Abstract
Background
This pilot study compared the risk predictive value of preoperative physiological capacity (PC: defined by gas exchange measured during cardiopulmonary exercise testing) with the ASA physical status classification in the same patients (n=32) undergoing major abdominal cancer surgery.
Methods
Uni- and multivariate logistic regression models were fitted to measurements of PC and ASA rank data determining their predictive value for postoperative morbidity. Receiver operating characteristic (ROC) curves were used to discriminate between the predictive abilities, exploring trade-offs between sensitivity and specificity.
Results
Individual statistically significant predictors of postoperative morbidity included the ASA rank [P=0.038, area under the curve (AUC)=0.688, sensitivity=0.630, specificity=0.750] and three newly identified measures of PC: PAT (% predicted anaerobic threshold achieved, <75% vs ≥75%), ΔHR1 (heart rate response from rest to the anaerobic threshold), and HR3 (heart rate at the anaerobic threshold). A two-variable model of PC measurements (ΔHR1+PAT) was also shown to be statistically significant in the prediction of postoperative morbidity (P=0.023, AUC=0.826, sensitivity=0.813, specificity=0.688).
Conclusions
Three newly identified PC measures and the ASA rank were significantly associated with postoperative morbidity; none showed a statistically greater association compared with the others. PC appeared to improve predictive sensitivity. The potential for new unidentified measures of PC to predict postoperative outcomes remains unexplored.
Keywords: assessment, preanaesthetic; complications, morbidity; measurement techniques, gas exchange metabolic; metabolism, oxygen consumption; oxygen uptake; risk; surgery, postoperative
Postoperative morbidity is a major healthcare concern that negatively impacts the cost of care, quality of life, and survival.1,2 An accurate preoperative risk assessment tool would make risk stratification available to both healthcare providers and patients, and allow better evaluation of the risks of postoperative morbidity unrelated to the surgical disease prognosis. Perioperative clinicians have traditionally used independent preoperative pulmonary and cardiac risk factors, consensus algorithms, empirical risk indices, and diagnostic tests to predict a surgical patient's risk of adverse postoperative outcomes. The results have been controversial, conflicting, and most importantly have fallen short of making the accurate clinical predictions expected in today's perioperative environment.3 At The University of Texas M.D. Anderson Cancer Center, the ASA physical status classification system (one type of empirical risk index) is used to assess preoperative risk. Thus, there remains a need to identify a preoperative risk assessment tool that is objective, accurate, and clinically valuable.4
Physiological capacity (PC) is a promising preoperative risk assessment tool that may provide the accuracy and precision desired. PC defines an individual's metabolic response, measured by gas exchange, during cardiopulmonary exercise testing (CPET). Gas exchange measurements reflect the efficiency of oxygen utilization and the integrated efficiency of the oxygen transport system.5 A CPET is an individual, non-invasive, evaluator-independent, controlled metabolic stress test. The results obtained from CPET may define the degree of physiological reserve that determines an individual's ability to adjust to perioperative stress.
The aim of this pilot study was to: (i) identify preoperative PC measurements that predict acute postoperative morbidity and (ii) compare the predictive value of these PC measurements with that of the assigned (ASA) physical status classification rank in the same (n=32) cancer patients undergoing major abdominal surgery at The University of Texas M.D. Anderson Cancer Center. This pilot study is intended to serve as the foundation for future studies exploring the relationship between measurements of PC and postoperative outcomes.
Methods
After approval by the Institutional Review Board of M.D. Anderson, patients undergoing elective major abdominal cancer surgery and meeting the inclusion and exclusion criteria for enrolment (Table 1) were enrolled in this prospective, blinded, observational study. Participation in this study did not alter the standard perioperative treatment plan but rather allowed a patient to proceed through two separate processes of preoperative evaluations: (i) the standard of care for preoperative evaluation process at M.D. Anderson and (ii) the study's process of identifying an individual's preoperative PC using CPET. The following were blinded to the CPET results: the patient, the clinicians performing the standard preoperative evaluation, all healthcare providers caring for the patients during the intra- and postoperative periods, and the individuals who collected the postoperative outcomes data. The following were blinded to the patients' postoperative outcomes: the individuals administering the CPET, the individual reviewing all ECGs, and the individual interpreting the CPET results. This methodology was used to maintain the current standard of care and reduce bias during the data gathering and analysis processes.
