Abstract
Looping is a crucial early phase of heart development, as the initially straight heart tube (HT) bends and twists into a curved tube to lay out the basic plan of the mature heart. The physical mechanisms that drive and regulate this process are not yet completely understood. This paper reviews our recent investigations into the mechanical mechanisms of cardiac torsion during the first phase of looping, called c-looping. Experiments on chick embryos and computational modeling show that torsion is caused primarily by forces exerted on the HT by the primitive atria and the splanchnopleure, a membrane that presses against the ventral surface of the heart. Results from various experiments and finite element models are described and integrated to propose a hypothesis for cardiac torsion. Then, key aspects of our hypothesis are tested using experiments that perturb normal looping. For each perturbation, our models predict the correct qualitative response of the heart, supporting our hypothesis. These studies provide new insight into the mechanical mechanisms that drive and regulate cardiac looping.
Keywords: heart development, morphogenesis, chick embryo, finite elements, biomechanics
Introduction
Cardiac looping is one of the earliest manifestations of left-right asymmetry in the vertebrate embryo. During looping, the initially straight heart tube deforms into a curved tube, creating the basic pattern of the mature heart. Because looping abnormalities often lead to congenital heart defects,1, 2 this problem has received a great deal of attention over the years. Numerous hypotheses have been proposed for physical mechanisms that create the looped heart tube, but few of these have escaped experimental testing unscathed.3 Hence, while the genetic and molecular signals that regulate looping are becoming clear, the mechanical mechanisms that drive looping have remained poorly understood.
During the last decade, we have undertaken a systematic study of the biomechanical mechanisms of looping. Our work involves a combination of mathematical modeling and experiments with chick embryos. The purpose of this paper is to summarize some of the current thinking on this important problem.
Background
Development of the chick heart is similar to that of the human heart.4 Hence, the chick embryo has been a popular model for studies of heart development. Based on external characteristics of the developing embryo, the system of Hamburger and Hamilton5 divides the 21-day incubation period of the chick into 46 stages.
In the early chick embryo, bilateral membranes fold and fuse together, creating by stage 10 (33 hr) a relatively straight heart tube (HT) composed of three layers: an inner layer of endocardium, a relatively thick middle layer of acellular extracellular matrix called cardiac jelly, and a two-cell-thick outer layer of myocardium (Fig. 1a′). Soon the first heartbeats appear and looping begins.
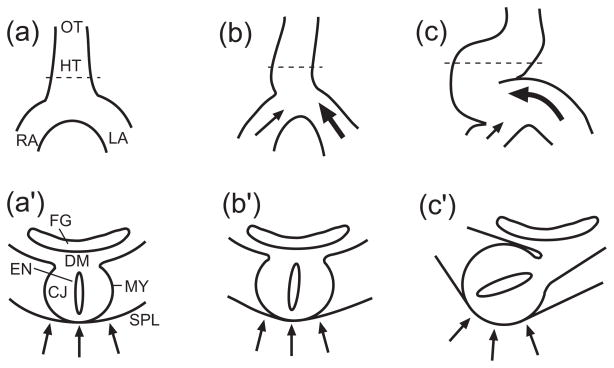
Figure 1.
Schematic for cardiac rotation hypothesis. Ventral views (a,b,c) and cross-sectional views (a′,b′,c′) are shown, with locations of cross sections indicated by dashed lines in a,b,c. (HT = heart tube, LA = primitive left atrium, RA = primitive right atrium, OT = outflow tract, CJ = cardiac jelly, MY = myocardium, EN = endocardium, SPL = splanchnopleure, DM = dorsal mesocardium, FG = foregut) (a,a′) Straight heart tube before looping. (b,b′) Both atria push against caudal end of the heart tube, and relatively greater force exerted by the left atrium displaces and rotates the heart tube slightly toward the right. (c,c′) The splanchnopleure pushes the heart tube dorsally, completing torsion. (from 24)
Looping consists of two main phases --- c-looping and s-looping.6 During normal c-looping (stages 9–13, 30–50 hr), the HT deforms into a c-shaped tube with its outer curvature normally directed toward the right side of the embryo. It is important to note that tissue markers placed on the ventral midline of the originally straight HT move to positions along the outer curvature of the looped heart (Fig. 2A–C). This result indicates that c-looping is not just rightward bending of a tube; rather it consists of a combination of ventral bending and rightward torsion.6 During s-looping (stages 14–18, 52–68 hr), the atrium moves superior to the ventricle, creating the basic form of the mature heart (not shown), and then septation divides the tube into four chambers (stages 21–36, 3.5–10 days).
