Abstract
Objectives
To examine mutations within the penA, mtrR, porB, ponA and pilQ genes of Neisseria gonorrhoeae to determine their contribution to cephalosporin resistance.
Methods
A total of 46 N. gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone (MICs ≥ 0.12 mg/L) and two susceptible isolates were selected. The full sequence of penA and partial sequences previously reported as hot mutation sites of the other genes were analysed. Genotyping by N. gonorrhoeae multiantigen sequence typing (NG-MAST) was also performed.
Results
A mosaic penicillin-binding protein 2 (PBP 2) was found in a single isolate that exhibited the highest cefixime MIC (0.5 mg/L). The majority of the isolates with reduced susceptibility to cephalosporins contained non-mosaic PBP 2 sequences, of which PBP 2 pattern XIII was most common (28/46). All isolates with reduced susceptibility to cephalosporins also had mtrR and porB mutations. Two susceptible isolates had the PBP 2 pattern XIV and an incomplete MtrR protein, which was a new mutation. Isolates with identical PBP 2 patterns comprised multiple NG-MAST sequence types.
Conclusions
Reduced susceptibility of N. gonorrhoeae to ceftriaxone and cefixime was associated with diverse penA mutations, particularly PBP 2 pattern XIII containing an Ala-501→Val substitution, together with mtrR and porB mutations. The existence of only one strain having the mosaic penA sequence indicated that ceftriaxone and cefixime resistance in Korea is mostly not associated with a mosaic penA sequence. Highly heterogeneous NG-MAST sequence types excluded the clonal expansion of a particular subtype.
Keywords: penicillin binding protein 2, genotyping, antibiotic resistance, cephalosporins
Introduction
Gonorrhea remains a major sexually transmitted disease (STD) in Korea, with 3115 reported cases in 2007 according to the STD Sentinel Surveillance System from the National Institute of Health, Korea (http://stat.cdc.go.kr). Effective treatment options for Neisseria gonorrhoeae infections have been significantly reduced over time by the emergence and spread of gonococci resistant to penicillins, tetracyclines, macrolides and quinolones.1 Cephalosporins are now recommended as the mainstay of treatment.2 The Korea Centers for Disease Control and Prevention (KCDC) has recommended a single dose of 125 mg of intramuscular ceftriaxone or a single dose of 400 mg of oral cefixime since 2006. However, the emergence of N. gonorrhoeae isolates with reduced susceptibility to cephalosporins in vitro,3–13 along with occasional treatment failures with cefixime10,14 and ceftibuten,12 have been reported.
Sequential alterations of the penA, mtrR, porB, ponA and pilQ genes induce chromosomally mediated high-level resistance to penicillin.15 A point mutation in the pilQ gene (pilQ2, previously named penC) was recently identified, but this mutation has not been observed in a clinical setting.15,16 Similarly, an association has been proposed between mutations in penA, mtrR, porB and ponA and reduced susceptibility of N. gonorrhoeae strains to broad-spectrum cephalosporins such as cefixime and ceftriaxone.6,9,17 The penA mutations usually comprise insertion of a single aspartic acid codon between Arg345 and Asp346 (R345_D346insD), along with a five or six amino acid substitution downstream.18,19 Recently, penA mosaic alleles different from the former non-mosaic penA mutations were identified,4,6,8,9,11,13 with mosaic alleles particularly linked to reduced susceptibility to cefixime.4,6,8,13 However, recent studies suggest that reduced susceptibility to ceftriaxone is not due to the presence of a mosaic penA allele.11,20 Accordingly, the aims of this study were to examine mutations in the penA, mtrR, porB, ponA and pilQ genes of N. gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone and to determine their contribution to resistance.
Materials and methods
N. gonorrhoeae isolates
N. gonorrhoeae isolated from patients attending STD clinics and public health centres during 2001–07 were studied. Of the 877 gonococci isolated, 208 were randomly selected for antimicrobial susceptibility testing and were stored at −70°C until further use.