Table 1.
Inclusion and exclusion criteria for participation in the study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients >18 yr of age | Any patient who is unable to exercise |
| Patients must sign informed consent | Patient is deemed unacceptable for surgery after evaluation in the Pre-anaesthesia Assessment Center |
| Patients screened in the Pre-anaesthesia Assessment Center | Surgery is cancelled for any reason |
| Patients must be scheduled for one of the following (frequency of surgery) | The patient suffers any of the following within 3 months before visiting the Pre-anaesthesia Assessment Center |
| Gastrectomy (3) | Myocardial infarction |
| Pancreatectomy (2) | Cerebrovascular event |
| Radical cystectomy (14) | Transient ischaemic attack |
| Radical nephrectomy (1) | Pulmonary embolic event |
| Radical transabdominal tumour debulking (2) | Existing acute or chronic deep vein thrombosis |
| Pelvic exenteration (5) | Pregnancy |
| Low anterior resection (1) | |
| Retroperitoneal lymph node dissection (4) |
Cardiopulmonary exercise test protocol
CPET was performed within 2 weeks of the patient's scheduled surgery to reduce the possibility of a significant change in patient activity levels before surgery that could potentially affect PC. Patients participating in the study were instructed not to eat or drink anything within 2 h of the scheduled CPET. Pretest data gathered on the day of the exercise test before CPET included the following: patient characteristics, co-morbidities, preoperative medications, and diagnostic and laboratory test results.
The exercise test was conducted using the following five-phase process
Phase 1: Pulmonary function testing (sitting): This measured maximum voluntary ventilation, forced expiratory volume in 1 s, and forced vital capacity.
Phase 2: Supine resting: After applying the electrodes for a 12-lead ECG, arterial pressure cuff, pulse oximeter, and gas exchange collection mouth piece, the patient lay quietly in the supine position for 10 min while resting gas exchange data were collected (CardiO2/CP System, Medical Graphics Corporation, USA).
Phase 3: Unloaded cycling: Patients cycled at 60 revolutions per minute (RPM) with no resistance for 3 min on a bicycle ergometer while their vital signs and gas exchange data were collected.
Phase 4: Ramp protocol:6 Patients continued cycling at 60 RPM while the pedal resistance was progressively increased at a predetermined rate (5–25 W min−1 increments according to the individual patient's physical strength). The test was stopped either when the patient fatigued or at the investigator's discretion (on the basis of signs or symptoms of cardiopulmonary distress).
Phase 5: Recovery: Patients continued pedalling at 60 RPM with no resistance for 5 min after the conclusion of phase 4.
The patients' vital signs along with gas exchange variables were continuously monitored during phases 2 through 5 of the exercise test. All ECGs were reviewed for ischaemic changes by a staff cardiologist at M.D. Anderson and the raw gas exchange data were interpreted at Harbor Medical Center, University of California, Los Angeles, USA.
Surgical procedures and anaesthetic technique
Patients in this study underwent one of eight different surgical procedures (Table 1). The standard of care at M.D. Anderson was maintained concerning the patients' surgical evaluation, indications for surgery, surgical technique, and postoperative care plans. All patients received a balanced, general anaesthetic consisting of propofol (1–2 mg kg−1), sufentanil (0.25–0.5 µg kg−1), and rocuronium (0.15 mg kg−1) during induction. Anaesthesia was maintained with an oxygen/volatile anaesthesia mixture (isoflurane or desflurane) and infusion of sufentanil (0.1–0.3 µg kg−1 h−1) and rocuronium (4 µg kg−1 min−1).