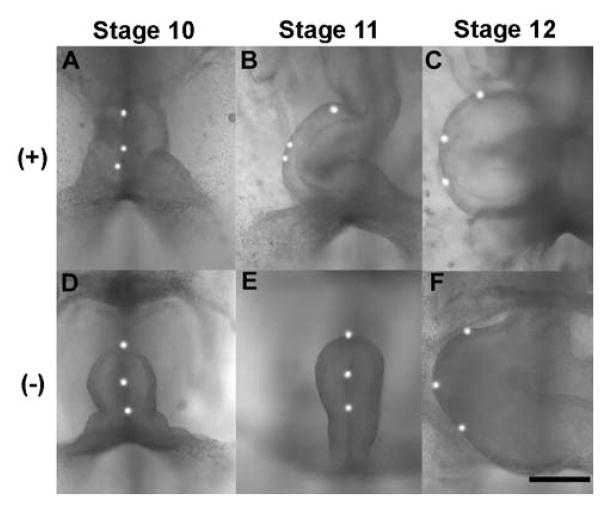
Figure 2.
Effects of removing splanchnopleure (SPL) on c-looping in embryonic chick heart. To visualize torsion, fluorescent labels were placed along the ventral midline. (Stages 10, 11, and 12 are 36, 42, and 48 hr of incubation, respectively.) (A to C) Control heart rotates rightward, as labels move to outer curvature. (D to F) SPL removal at stage 10 results in little or no rotation at stage 11 (E), but full rotation by stage 12 (F). (+) = SPL intact, (−) = SPL removed. Scale bar = 300 μm. (from 20)
In studying the mechanics of looping, we have found it important to consider bending and torsion separately. Studies have shown that ventral bending is a process intrinsic to the HT,7, 8 and we have speculated that it is driven by active changes in cell shape caused by the forces of actin polymerization.9 (This proposed mechanism warrants further scrutiny.) In contrast, we have found that torsion is driven primarily by loads external to the HT. This paper focuses on the torsional component of c-looping.
Hypothesis for Cardiac Torsion
A number of structures exert forces on the early HT or constrain its motion. The heart is constrained by connections to the primitive left and right atria (or omphalomesenteric veins) at its caudal end and to the outflow tract at its cranial end (Fig. 1a). A membrane called the splanchnopleure (SPL) presses against the ventral surface of the heart10, 11 (Fig. 1a′). Finally, the dorsal mesocardium (DM) attaches the entire length of the stage-10 heart tube to the foregut of the embryo (Fig. 1a′). Later, however, the DM ruptures (by stage 12, 48 hr), leaving the heart attached to the embryo only at its ends.
During looping, the primitive atria gradually fuse together, adding new segments to the caudal end of the HT.12 From top to bottom, this process creates a stage-12 HT composed of a series of segments representing the conotruncus (outflow tract), ventricle (future left and right ventricles), and atrium (future left and right atria). This lengthening facilitates both bending and torsion of the HT.
As described by Voronov et al.,13, 14 a relatively simple experiment provides insight into the mechanics of cardiac torsion. When the SPL is removed from a stage-11 chick heart that already has undergone considerable torsion (Fig. 2B), the heart immediately springs back partially toward the midline of the embryo and then slowly untwists until most of the torsion is lost (not shown). If the atria are then severed, the remainder of the twist disappears.13, 14 These results suggest that forces supplied by the SPL and atria play major roles in the torsional component of c-looping.