A total of 48 isolates were selected for further investigation on the basis of their ceftriaxone or cefixime MICs: 46 had reduced susceptibility (MICs 0.12–0.5 mg/L); and 2 were susceptible (MICs ≤ 0.008 mg/L).
MIC determinations
MICs of penicillin G, cefixime and ceftriaxone were determined by the agar dilution method.21 The medium used was GC agar base (BBL; Becton Dickinson, Cockeysville, MD, USA) supplemented with 1% haemoglobin and 1% IsoVitaleX (BBL). Penicillin G (Sigma Chemical Co., St Louis, MO, USA), cefixime (Dong A Pharmaceutical Co., Seoul, Korea) and ceftriaxone (Hanmi Pharmaceutical Co., Seoul, Korea) were used. Inocula of 104 cfu were applied using a Steers replicator (Craft Machine Inc., Woodline, PA, USA). Plates were incubated in a 5% CO2 incubator at 35°C for 24 h after which the results were read. N. gonorrhoeae ATCC 49226 was used as a control strain.
Nucleotide sequencing of penA genes
Full-length penA nucleotide sequences from the N. gonorrhoeae strains were determined as reported previously.13 The primers used in this study are listed in Table 1. PCR was carried out in a total volume of 20 µL, with 1 µL of heat-extracted template DNA, 10 pmol of each primer and PreMix (Bioneer, Cheongwon, Korea) containing 1 U of Taq DNA polymerase. A thermal cycler (Eppendorf, Hamburg, Germany) was used with the following conditions: 35 cycles of 94°C for 1 min; 50°C (penA-A1, B1), 56°C (penA-A2, B2) or 52°C (penA-A3, B3) for 1 min; and 72°C for 1 min. The PCR products were extracted using a DNA extraction kit (Qiagen, Hilden, Germany) and subjected to direct sequencing. All PCR products were sequenced twice with forward and reverse primers using an automatic sequencer (model 3730xl; Applied Biosystems, Weiterstadt, Germany). Multiple-sequence alignments of nucleotide and amino acids were performed using BioEdit (version 7.0.9.0). The amino acid sequence patterns of PBP 2 were classified according to those previously described by Ito et al.13 (I–X) and Whiley et al.11 (XI–XXIII).
Table 1.
Primers used for PCR amplification and sequencing of the penA, mtrR, porB, ponA and pilQ genes
| Primer | Nucleotide sequence (5′ to 3′) | Nucleotide positions | Reference |
|---|---|---|---|
| penA-A1 | CGGGCAATACCTTTATGGTGGAAC | 8 to 31 | 13 |
| penA-B1 | AACCTTCCTGACCTTTGCCGTC | 655 to 676 | 13 |
| penA-A2 | AAAACGCCATTACCCGATGGG | 597 to 617 | 13 |
| penA-B2 | TAATGCCGCGCACATCCAAAG | 1157 to 1177 | 13 |
| penA-A3 | GCCGTAACCGATATGATCGA | 1003 to 1022 | 13 |
| penA-B3 | CGTTGATACTCGGATTAAGACG | 1844 to 1865 | 13 |
| mtrR-F | GCCAATCAACAGGCATTCTTA | −210 to −191 | 5 |
| mtrR-R | GTTGGAACAACGCGTCAAAC | 190 to 171 | 5 |
| porB-F | CCGGCCTGCTTAAATTTCTTA | −41 to −22 | 22 |
| porB-R | TATTAGAATTTGTGGCGCAG | 1030 to 1049 | 22 |
| ponA-F | GAGAAAATGGGGGAGGACCG | 1171 to 1190 | 5 |
| ponA-R | GGCTGCCGCATTGCCTGAAC | 1395 to 1376 | 5 |
| pilQ-F | CGTTACGCCGAACATCACG | 1833 to 1851 | this study |
| pilQ-R | TGACCGAAACTGAACGGACTG | 2358 to 2338 | this study |
Mutations in mtrR, porB, ponA and pilQ
The promoter and coding regions of mtrR, porB, ponA and pilQ were amplified using previously described mtrR,5 porB22 and ponA5 primers and pilQ primers newly designed from the nucleotide sequence of N. gonorrhoeae FA 1090 (GenBank accession no. AE004969) (Table 1). The primers were intended to amplify the partial sequences previously reported as hot mutation sites. The parameters of the amplifications were as follows: 35 cycles of 94°C for 30 s; 50°C (mtrR), 46°C (porB), 56°C (ponA) or 52°C (pilQ) for 30 s; and 72°C for 1 min. PCR products were extracted and sequenced using the PCR primers mentioned above.