Postoperative outcome
Patients were monitored up to the seventh postoperative day for acute morbid events. Postoperative events were defined before commencement of the study, according to the standard diagnostic criteria used to identify these conditions at M.D. Anderson (Supplementary material, Appendix 1). Patients who suffered one or more events (both in and out of hospital) during the 7 day observation period were assigned to the all-event group. Patients discharged before the seventh day were followed up by telephone and outpatient clinic notes. The absence of an event defined the non-event group.
Statistical analysis
The preoperative data, co-morbidities, the ASA rank, and the parameters of PC were evaluated using uni- and multivariate logistic regression. Owing to the limited number of patients enrolled in this study, only a two-variable multivariate logistic regression model was considered as to not over-fit the data. The statistical package SAS7 was used to fit all logistic regression models. The significance of the ASA rank and each gas exchange measure as a predictor of postoperative events, along with the sensitivity and specificity for postoperative risk prediction, were determined. Receiver operating characteristic (ROC) curves were used to describe the discrimination ability and to explore the trade-offs between sensitivity and specificity for each potential predictor of postoperative morbidity. The area under the curve (AUC) for each ROC curve was calculated because of its robust indication of performance for classification models, and also its usefulness as an overall index of diagnostic accuracy which is not dependent on a decision threshold.8 AUC values, which range from 0.5 (no predictive power) to 1.0 (total predictive power), were used to estimate the discriminating power of the predictors. Thus, the AUC is a performance indicator equivalent to the non-parametric concordance measure, Somers' D, and the difference between two AUCs is half the difference between the corresponding Somers' D values.9 STATA version 9 (Stata Corporation, College Station, TX, USA)10 was used to assess the difference between the AUC values of two models on the basis of the χ2 test developed from the generalized U-statistics theory.11 A multivariate logistic regression model evaluated the predictive power or synergism gained from linear combinations of parameters. In this study, a P-value of ≤0.05 was considered statistically significant.
Results
Of the 32 patients enrolled in the study, two were classified as ASA I, 16 as ASA II, and 14 as ASA III. All patients completed an interpretable, maximal effort CPET (Table 2). No adverse events occurred during CPET. Patient characteristics, preoperative co-morbidities, and medications, with the exception of diabetes mellitus and β-blocker therapy, did not appear to associate with postoperative outcomes (Table 3). Preoperative diabetes mellitus and the use of β-blocker therapy were more common in the all-event group than in the non-event group. Pulmonary function tests performed before CPET were not associated with adverse outcomes.
Table 2.
Study population standard gas exchange measurements
| Measurement | Median | Range |
|---|---|---|
| Peak oxygen uptake (ml min−1) | 1337 | 659–3565 |
| Peak oxygen uptake/ideal body weight (kg) | 18.5 | 8.8–42.6 |
| Anaerobic threshold (ml min−1) | 779 | 470–1560 |
| Anaerobic threshold/ideal body weight (kg) | 11.1 | 6.0–18.6 |
Table 3.