Based on these and other results, we proposed the following hypothesis for the mechanism for c-looping.14 First, cells along the lower regions of both atria contract and pull precardiac cells in the atria toward the caudal end of the heart. Tension caused by the contraction also may induce additional cells to migrate toward the heart (via tensotaxis) along stress-aligned fibronectin tracts.15–19
Second, the migrating cells in the atria push against the caudal end of the HT. Due possibly to asymmetric atrial geometry or contractile strength, the left atrium pushes with more total force than the right atrium, displacing the heart slightly rightward (Fig. 1b). The DM constrains this motion on the dorsal side of the heart, producing a small rightward rotation about the DM (Fig. 1b′).
Third, as the heart bends ventrally via intrinsic forces, the SPL exerts a compressive load against the ventral surface of the ventricle, enhancing the intitial rotation (Fig. 1c,c′). Further bending and torsion causes the DM to rupture, allowing the heart to deform into a c-shaped tube.
Testing the Looping Hypothesis
To test our hypothesis for c-looping, we experimentally perturbed the normal loads on the heart and examined whether the observed response is consistent with the hypothesis. According to our hypothesis, for example, rightward torsion is initiated by a net rightward force applied on the HT by the atria. If this idea is valid, then reducing the force exerted by the left atrium below that exerted by the right atrium should yield leftward looping. Accordingly, we removed a portion of the left atrium via dissection and found that a significant number of these operations produced left looping,14 which rarely occurs under normal conditions using our culture method.13 (Rightward looping occurred in all hearts for which only a segment of the right atrium was removed.)
Another perturbation involved dissecting the SPL from the ventral side of the heart. When the SPL is removed from stage 10 embryos, the heart bends ventrally but rotates little during six hours of culture to stage 1120 (Fig. 2E). This behavior is consistent with the crucial role of the SPL in our hypothesis, as well as with our previous result showing that the heart untwists when the SPL is removed from a stage-11 heart.13, 14
To determine whether the hypothesis is consistent with the laws of physics, we developed a 3-D computational model for the looping heart. Mathematical modeling can help sort conjecture from reality in complex 3-D problems such as looping, where physical intuition can be misleading.
In a model for the stage-10 heart, the HT and primitive atria are taken as tubes consisting of a thin layer of myocardium surrounding a thick layer of cardiac jelly.21 All tissues are taken as pseudoelastic, with mechanical properties provided by published data,22 and morphomechanical forces are simulated by specifying time-dependent changes in the zero-stress reference length of each material element, i.e., growth and cytoskeletal contraction are given by increased and decreased zero-stress lengths, respectively.23 The atria provide the only initial asymmetry in this model, with the diameter of the left atrium being slightly greater than that of the right atrium, e.g., see Fig. 2A. The model was analyzed using the finite element software ABAQUS (ABAQUS, Inc.); details of the methodology are described in other reports.21, 24
Consistent with experimental findings, differential growth (simulating actin polymerization) is specified in the HT, longitudinal growth (representing the influx of cells) is specified in the presumptive myocardial layer of the upper half of the atria, and cytoskeletal contraction is prescribed in the lower half of the atria. When these morphogenetic forces are activated, the model correctly predicts the gross morphological shape changes of the heart during the first three hours of looping, as well as distributions of morphogenetic stresses and strains measured in embryonic chick hearts.21 Stress was characterized using tissue dissection, and strains were computed from the measured motions of surface markers. Overall, the results from the model support our looping hypothesis.