Genotyping
N. gonorrhoeae multiantigen sequence typing (NG-MAST) was conducted by the sequencing of internal fragments of two highly polymorphic antigen-encoding loci, por and tbpB.23 The sequence data were uploaded onto the NG-MAST website (www.ng-mast.net) to obtain the allele numbers and the sequence types.
Nucleotide sequence accession numbers
Nucleotide sequence data for the penA gene encoding PBP 2 with amino acid pattern XXIV, the mtrR gene encoding the incomplete MtrR protein and the mtrR gene encoding the MtrR protein with new amino acid substitutions (Ala-39→Thr, Leu-47→Pro) were submitted to the GenBank nucleotide database under accession numbers FJ465093, FJ465094 and FJ465095, respectively.
Results
Alterations of PBP 2 in clinical isolates
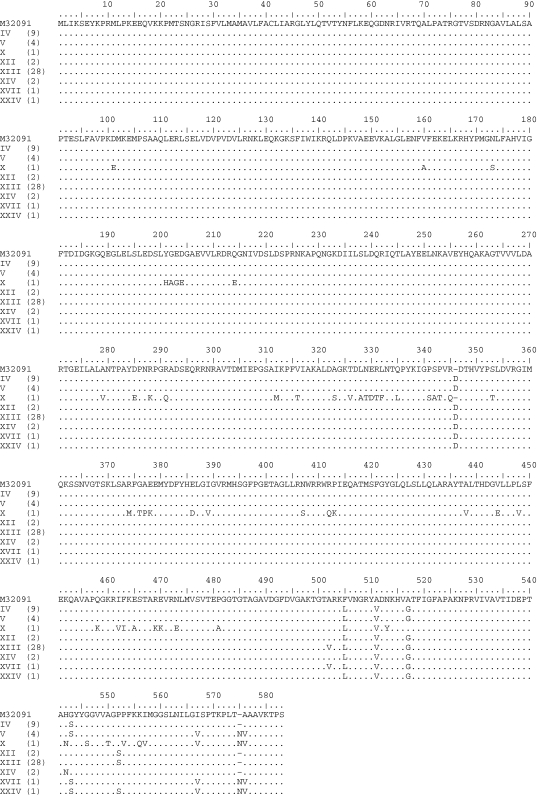
The complete nucleotide sequences of the penA genes from 48 clinical isolates were determined. Eight amino acid sequence patterns in PBP 2 were identified. These sequences were compared with those of penicillin- and cephalosporin-susceptible N. gonorrhoeae strain LM306 (GenBank accession no. M32091) (Figure 1). Overall, sequence pattern XIII was most common (28 strains), followed by pattern IV (9 strains) and pattern V (4 strains). Sequence pattern X, a previously described mosaic PBP 2 sequence,13 was found in only one strain. Pattern XXIV was newly identified in this study, which was different from pattern V by a single amino acid at position 551 (Pro-551→Ser).
Figure 1.
Amino acid sequences of PBP 2 from the 48 N. gonorrhoeae clinical isolates used in this study. The sequences are classified into different amino acid patterns (patterns I–XXIV) and are aligned with a GenBank sequence (accession number M32091). Sequence patterns I–X and XI–XXIII were previously described by Ito et al.13 and Whiley et al.,11 respectively, while pattern XXIV was identified in this study. The number of isolates displaying each pattern is indicated in parentheses.