Comparison of the preoperative characteristics, co-morbidities, and medication data for the study population. IBW, ideal body weight
| Total | Non-event | All-event | P-value | |
|---|---|---|---|---|
| Characteristic | ||||
| Patients (n) | 32 | 16 | 16 | |
| Age (yr) | 63 (22–80) | 63 (38–80) | 63 (22–78) | |
| Gender (M/F) | 21/11 | 11/5 | 10/6 | 1.00 |
| Weight (kg) | 83 (55–167) | 80 (67–129) | 84 (55–167) | |
| Predicted IBW (kg) | 77 (53–90) | 78 (52–88) | 80 (61–90) | |
| Co-morbidities | ||||
| Cardiovascular disease | 16 (50.0%) | 8 | 8 (50.0%) | 1.00 |
| Hypertension | 14 (43.7%) | 7 | 7 (50.0%) | 1.00 |
| Diabetes mellitus | 5 (15.6%) | 1 | 4 (80.0%) | 0.033 |
| Respiratory disease | 3 (9.4%) | 2 | 1 (33.3%) | 1.00 |
| Chemotherapy | 21 (65.6%) | 11 | 10 | 1.00 |
| Radiotherapy | 9 (28.1%) | 4 | 5 | 1.00 |
| Preoperative medication | ||||
| β-blockers | 5 (15.6%) | 0 | 5 | 0.04 |
Sixteen (50%) of the study patients suffered at least one postoperative event. Hospital (P=0.0007) and intensive care unit (P=0.0002) lengths of stay were significantly associated with postoperative events (Table 4). Reasons for ICU admissions ranged from for observation to patient's who experienced one or more events (Table 5). The decision to admit patients to the ICU was made without knowledge of a patient's CPET results.
Table 4.
Hospital and intensive care unit length of stay
| Total | Non-event (n=16) |
Any-event (n=16) |
P-value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| ICU length of stay (days) | 0 | 0–2 | 2 | 0–7 | 0.0002 |
| Hospital length of stay (days) | 8 | 3–14 | 12 | 7–36 | 0.0007 |
Table 5.
Postoperative events identified during pilot study (frequency of event)
| Cardiac events (6 total) |
| Myocardial infarction (1) |
| Dysrhythmia or conduction abnormality (1) |
| Congestive heart failure (2) |
| Postoperative vasopressors (2) |
| Respiratory event (8 total) |
| Prolonged intubation >24 h (5) |
| Acute respiratory distress syndrome (2) |
| Acute respiratory failure (1) |
| Vascular events (1 total) |
| Venous thrombus (1) |
| Renal events (2 total) |
| Renal insufficiency (1) |
| Renal failure (1) |
| Infectious events (5 total) |
| Wound infection (4) |
| Sepsis (1) |
The use of epidural analgesia had no significant association with postoperative events. No deaths occurred during the 7 day postoperative observation period. Of the variables evaluated for an association with postoperative morbidities (Table 6), those significantly associated with postoperative morbidity were ASA rank (P=0.038, AUC=0.688, 95% CI=0.52315, 0.85185) and three PC measurements, heart rate at the anaerobic threshold (HR3; P=0.025, AUC=0.734, 95% CI=1.008, 1.133), heart rate response from rest to the anaerobic threshold (ΔHR1; P=0.010, AUC=0.799, 95% CI=0.64510, 0.95256), and per cent predicted anaerobic threshold achieved <75% vs ≥75% (PAT; P=0.016, AUC=0.719, 95% CI=0.56789, 0.86961), were significantly associated with postoperative morbidity (Table 7). Both ΔHR1 and PAT were more sensitive than the ASA rank, and HR3 yielded a greater specificity than the ASA rank. ΔHR1 produced the greatest AUC (0.799). A pairwise comparison of the AUC for ASA rank to the AUCs for each of the three PC measurements showed none to be statistically significantly better than any of the others.
Table 6.
Definitions of significant measurements associated with postoperative morbidity
| Variable | Definition |
|---|---|
| ASA | ASA classification rank, <3 vs ≥3 (dichotomized) |
| HR1 | Average resting heart rate (beats min−1) |
| HR2 | Average unloaded cycling heart rate (beats min−1) |
| HR3 | Heart rate at AT (beats min−1) |
| HR4 | Heart rate at peak oxygen uptake, VO2 (beats min−1) |
| ΔHR1 | The difference in heart rate (HR) between HR3 (HR at the anaerobic threshold) and HR1 (HR at rest) |
| ΔHR2 | The difference in heart rate between HR4 (heart rate at peak oxygen uptake) and HR1 |
| RER1 | Respiratory exchange ratio (VCO2/VO2) at rest |
| AT/IBW | Anaerobic threshold (AT; ml min−1) per ideal body weight (IBW; kg) |
| AT | AT/IBW, <11 vs ≥11 ml min−1 (dichotomized) |
| %AT | Percentage of predicted AT achieved |
| PAT | Percentage of predicted AT achieved, <75% vs ≥75% (dichotomized) |
| PVO2/IBW | Peak oxygen uptake (PVO2; ml min−1) per ideal body weight (kg) |
| %PVO2 | Percentage of predicted peak VO2 achieved |
| PPVO2 | Percentage of peak VO2 achieved; <75% vs ≥75% (dichotomized) |
| VE3 | Minute ventilation at AT |
| ΔHR1+PAT | Multivariate model combining ΔHR1 and PAT |
Table 7.