Morphogenetic Adaptation of the Looping Heart
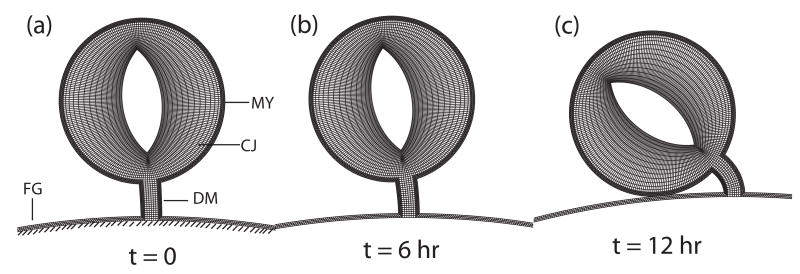
In the SPL-removal experiments discussed above, torsion is suppressed during six hours of culture from stage 10 to 11 (Fig. 2D,E). However, when the culture time is extended another six hours to stage 12, the heart slowly twists into its normal rightward position (Fig. 2F). As this finding appeared to contradict the crucial role for the SPL in our looping hypothesis, we explored this phenomenon in some detail.20
Mechanical testing via microindentation revealed that that the right side of a heart cultured from stage 10 without the SPL stiffens considerably at approximately the same time that the late-onset torsion begins, while the left side maintains its normal stiffness. Moreover, similarly treated hearts exposed to the Rho kinase inhibitor Y-27632 (to inhibit cytoskeletal contraction) did not stiffen or twist, even at stage 12.20 These results suggest that the embryonic heart adapts to the loss of loads normally supplied by the SPL by generating an asymmetric (right-sided) cytoskeletal contraction that belatedly pulls the HT rightward, restoring the normal morphology of the c-looped heart.
A finite element model was used to simulate this experiment.24 The model is similar to the model described above, but the initial configuration corresponds to an SPL-lacking stage-11 heart, which is bent ventrally not yet twisted (Fig. 2E; see model in Fig 3a). In addition, the outflow tract (conotruncus) is included as a straight extension of the HT attached dorsally to the foregut of the “embryo” by a longitudinal strut representing the DM. The dorsal side of the DM is constrained to move only vertically (see insert in Fig. 3a), and the atria are fixed at their distal ends.
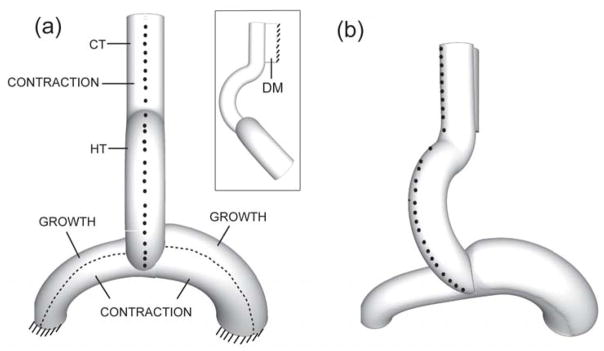
Figure 3.
Three-dimensional finite element model for looping heart without splanchnopleure (ventral view). Nodes on the ventral midline are marked to visualize rotation. (a) Undeformed configuration with morphogenetic loads indicated; insert shows side view. (HT = heart tube, CT = conotruncus, DM = dorsal mesocardium) (b) Deformed configuration. Midline nodes move rightward as HT rotates, similar to experiment (see Fig. 2B). (from 24)
As in the prior model, growth and contraction are specified in the upper and lower regions of the atria, respectively. Moreover, consistent with our SPL-removal experiments, circumferential contraction is stipulated in the myocardium along the right side of the HT (Fig. 3a). When these forces reach their peaks at the end of c-looping, rightward torsion (visualized from the motions of ventral markers), downward deflection of the atria, and increased curvature of the left atrium agree reasonably well with observed behavior of embryonic hearts following SPL removal (compare Figs. 3b and 2F).
To test the predictive ability of our model (and hypothesis), we conducted additional experiments in which the SPL was removed at stage 10, along with one or both atria.24 When the left atrium is removed, most hearts loop leftward (Fig. 4a′), while removing the right atrium yields rightward looping (Fig. 4b′). These results are consistent with the aforementioned experiments in which part of one atrium was removed with the SPL intact. Without the SPL, however, the shape of the looped heart appears abnormal, as the remaining atrium undergoes excessive bending (Fig. 4a′,b′). Finally, removing both atria results in rightward looping (Fig. 4c′).