Association of alteration in PBP 2 with antimicrobial susceptibility
The distributions of the MICs of antimicrobial agents for the clinical isolates associated with the PBP 2 sequence patterns are shown in Table 2. The penicillin MIC for the isolate with the mosaic PBP 2 structure (pattern X) was the highest for the β-lactamase non-producing strains (Table 3). This isolate also had the highest ceftriaxone and cefixime MICs (0.25 and 0.5 mg/L). However, four other strains with non-mosaic penA mutations also had ceftriaxone MICs of 0.25 mg/L. The two susceptible isolates had PBP pattern XIV and displayed the lowest MICs of the antimicrobial agents.
Table 2.
MICs of penicillin G, cefixime and ceftriaxone for the 48 N. gonorrhoeae clinical isolates with various patterns of alterations in PBP 2
| Isolate (no. of isolates tested) |
No. of isolates with MIC (mg/L) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactamase | PBP 2 pattern | No. of NG-MAST STs | Antibiotic | ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 32 | 64 | ≥128 |
| Positive | all (11) | PEN | 3 | 1 | 7 | ||||||||||||
| Negative | all (37) | PEN | 1 | 2 | 6 | 21 | 6 | 1 | |||||||||
| All | IV (9) | 8 | CFX | 2 | 3 | 4 | |||||||||||
| CRO | 7 | 2 | |||||||||||||||
| V (4) | 4 | CFX | 1 | 1 | 1 | 1 | |||||||||||
| CRO | 3 | 1 | |||||||||||||||
| X (1) | 1 | CFX | 1 | ||||||||||||||
| CRO | 1 | ||||||||||||||||
| XII (2) | 2 | CFX | 1 | 1 | |||||||||||||
| CRO | 1 | 1 | |||||||||||||||
| XIII (28) | 19 | CFX | 3 | 22 | 3 | ||||||||||||
| CRO | 16 | 11 | 1 | ||||||||||||||
| XIV (2) | 2 | CFX | 2 | ||||||||||||||
| CRO | 2 | ||||||||||||||||
| XVII (1) | 1 | CFX | 1 | ||||||||||||||
| CRO | 1 | ||||||||||||||||
| XXIV (1) | 1 | CFX | 1 | ||||||||||||||
| CRO | 1 | ||||||||||||||||
PEN, penicillin G; CFX, cefixime; CRO, ceftriaxone; NG, Neisseria gonorrhoeae; MAST, multiantigen sequence typing; STs, sequence types.
Table 3.
Antibiogram, mutations of penA, mtrR, porB and ponA genes and genotypes of the 48 N. gonorrhoeae clinical isolates
| MIC (mg/L) |
Mutations in |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Penicillinase | Isolate no. | PEN | CFX | CRO | penA (PBP 2) | mtrR | porB | ponA | NG-MAST ST |
| Positive | 2001-12 | >128 | 0.12 | 0.06 | XIII | deletion of Aa | G101K, A102D | WT | 3946b |
| 2002-1 | 128 | 0.12 | 0.06 | XIII | A39T, L47P | G101K, A102D | L421P | 495 | |
| 2004-2 | 32 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 270 | |
| 2004-3 | 32 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 270 | |
| 2006-39 | 128 | 0.25 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3956b | |
| 2006-43 | >128 | 0.25 | 0.25 | IV | deletion of A | G101K, A102D | L421P | 3956b | |
| 2007-17 | 128 | 0.12 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3485 | |
| 2007-32 | 64 | 0.06 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3965b | |
| 2007-38 | 128 | 0.25 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3966b | |
| 2007-53 | 128 | 0.12 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3965b | |
| 2002-8 | 32 | 0.12 | 0.06 | XII | A39T, L47P | G101K, A102D | L421P | 3948b | |
| Negative | 2003-9 | 0.25 | ≤0.008 | ≤0.008 | XIV | truncated MtrR | WT, WT | WT | 1798 |
| 2006-17 | 0.5 | ≤0.008 | ≤0.008 | XIV | truncated MtrR | G101K, A102D | WT | 3945b | |
| 2001-2 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 1623 | |
| 2002-3 | 4 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 1623 | |
| 2002-5 | 4 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102N | L421P | 3947b | |
