Dinapoli RP, O’Fallon JR. Thromboembolism in patients with high-grade glioma. Mayo Clin Proc. 1994;69:329–332. doi: 10.1016/s0025-6196(12)62216-2. [DOI] [PubMed] [Google Scholar]
- 22.Gerotziafas GT, Petropoulou AD, Verdy E, Samama MM, Elalamy I. Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular-weight heparins upon the phases of thrombin generation. J Thromb Haemost . 2007;5:955–962. doi: 10.1111/j.1538-7836.2007.02477.x. [DOI] [PubMed] [Google Scholar]
- 23.Brotman DJ, Deitcher SR, Lip GY, Matzdorff AC. Virchow’s triad revisited. South Med J . 2004;97:213–214. doi: 10.1097/01.SMJ.0000105663.01648.25. [DOI] [PubMed] [Google Scholar]
- 24.Bick RL. Cancer-associated thrombosis. N Engl J Med . 2003;349:109–111. doi: 10.1056/NEJMp030086. [DOI] [PubMed] [Google Scholar]
- 25.Letai A, Kuter DJ. Cancer, coagulation, and anticoagulation. Oncologist. 1999;4:443–449. [PubMed] [Google Scholar]
- 26.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med . 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 27.Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993;43:1111–1114. doi: 10.1212/wnl.43.6.1111. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt F, Faul C, Dichgans J, Weller M. Low molecular weight heparin for deep vein thrombosis in glioma patients. J Neurol . 2002;249:1409–1412. doi: 10.1007/s00415-002-0855-5. [DOI] [PubMed] [Google Scholar]
- 29.Nghiemphu PL, Green RM, Pope WB, Lai A, Cloughesy TF. Safety of anticoagulation use and bevacizumab in patients with glioma. Neurooncology . 2008;10:355–360. doi: 10.1215/152285 17-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry JR, Rogers L, Laperriere N, Julian JA, Geerts WH, Agnelli G, Malkin M, Sawaya R, Baker R, Levine MN. PRODIGE: a phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma. J Clin Oncol. 2007;25:77s. doi: 10.1111/j.1538-7836.2010.03973.x. (Abstract 2011) [DOI] [PubMed] [Google Scholar]
- 31.Lieu AS, Hwang SL, Howng SL, Chai CY. Brain tumors with hemorrhage. J Formos Med Assoc. 1999;98:365–367. [PubMed] [Google Scholar]
- 32.Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M, Soyuer S. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost . 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 33.Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol . 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Buller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol . 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 35.Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol . 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]