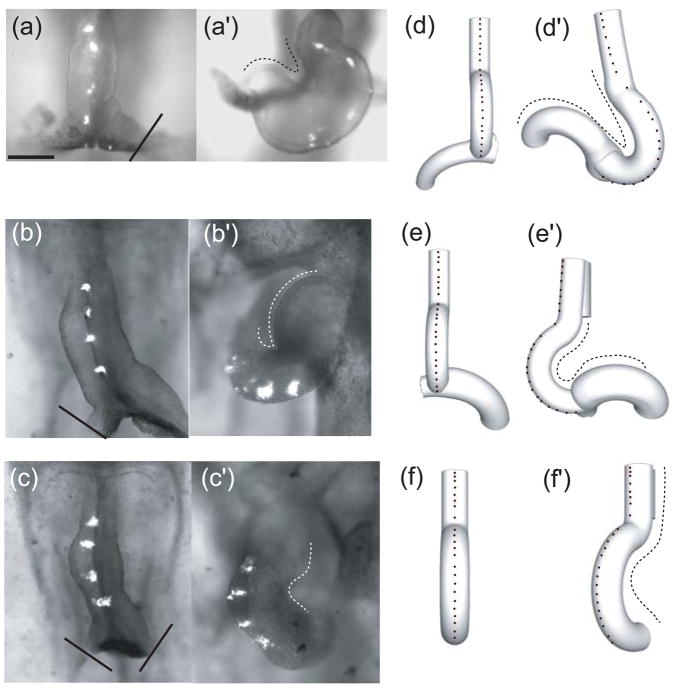
Figure 4.
Experimental and computational effects of atria removal on looping. (a–c,d–f) Initial configuration (stage 10) with splanchnopleure (SPL) and at least one atrium removed (solid lines indicate cut locations). (a′–c′, d′–f′) Final configuration (approximately 12 hr later). Midline labels (experiment) and nodes (model) are used to visualize rotation. Top row: Left atrium is removed; heart loops leftward. Middle row: Right atrium is removed; heart loops rightward with abnormal morphology. Bottom row: Both atria are removed; rightward rotation occurs. In each case, the model predicts approximately the correct heart shape, as indicated by dotted traces. (from 24)
We simulated each of these experiments using our 3-D model. All parameter values and morphogenetic forces are the same as those used in the complete model described above, with the only difference being the removal of the corresponding atrium. In each case, the model-predicted morphology at stage 12 agrees reasonably well with experimental results (Fig. 4d′-f′). Note that the model correctly predicts the correct looping direction, including the rightward looping that occurs when both atria are removed, as well as the abnormal shape of the remaining atrium. The rotation in the model without atria is caused by contraction on the right side of the conotruncus.
Mechanical Regulation of Looping
In all the models discussed thus far, growth and contraction are specified in various regions of the heart. The SPL removal experiments suggest, however, that the heart responds actively to load perturbations, indicating that mechanical feedback is involved. To explore this idea, we developed a plane-strain (2-D) model for a cross section of the stage-10 HT. Representing the structure of the HT, the model consists of a layer of myocardium surrounding a relatively thick layer of cardiac jelly (Fig. 5a). The HT is connected to rectangular DM, which is fixed on its opposite side to the rigid “embryo.” Rather than being specified, growth and contraction in the myocardium of this model are assumed to be governed by morphomechanical laws of the form24, 25
Figure 5.
Two-dimensional model for cardiac rotation including mechanical feedback in the myocardium. The time points shown in each case correspond to the experimental time points shown in Fig. 2D–F. (a) Model geometry for stage-10 heart (MY = myocardium, DM = dorsal mesocardium, CJ = cardiac jelly, FG = foregut wall). The heart is given a small initial rightward push, and then the load is removed. (b) Little rotation occurs during the first six hours. (c) After 12 hours, the heart has rotated fully. (from 24)
where G is the growth (G > 1) or contraction (G < 1) stretch ratio in the circumferential direction, σ is the circumferential Cauchy stress, dot denotes time differentiation, subscript zero indicates a “target” stress, and a and b are positive constants. The form of these relations is motivated by the Hyper-restoration (HR) Hypothesis of Beloussov,26 who found that perturbing stress in a developing tissue induces an active response that tends to restore the original (target) stress, but usually overshoots (hyper-restores) the stress to the opposite side. The second equation yields such an overshoot through an evolving target stress.