| 2002-15 | 4 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 1623 | |
| 2002-21 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 1623 | |
| 2003-8 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 3950b | |
| 2003-25 | 2 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3951b | |
| 2004-6 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102N | L421P | 3952b | |
| 2004-28 | 1 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 3954b | |
| 2006-6 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 972 | |
| 2006-12 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 3955b | |
| 2006-19 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 3955b | |
| 2006-20 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 3955b | |
| 2006-21 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 1623 | |
| 2006-23 | 2 | 0.12 | 0.06 | XIII | deletion of A | G101K, A102D | L421P | 437 | |
| 2006-34 | 2 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 437 | |
| 2006-35 | 1 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102G | L421P | 3976b | |
| 2006-42 | 0.5 | 0.25 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3957b | |
| 2007-14 | 2 | 0.25 | 0.25 | XIII | deletion of A | G101K, A102D | L421P | 3962b | |
| 2007-19 | 2 | 0.25 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3963b | |
| 2007-45 | 1 | 0.12 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3967b | |
| 2007-50 | 1 | 0.06 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3967b | |
| 2007-51 | 1 | 0.06 | 0.12 | XIII | deletion of A | G101K, A102D | L421P | 3968b | |
| 2007-61 | 1 | 0.06 | 0.12 | XIII | deletion of A | G101K, A102D | WT | 3970b | |
| 2007-9 | 4 | 0.12 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3961b | |
| 2007-60 | 2 | 0.25 | 0.25 | IV | deletion of A | G101N, A102D | L421P | 3969b | |
| 2007-64 | 4 | 0.06 | 0.12 | IV | deletion of A | G101K, A102D | L421P | 3971b | |
| 2006-44 | 2 | 0.06 | 0.12 | V | deletion of A | G101K, A102D | L421P | 3959b | |
| 2006-45 | 4 | 0.25 | 0.25 | V | deletion of A | G101K, A102D | L421P | 1868 | |
| 2007-6 | 2 | 0.12 | 0.12 | V | deletion of A | G101K, A102D | L421P | 3960b | |
| 2007-65 | 2 | 0.03 | 0.12 | V | deletion of A | G101K, A102D | L421P | 3972b | |
| 2004-16 | 8 | 0.5 | 0.25 | X | deletion of A | G101K, A102D | L421P | 3953b | |
| 2006-38 | 2 | 0.06 | 0.12 | XII | deletion of A | G101K, A102D | L421P | 1614 | |
| 2007-30 | 2 | 0.12 | 0.12 | XVII | deletion of A | G101N, A102D | L421P | 3964b | |
| 2002-37 | 2 | 0.12 | 0.12 | XXIV | deletion of A | G101K, A102D | L421P | 3949b | |
PEN, penicillin G; CFX, cefixime; CRO, ceftriaxone; WT, wild-type; NG, Neisseria gonorrhoeae; MAST, multiantigen sequence typing; ST, sequence type.
aNucleotide (A) deletion within the 13 bp inverted repeat located between the −10 and −35 sequences of the mtrR promoter.
bNew sequence types found in this study.
Mutations in mtrR, porB, ponA and pilQ
Forty-four of the 46 N. gonorrhoeae isolates with reduced susceptibility to cephalosporins harboured a single nucleotide (A) deletion in the 13 bp inverted repeat located between the −10 and −35 sequences of the mtrR promoter (c.–57delA). The other two isolates contained missense mutations at codons 39 and 47 in the coding segments of mtrR that resulted in Ala-39→Thr, Leu-47→Pro amino acid substitutions. The two susceptible isolates contained a single nucleotide (G) deletion at position 81 of the mtrR coding sequence (c.81delG). This deletion induced a frame shift and produced a premature stop codon in mtrR, resulting in an incomplete MtrR protein consisting of 41 amino acids.