Uni- and multivariate logistic regression model analysis related to postoperative outcomes
| Variable | AUC | P-value | Sensitivity | Specificity |
|---|---|---|---|---|
| ASA | 0.688 | 0.038 | 0.630 | 0.750 |
| HR1 | 0.611 | 0.412 | 0.625 | 0.750 |
| HR2 | 0.678 | 0.101 | 0.625 | 0.625 |
| HR3 | 0.734 | 0.025 | 0.563 | 0.938 |
| HR4 | 0.711 | 0.061 | 0.688 | 0.688 |
| ΔHR1 | 0.799 | 0.010 | 0.813 | 0.625 |
| ΔHR2 | 0.648 | 0.155 | 0.563 | 0.875 |
| RER1 | 0.713 | 0.065 | 0.625 | 0.875 |
| AT/IBW | 0.664 | 0.094 | 0.875 | 0.500 |
| AT | 0.594 | 0.291 | 0.563 | 0.625 |
| %AT | 0.705 | 0.061 | 0.687 | 0.688 |
| PAT | 0.719 | 0.016 | 0.875 | 0.563 |
| PVO2/IBW | 0.605 | 0.190 | 0.750 | 0.438 |
| %PVO2 | 0.648 | 0.108 | 0.750 | 0.500 |
| PPVO2 | 0.656 | 0.062 | 0.875 | 0.438 |
| VE3 | 0.670 | 0.086 | 0.688 | 0.625 |
| ΔHR1+PAT | 0.826 | 0.023 | 0.813 | 0.688 |
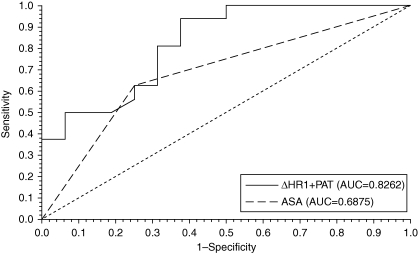
A two-variable model utilizing ΔHR1 and PAT was fitted to the data. The multivariate PC model yielded a significance of P=0.023, AUC=0.826, 95% CI=0.68363, 0.96871, sensitivity=0.813, and specificity=0.688 (Fig. 1). Although the AUC for the multivariate model was greater than that for the ASA rank model, the difference was not statistically significant.
Fig 1.
ROC curve for PC model (ΔHR1+PAT) vs ASA in relation to postoperative outcomes (P=0.27).
Discussion
Our study has identified three measurements of PC previously unidentified, two of which were incorporated into a multivariate model, all of which may be significantly associated with a broad range of acute postoperative outcomes and suggests the possibility that other, yet undetermined, parameters of PC may also be useful indicators of risk. We also showed that both the ASA rank assignment and preoperative measurements of PC are significantly associated with a broad range of acute postoperative morbidities in the study patient population, and suggest that, compared with the ASA rank assignment, the multivariate model of PC may be a more sensitive predictor of risk, defining more accurately the patient population most likely to experience an acute postoperative morbid events.