The initial conditions are G = 1 and σ = 0 everywhere. At t = 0 (stage 10), a small rightward push representing the net atrial force is applied briefly to the left side of the heart. Then, after the applied force is removed, the myocardium responds on its own according to the morphomechanical laws listed above. First, the heart begins to rotate slowly as it approaches stage 11 (Fig. 5b), and then the rotation rate increases until the HT is rotated fully (Fig. 5c). This behavior agrees with the delayed torsion observed after SPL removal at stage 10 (Fig. 2D–F).
Discussion
In this paper, we have summarized some key results from our experimental and theoretical studies of the looping heart. The main finding is that the primitive atria and the splanchnopleure exert forces on the heart tube that cause it to loop rightward in the normal embryo. Perturbing these forces can alter the direction and timing of looping, as well as trigger an adaptive response that tends to restore normal looping. These results led us to propose a new hypothesis for looping that awaits further testing and possible refinement.
The experimental methods used in the present study are somewhat of a throwback to the days prior to the advent of modern molecular biology. Before the 1980s, embryologists often used tissue dissection, along with other mechanical and chemical perturbations, to study morphogenetic mechanisms. For many problems, these techniques have provided valuable insight. In the case of cardiac looping, however, classical methods have not provided clear answers on many aspects of looping mechanisms, but neither have the techniques of genetics and molecular biology. In fact, knowing every gene and molecular signal involved in looping still would not give a complete understanding of how the complex 3-D geometry of the looped heart is created.
There are several reasons why, despite intensive study for nearly a century, looping has remained a relatively poorly understood morphogenetic process. First, although it has been known for decades that c-looping involves both bending and torsion,7 many researchers have not fully appreciated (or been aware of) this fact when interpreting experimental results. Indeed, some researchers still speak of looping as a rightward bending of the heart tube. Second, visualizing deformation of such a complex 3-D structure can be difficult,27 leading to misinterpretation of data. Third, looping may involve a combination of several different morphogenetic mechanisms, some of which may be redundant and compensate when others fail.28 The apparent adaptive response to SPL removal is one possible example. While such redundancy offers advantages to the embryo, it can complicate matters considerably for investigators.
Bending and torsion of a tube clearly involves mechanical forces, and engineering analysis provides important clues to the looping puzzle. However, obtaining a full understanding of this problem will ultimately require integrating data from engineers with that provided by cell, molecular, and developmental biologists across multiple spatial and temporal scales.
Acknowledgments
This research was supported by grants R01 GM075200 and R01 HL083393 (LAT) from the National Institutes of Health. This support is gratefully acknowledged.
