Abstract
Objectives
Invasive fungal infections (IFIs) contribute significantly to mortality and morbidity in patients receiving myelosuppressive chemotherapy for haematological malignancies. The present study investigates the overall survival (OS), infection-related mortality and changes in treatment of IFIs in our department from 1995 until 2006.
Methods
Outcomes of all chemotherapy courses were retrospectively evaluated using a standard questionnaire. Modified EORTC/MSG criteria for IFIs were applied: a positive PCR result for Aspergillus spp. in bronchoalveolar lavage was also defined as probable IFI.
Results
In total, 1693 chemotherapy courses in 592 patients were evaluated. Sixty-three percent of chemotherapy courses were given to treat acute myeloid leukaemia, with the rest for acute lymphoblastic leukaemia or aggressive lymphoma. IFIs were observed in 139/592 patients [23.5%, 95% confidence interval (CI) 20%–27%] and in 149/1693 courses (8.8%, 95% CI 8%–10%). IFI-related mortality was 56.9% in 1995–2001 and 28.6% in 2002–06, P < 0.001. Accordingly, median OS in patients with IFI increased: 54 days (95% CI 26–82 days) in 1995–2001 versus 229 days (95% CI 35–423 days) in 2002–06, P = 0.001. By multivariate analysis, factors predictive for better OS were controlled disease after chemotherapy [hazard ratio (HR) 0.228, P < 0.001], possible IFI (in contrast to proven/probable IFI, HR 0.537, P = 0.005), age <60 years (HR 0.583, P = 0.008), time period 2002–06 (HR 0.612, P = 0.021) and use of novel antifungals (HR 0.589, P = 0.033).
Conclusions
Compared with 1995–2001, IFI-related mortality decreased and OS in patients with IFI increased significantly in recent years. Improved OS was associated with controlled haematological disease, certainty of IFI diagnosis (possible), younger age, time period 2002–06 and the use of novel antifungals.
Keywords: outcomes research, prognostic factors, antifungal agents, acute leukaemia
Introduction
Invasive fungal infections (IFIs) are a leading cause of mortality and morbidity in patients with haematological malignancies and prolonged neutropenia after chemotherapy.1–3 The prevalence of IFI ranges from 2% to 40%, depending upon a variety of factors, including the underlying disease and required treatment.4–6 In patients with febrile neutropenia as well as in autopsies from patients with haematological malignancies, incidences of 13%–21% have been reported.7,8 Others deduce a much higher infection rate from the fact that 60% of patients with pulmonary infiltrates respond to antimycotic treatment, whereas only ∼30% respond to antibiotics alone.9 In haematological malignancies, the most common manifestation of IFI is fungal pneumonia, often caused by moulds such as Aspergillus species.10
It has been shown that patients with pulmonary infiltrates in their neutropenic phase have a much worse prognosis than those without.11 This is why empirical and pre-emptive antimycotic treatment in persistently febrile neutropenic patients have become well-recognized parts of current treatment strategies.12,13
Amphotericin B has been the gold standard for the treatment of IFI for many years. However, this treatment is associated with a considerable toxicity, particularly regarding renal function.14 Newer antimycotic drugs with better tolerability, such as echinocandins and azoles, can be regarded as alternatives to amphotericin B.15,16 Although it is widely accepted that these drugs have a considerably favourable toxicity profile compared with conventional amphotericin B, it is as yet unknown if the use of the newer antifungal agents has an influence on overall survival (OS) or fungal infection-related mortality. At least for some haematological malignancies a clear survival benefit due to improved supportive care has been shown in the past,17 but epidemiological studies investigating the effects of novel antifungals in patients with acute leukaemia who did not undergo allogeneic transplantation are lacking.
In our department (tertiary care centre, University of Bonn), all patients with acute leukaemia were treated on the same ward. Antileukaemic treatment has not changed significantly during the past 12 years and comprehensive diagnostic procedures have been performed in patients with febrile neutropenia since 1995. In this uniform setting, we evaluated the incidence of IFI as well as IFI-related mortality and OS in patients with IFI, and analysed time trends in IFI-related mortality.