It is difficult to draw a conclusion as to the predictive value of PC measures by comparing the results of the previous studies due to the diversity of: surgical procedures, postoperative endpoints, and the variety of measures evaluated. The use of PC as a preoperative cardiac risk assessment for elderly patients undergoing various abdominal operations identified an anaerobic threshold of <11 ml kg−1 min−1 as the critical measure of PC defining high risk for postoperative death resulting from cardiac dysfunction.12,13 This was the only study to identify the anaerobic threshold as the critical parameter of PC defining high risk. In a population of 82 morbidly obese patients undergoing bariatric surgery, peak oxygen uptake ≤15.8 ml kg−1 min−1 was identified as a critical measure of PC that defined high risk of overall postoperative morbidity.14 This study used a single surgical procedure, evaluated a variety of postoperative outcomes, and identified the peak oxygen uptake as the critical parameter of PC defining high risk.14 A peak oxygen uptake of <800 ml min−1 m−1 was proposed as a predictor of postoperative cardiopulmonary morbidity in 91 patients undergoing oesophagectomy.15 In contrast, an anaerobic threshold <11 ml kg−1 min−1 was a poor predictor of postoperative cardiopulmonary morbidity in 78 patients undergoing oesophagectomy.16 The peak oxygen uptake was significantly lower in patients developing cardiopulmonary complications, but CPET was thought to be of limited value in predicting postoperative cardiopulmonary morbidity. These studies15,16 evaluated the same surgical procedure and the same postoperative endpoint and identified the peak oxygen uptake as the measure defining high risk. However, they arrived at conflicting opinions about the value of PC as a preoperative risk assessment tool.
In our study, we evaluated 32 patients undergoing a mix of surgical procedures, and looked at a variety of postoperative outcomes. In our study, in contrast to the previous studies, peak oxygen uptake and the anaerobic threshold were not significantly associated with postoperative morbidity in our patient population. We identified three new and previously unreported measurements of PC that are potentially significant predictors of postoperative morbidity: HR3, ΔHR1, and PAT. ΔHR1 was identified based on a previous study17 which reported that a heart rate <99 beats min−1 after supine pedalling for 2 min (not measuring gas exchange) was a preoperative indicator of postoperative cardiac and pulmonary complications. These findings encouraged us to investigate such a measure in our data using a metabolic endpoint. PAT is a modification of the anaerobic threshold and was used in a multivariate model with ΔHR1 (PAT+ΔHR1). If our study had been limited to peak oxygen uptake and the anaerobic threshold, these additional measurements of PC would not have been identified as being significantly associated with postoperative morbidity in our study population. More importantly, we would have suspected that there was no relationship between measures of PC and postoperative outcomes in this study population. Taken together, all of these studies suggest that some measurements of PC are associated with postoperative morbidity. The specific details concerning which measures are associated with which postoperative endpoints, and under what conditions are not clear. Clarification of these details will require further studies of these potentially complex relationships.
CPET could have a significant value for patients undergoing a broad range of surgical procedures. Patients with cancer are frequently treated with cardiopulmonary toxic chemotherapy, radiation therapy, or both before surgery.18,19 Currently, there is no preoperative risk assessment tool that accurately assesses the physiological effect of preoperative chemotherapy or radiotherapy on postoperative outcomes. In the attempt to maximize the effects of cancer controlling surgical therapy, postoperative morbidities unrelated to the cancer prognosis may result in decreased patient survival.1,20 A patient's PC may be an effective means to assess the physiological effect of preoperative anti-cancer therapies and establish the recovery time needed after these therapies before surgical intervention to achieve the best therapeutic outcomes. The potential clinical values of data obtained from CPET are largely unexplored at this time.
The small number of patients in this pilot study and the number of measurements evaluated increase the possibility that some reported associations may be due to chance. This caveat notwithstanding, such limitations of statistical power should not be used as a pretence to ignore the potential implications of the newly reported measurements of PC. Given the inconsistencies in the literature regarding the predictive value of the reported measurements and this pilot study's identification of new potentially predictive measurements raises the possibility of others that predict postoperative outcomes. Systematic expression of CPET data may provide an opportunity to identify even more accurate and precise measures predictive of postoperative outcomes for specific types of surgeries, illnesses, or both. It is becoming increasingly clear that identifying which measurements of PC are associated with postoperative outcomes will require further studies to understand these critical and complex relationships.