References
- 1.Harvey RP. Cardiac looping --- an uneasy deal with laterality. Semin Cell Dev Biol. 1998;9:101–108. doi: 10.1006/scdb.1997.0188. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava D, Olson EN. Knowing in your heart what’s right. Trends Cell Biol. 1997;7:447–453. doi: 10.1016/S0962-8924(97)01150-1. [DOI] [PubMed] [Google Scholar]
- 3.Taber LA. Biophysical mechanisms of cardiac looping. Int J Dev Biol. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- 4.DeHaan RL. Development of form in the embryonic heart. An experimental approach. Circulation. 1967;35:821–833. doi: 10.1161/01.cir.35.5.821. [DOI] [PubMed] [Google Scholar]
- 5.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 6.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec. 2000;259:248–262. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Butler JK. MS Thesis. University of Texas; 1952. An experimental analysis of cardiac loop formation in the chick. [Google Scholar]
- 8.Manning A, McLachlan JC. Looping of chick embryo hearts in vitro. J Anat. 1990;168:257–263. [PMC free article] [PubMed] [Google Scholar]
- 9.Latacha KS, Remond MC, Ramasubramanian A, Chen AY, Elson EL, Taber LA. The role of actin polymerization in bending of the early heart tube. Dev Dyn. 2005;233:1272–1286. doi: 10.1002/dvdy.20488. [DOI] [PubMed] [Google Scholar]
- 10.Patten BM. Early Embryology of the Chick. McGraw-Hill; New York: 1951. [Google Scholar]
- 11.Romanoff AL. The Avian Embryo: Structural and Functional Development. Macmillan; New York: 1960. [Google Scholar]
- 12.de la Cruz MV, Sanchez-Gomez C, Markwald RR. Living Morphogenesis of the Heart. Birkhauser; Boston: 1998. Straight heart tube. Primitive cardiac cavities vs. primitive cardiac segments; pp. 85–98. [Google Scholar]
- 13.Voronov DA, Taber LA. Cardiac looping in experimental conditions: the effects of extraembryonic forces. Dev Dyn. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- 14.Voronov DA, Alford PW, Xu G, Taber LA. The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev Biol. 2004;272:339–350. doi: 10.1016/j.ydbio.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Linask KK, Lash JW. Precardiac cell migration: fibronectin localization at mesoderm-endoderm interface during directional movement. Dev Biol. 1986;114:87–101. doi: 10.1016/0012-1606(86)90385-4. [DOI] [PubMed] [Google Scholar]
- 16.Linask KK, Lash JW. A role for fibronectin in the migration of avian precardiac cells. II. Rotation of the heart-forming region during different stages and its effects. Dev Biol. 1988;129:324–329. doi: 10.1016/0012-1606(88)90379-x. [DOI] [PubMed] [Google Scholar]
- 17.Linask KK, Lash JW. A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev Biol. 1988;129:315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- 18.Toyoizumi R, Shiokawa K, Takeuchi S. The behavior and cytoskeletal system of chick gastrula mesodermal cells on substrata coated with lines of fibronectin. J Exp Zool. 1991;260:345–353. doi: 10.1002/jez.1402600309. [DOI] [PubMed] [Google Scholar]
- 19.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 20.Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Dev Dyn. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- 21.Ramasubramanian A, Latacha KS, Benjamin JM, Voronov DA, Ravi A, Taber LA. Computational model for early cardiac looping. Ann Biomed Eng. 2006;34:1355–1369. doi: 10.1007/s10439-005-9021-4. [DOI] [PubMed] [Google Scholar]
- 22.Zamir EA, Taber LA. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J Biomech Eng. 2004;126:823–830. doi: 10.1115/1.1824129. [DOI] [PubMed] [Google Scholar]
- 23.Taber LA. Biomechanics of cardiovascular development. Ann Rev Biomed Eng. 2001;3:1–25. doi: 10.1146/annurev.bioeng.3.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Ramasubramanian A, Nerurkar NL, Achtien KH, Filas BA, Voronov DA, Taber LA. On Modeling Morphogenesis of the Looping Heart Following Mechanical Perturbations. J Biomech Eng. 2008;130:061018. doi: 10.1115/1.2978990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taber LA. Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech Model Mechanobiol. 2008;7:427–441. doi: 10.1007/s10237-007-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beloussov LV. The Dynamic Architecture of a Developing Organism: An Interdisciplinary Approach to the Development of Organisms. Kluwer Dordrecht; the Netherlands: 1998. [Google Scholar]
- 27.Manner J. On rotation, torsion, lateralization, and handedness of the embryonic heart loop: new insights from a simulation model for the heart loop of chick embryos. Anat Rec. 2004;278A:481–492. doi: 10.1002/ar.a.20036. [DOI] [PubMed] [Google Scholar]
- 28.Stalsberg H. Mechanism of dextral looping of the embryonic heart. Am J Cardiol. 1970;25:265–271. doi: 10.1016/s0002-9149(70)80002-9. [DOI] [PubMed] [Google Scholar]