Non-synonymous substitutions at Gly-101 and Ala-102 of porin encoded by porB were found in 47 clinical isolates. Among the amino acid substitutions, Gly-101→Lys and Ala-102→Asp were the most common (42 strains), followed by Gly-101→Lys, Ala-102→Asn (2 strains) and Gly-101→Asn, Ala-102→Asp (2 strains). The ponA1 allele (Leu-421→Pro in PBP 1) was detected in 44 of the 46 clinical isolates with reduced susceptibility and not in the two susceptible isolates. Finally, the pilQ mutation was not found in any of the clinical isolates tested.
Genotyping
A total of 38 NG-MAST sequence types were observed, of which 26 were new. The identical PBP 2 patterns were divided into multiple NG-MAST sequence types (Table 2). The 28 strains with PBP 2 pattern XIII, the most common PBP 2 alteration, resolved into 19 NG-MAST sequence types.
Discussion
In the present study, the reduced susceptibility of N. gonorrhoeae isolates to cefixime or ceftriaxone was associated with mutations in the penA, mtrR, porB and ponA genes (Table 3). We found the penA mosaic allele in a single clinical isolate, for the first time in Korea. This penA mosaic sequence was identical to the sequence (PBP 2 pattern X) identified in N. gonorrhoeae strains with reduced susceptibility to cefixime and ceftriaxone in Japan4,8,9,13 and to ceftriaxone in Australia.11 However, the mosaic allele was found in only one of the 46 isolates with reduced susceptibility to cephalosporins. This finding emphasizes the importance of non-mosaic penA alleles in mediating reduced susceptibility to broad-spectrum cephalosporins. Among non-mosaic penA alleles, PBP 2 pattern XIII was observed in a large proportion of the isolates with reduced susceptibility (28 out of 46). This PBP 2 pattern contained a substitution at position 501 (Ala-501→Val), which was considered to cause reduced susceptibility to cephems in a homology modelling study.24 PBP 2 pattern XVII, which also contained the Ala-501→Val substitution, was found in one isolate. In a modelling study, the Ala-501→Val substitution and mosaic structure induced a conformational alteration of the β-lactam-binding pocket in PBP 2 making a major contribution to the reduced susceptibility to cephems.24 In other transformation experiments,8 the Ala-501→Val substitution led to a 2- to 4-fold increase in the MICs of cefixime and other cephems. Whiley et al.11 suggested that the Ala-501→Val substitution is more significant as a contribution to ceftriaxone susceptibility than a mosaic sequence. Our data showed the possibility that the Ala-501→Val substitution contributed to the reduced susceptibility to broad-spectrum cephalosporins although the penA mosaic allele has received attention with regard to reduced susceptibility to cephalosporins.4,8,12,13,17 In contrast, two isolates that were fully susceptible to cephalosporins contained PBP 2 pattern XIV, consistent with the previous study.11 This suggests that pattern XIV is not related to reduced susceptibility to cephalosporins.
The isolates with reduced susceptibility to cephalosporins were not restricted to any particular genotype. The isolates with identical PBP 2 patterns further resolved into multiple NG-MAST sequence types. These results showed the heterogeneity of N. gonorrhoeae strains with reduced susceptibility to cephalosporins, as has been observed in a recent study.11 Both the previous study11 and our present study included gonococci isolated at different times and in diverse geographical locations and comprised multiple subtypes.25 The heterogeneity of NG-MAST sequence types suggests that the common occurrence of mutations in the penA gene rather than clonal spread of a single strain contributes to the reduced susceptibility to cephalosporins. The NG-MAST sequence type 835, which was reported in the strains with reduced susceptibility to cephalosporins from the nearby countries of Hong Kong12 and Taiwan,26 was not found in Korea.