In summary, assessing surgical risk requires consideration of both the anticipated therapeutic outcome related to the surgical prognosis and the probability of postoperative adverse outcomes unrelated to the surgical prognosis. This initial study was not designed to supply a definitive answer regarding the relationship between PC and postoperative outcome. The definitive answer will only result from more thorough, detailed studies of this potentially valuable, complex relationship. These studies must first identify measurements of potential risk, and then validate these as risk predictors. Continued investigations may ultimately result in the pre-emptive preoperative management of more precisely defined physiological risk status, thereby reducing postoperative complications.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Supplementary Material
References
- 1.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA., Jr Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 3.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4:172–7. [PubMed] [Google Scholar]
- 4.Garcia-Miguel FJ, Serrano-Aguilar PG, Zlopez-Bastida J. Preoperative assessment. Lancet. 2003;362:1749–57. doi: 10.1016/s0140-6736(03)14857-x. [DOI] [PubMed] [Google Scholar]
- 5.Wasserman K, Hansen J, Sue D, et al. Physiology of exercise. In: Wasserman K, editor. Principles of Exercise Testing and Interpretation. Baltimore, MD: Lippincott Williams and Wilkins; 1999. pp. 10–61. [Google Scholar]
- 6.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol. 1983;55:1558–64. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 7.Cary, NC: SAS Institute Inc.; 2003. SAS Release 9.1.3. [Google Scholar]
- 8.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 9.Somers R. A new asymmetric measure of association for ordinal variables. Am Sociol Rev. 1962;27:799–811. [Google Scholar]
- 10.College Station, TX: Stata Corporation; 2005. STATA version 9. [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 12.Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993;104:701–4. doi: 10.1378/chest.104.3.701. [DOI] [PubMed] [Google Scholar]
- 13.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–62. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 14.McCullough PA, Gallagher MJ, Dejong AT, et al. Cardiorespiratory fitness and short-term complications after bariatric surgery. Chest. 2006;130:517–25. doi: 10.1378/chest.130.2.517. [DOI] [PubMed] [Google Scholar]
- 15.Nagamatsu Y, Shima I, Yamana H, Fujita H, Shirouzu K, Ishitake T. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg. 2001;121:1064–8. doi: 10.1067/mtc.2001.113596. [DOI] [PubMed] [Google Scholar]
- 16.Forshaw MJ, Strauss CF, Davies AR, et al. Is cardiopulmonary exercise testing a useful test before esophagaectomy. Ann Thorac Surg. 2008;85:294–9. doi: 10.1016/j.athoracsur.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 17.Gerson MC, Hurst JM, Hertzberg VS, Baughman R, Rouan GW, Ellis K. Prediction of cardiac and pulmonary complications related to elective abdominal and noncardiac thoracic surgery in geriatric patients. Am J Med. 1990;88:101–7. doi: 10.1016/0002-9343(90)90456-n. [DOI] [PubMed] [Google Scholar]
- 18.Lefor AT. Perioperative management of the patient with cancer. Chest. 1999;115:165S–71S. doi: 10.1378/chest.115.suppl_2.165s. [DOI] [PubMed] [Google Scholar]
- 19.Thorsen L, Nystad W, Stigum H, et al. Cardiorespiratory fitness in relation to self-reported physical function in cancer patients after chemotherapy. J Sports Med Phys Fitness. 2006;46:122–7. [PubMed] [Google Scholar]
- 20.Mangano DT. Adverse outcomes after surgery in the year 2001—a continuing odyssey. Anesthesiology. 1998;88:561–4. doi: 10.1097/00000542-199803000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.