The isolate with a mosaic penA allele showed the highest cefixime MIC (0.5 mg/L), but other isolates with non-mosaic penA alleles also had increased cefixime MICs. The ceftriaxone MIC for the isolate with the mosaic allele (0.25 mg/L) overlapped with MICs for the isolates with non-mosaic penA alleles. These results suggest that non-mosaic penA mutations are also able to increase cephalosporins MICs to a level similar to that mediated by the mosaic allele. Recently, a clinical isolate with a different mosaic PBP 2, pattern XXIII, was reported to be fully susceptible to ceftriaxone.11,20 This indicates that the mosaic PBP 2 is not sufficient for reduced susceptibility to ceftriaxone. Transformation experiments17 have shown that a significant level of cefixime resistance was conferred by the mosaic penA allele, with only a small contribution from mtrR and porB, whereas ceftriaxone resistance was equally dependent upon both. It is possible that besides penA, mtrR, porB and ponA, additional genetic mutations in an as yet unidentified loci also contribute to increased resistance of N. gonorrhoeae to cephalosporins.17
All isolates with reduced susceptibility to cephalosporins had mtrR, porB and ponA mutations except two, which did not have the ponA mutation (Table 3). We set the cefixime or ceftriaxone MIC cut-offs for selecting strains for this study at 0.12 mg/L. This cut-off was chosen based on the study by Ito et al.13 which reported mosaic penA (type X) in strains for which the cefixime MIC was ≥0.12 mg/L. This high MIC cut-off would make it very difficult to find different combinations of several gene mutations that can be observed in N. gonorrhoeae strains with lower MICs and to deduce the effect of each gene mutation. However, we expect that the ponA mutation (ponA1 allele) is not important for cephalosporin resistance because cephalosporin susceptibility was not different between isolates with ponA mutation and isolates without the mutation if other gene mutations were the same, supporting a recent transformation study.17
Several new mutations of the mtrR gene were found in this study. The two susceptible isolates had an incomplete MtrR protein due to a single nucleotide (G) deletion at position 81. In contrast, the isolates with reduced susceptibility had a deletion in the promoter region (44 isolates) or newly found amino acid substitutions (Ala-39→Thr, Leu-47→Pro) within MtrR (2 isolates). Hagman and Shafer27 reported that the loss of MtrR resulted in enhanced expression of mtrC, but not to the same extent as that caused by the single bp deletion in the mtrR promoter region. The mtrR promoter overlaps the −35 sequence of mtrC, so mutation in the promoter region can enhance binding of either RNA polymerase or an activator to the mtrC promoter due to decreased competition for binding on the same region of the DNA, which induces higher resistance.27
In conclusion, reduced susceptibility of N. gonorrhoeae clinical isolates to ceftriaxone and cefixime was associated with diverse penA mutations, particularly PBP 2 pattern XIII containing an Ala-501→Val substitution. The existence of only one isolate having the mosaic penA sequence indicated that ceftriaxone and cefixime resistance in Korea is mostly not associated with a mosaic penA sequence. The mutations in mtrR and porB observed in all strains with increased MICs indicate their contribution to ceftriaxone and cefixime resistance. The considerable diversity in NG-MAST sequence types excluded the clonal expansion of a particular subtype.
Funding
This work was supported by the National Institute of Health, Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (grant number 2008-E00252-00).
Transparency declarations
None to declare.
Acknowledgements
We are grateful to Ms Hayeon Kim for collecting the isolates and to Ms Yunghee Seo and Hyeong-Mi Kim for technical assistance.
References
- 1.Tapsall J. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert Rev Anti Infect Ther. 2006;4:619–28. doi: 10.1586/14787210.4.4.619. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332–6. [PubMed] [Google Scholar]
- 3.Australian Gonococcal Surveillance Programme. Annual report of the Australian Gonococcal Surveillance Programme. Commun Dis Intell 2006. 2005;30:205–10. doi: 10.33321/cdi.2006.30.16. [DOI] [PubMed] [Google Scholar]
- 4.Ameyama S, Onodera S, Takahata M, et al. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002;46:3744–9. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilina EN, Vereshchagin VA, Borovskaya AD, et al. Relation between genetic markers of drug resistance and susceptibility profile of clinical Neisseria gonorrhoeae strains. Antimicrob Agents Chemother. 2008;52:2175–82. doi: 10.1128/AAC.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindberg R, Fredlund H, Nicholas R, et al. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother. 2007;51:2117–22. doi: 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin IM, Hoffmann S, Ison CA. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J Antimicrob Chemother. 2006;58:587–93. doi: 10.1093/jac/dkl265. [DOI] [PubMed] [Google Scholar]
- 8.Takahata S, Senju N, Osaki Y, et al. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2006;50:3638–45. doi: 10.1128/AAC.00626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Nakayama H, Huruya K, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents. 2006;27:20–6. doi: 10.1016/j.ijantimicag.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang SA, Lee MV, O'Connor N, et al. Multidrug-resistant Neisseria gonorrhoeae with decreased susceptibility to cefixime—Hawaii, 2001. Clin Infect Dis. 2003;37:849–52. doi: 10.1086/377500. [DOI] [PubMed] [Google Scholar]
- 11.Whiley DM, Limnios EA, Ray S, et al. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob Agents Chemother. 2007;51:3111–6. doi: 10.1128/AAC.00306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo JY, Ho KM, Leung AO, et al. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob Agents Chemother. 2008;52:3564–7. doi: 10.1128/AAC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito M, Deguchi T, Mizutani KS, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother. 2005;49:137–43. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deguchi T, Yasuda M, Yokoi S, et al. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J Infect Chemother. 2003;9:35–9. doi: 10.1007/s10156-002-0204-8. [DOI] [PubMed] [Google Scholar]
- 15.Ropp PA, Hu M, Olesky M, et al. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46:769–77. doi: 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao S, Tobiason DM, Hu M, et al. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol Microbiol. 2005;57:1238–51. doi: 10.1111/j.1365-2958.2005.04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Duncan M, Tomberg J, et al. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2009;53:3744–51. doi: 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowson CG, Jephcott AE, Gough KR, et al. Penicillin-binding protein 2 genes of non-β-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989;3:35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 19.Dougherty TJ. Genetic analysis and penicillin-binding protein alterations in Neisseria gonorrhoeae with chromosomally mediated resistance. Antimicrob Agents Chemother. 1986;30:649–52. doi: 10.1128/aac.30.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiley DM, Limnios EA, Ray S, et al. Further questions regarding the role of mosaic penA sequences in conferring reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2007;51:802–3. doi: 10.1128/AAC.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Seventh Edition: Approved Standard M7-A7. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 22.Liao M, Bell K, Gu WM, et al. Clusters of circulating Neisseria gonorrhoeae strains and association with antimicrobial resistance in Shanghai. J Antimicrob Chemother. 2008;61:478–87. doi: 10.1093/jac/dkm544. [DOI] [PubMed] [Google Scholar]
- 23.Martin IM, Ison CA, Aanensen DM, et al. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189:1497–505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 24.Osaka K, Takakura T, Narukawa K, et al. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J Infect Chemother. 2008;14:195–203. doi: 10.1007/s10156-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 25.Tapsall J, Whiley D, Sloots T. Applications of molecular testing in clinical laboratories for the diagnosis and control of gonorrhea. Future Microbiol. 2006;1:317–24. doi: 10.2217/17460913.1.3.317. [DOI] [PubMed] [Google Scholar]
- 26.Wong WW, Huang CT, Li LH, et al. Molecular epidemiological identification of Neisseria gonorrhoeae clonal clusters with distinct susceptibility profiles associated with specific groups at high risk of contracting human immunodeficiency virus and syphilis. J Clin Microbiol. 2008;46:3931–4. doi: 10.1128/JCM.00577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagman KE, Shafer WM. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol. 1995;177:4162–5. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]