Patients and methods
A retrospective cohort study was performed in all patients treated with myelosuppressive chemotherapy for haematological malignancies or autologous stem cell transplantation (SCT) at a tertiary care centre (University of Bonn, Germany) from 1995 to 2006. Data of chemotherapy courses were collected with a standard questionnaire. For episodes with occurrence of proven, probable or possible IFI, an additional validated and tested structured questionnaire was implemented to document data of medical records, imaging reports, histopathology reports, microbiological reports and use of antifungal agents. All applied chemotherapy courses were documented, except for courses given for palliative treatment, intrathecal therapy or courses with cyclophosphamide given for stem cell harvest. The pre-planned criteria for diagnosis of IFI were based on the 2002 version of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria.18 To ensure comparability with previously published data, the criteria were not adapted to the revised EORTC/MSG criteria published in 2008.19 In addition to these criteria, a positive PCR for Aspergillus spp. in bronchoalveolar lavage (BAL) was also defined as probable IFI. Documentation and data entry were performed by different persons; a data check was done during this process. The last follow-up data for patients with IFI were collected in July 2008.
Treatment and prophylaxis protocols
All patients were treated according to standard protocols of multicentric German study groups, i.e. the German Multicentre Study Group on Adult Acute Lymphoblastic Leukaemia (GMALL) protocol was applied in patients with acute lymphoblastic leukaemia (ALL), and the AML Cooperative Group (AMLCG) (1995–97) and AML Study Group (AMLSG) (1998–2006) protocols were applied for the treatment of acute myeloid leukaemia (AML).20,21 Antimicrobial prophylaxis consisted of colistin, co-trimoxazole and oral amphotericin B (in patients with ALL) or itraconazole (in patients with AML as described by Glasmacher et al.22).
Conventional amphotericin B as primary antifungal therapy was routinely administered throughout the whole observation period. If patients presented with clinically significant renal insufficiency they were treated with liposomal amphotericin B. Since 1999, novel antifungal agents were increasingly used in these patients. Intravenous itraconazole was introduced in 1999, but was given only occasionally due to oral itraconazole prophylaxis in most patients. Caspofungin was used in clinical trials from 1999 to 2003 and routinely from 2002 onwards. Use of voriconazole started in 2003.
At the onset of fever, blood cultures and a chest radiograph were performed. If pulmonary infiltrates were seen or antibiotic refractory fever lasted for >72 h, a computer tomography (CT) was done to identify suspected fungal infection. From 2000 onwards, blood samples were collected twice weekly as routine to detect the galactomannan antigen using the Platelia Aspergillus galactomannan enzyme-linked immunoassay.23 Panfungal PCR from BAL24 was applied in individual cases in 2001. In 2002, this changed to routine practice whenever a bronchoscopy could be done. Renal dysfunction was defined as serum creatinine above the upper limit of normal (ULN). Hepatic dysfunction was defined as bilirubin or alanine aminotransferase or aspartate transaminase above the ULN. IFI-related death was defined as death with continuing signs of IFI.
Statistical analysis
According to a pre-specified analysis plan, two cohorts of patients were defined: patients diagnosed with IFI from 1995 to 2001 and from 2002 to 2006. This year was selected in advance because of a change in diagnostic practice (Aspergillus galactomannan test, PCR) and availability of novel antifungal agents (caspofungin, voriconazole).
Fisher's exact, χ2 and Mann–Whitney tests were used to test for differences where appropriate. Survival was analysed using the Kaplan–Meier method and the log-rank test was used to test for differences. A two-sided P value of <0.05 was considered statistically significant. OS was calculated from the day of start of chemotherapy to the date of death or last follow-up. The multivariate Cox regression model was used to analyse risk factors for death. Variables with P values of <0.1 in univariate analysis and less than or equal to three missing values were entered in backward stepwise selection models, and hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. Statistical analyses were performed by SPSS v. 17 (Munich, Germany). The 95% CIs were calculated with GraphPad InStat V2.05.
Results
Study population
All 1693 chemotherapy courses from 592 patients were included in the analysis. Data were incomplete in 23/1693 courses (1.4%). The number of courses per year ranged between 83 and 195.
Baseline characteristics of courses and patients are given in Table 1. Standard intensive chemotherapy was given in 1142/1693 courses (67.5%), high-dose cytarabine in 404 courses (23.9%) and autologous SCT in 147 courses (8.7%). In 1433/1693 courses (84.6%) the patients were neutropenic (leucocytes < 1.0/nL or neutrophils < 0.5/nL), with a duration of ≥10 days in 1085/1693 courses (64.1%).
Table 1.
Baseline characteristics of courses and patients
| Characteristic | Courses (N = 1693) | Patients (N = 592)a |
|---|---|---|
| Median age (years, min.–max.) | 57 (17–82) | 59 (17–81) |
| Male sex (n, %) | 976 (57.6%) | 350 (59.1%) |
| Diagnosis (n, %) | ||
| AML | 1071 (63.3%) | 408 (68.9%) |
| ALL/B-ALL/B-NHL | 504 (29.8%) | 109 (18.4%) |
| others | 118 (7.0%) | 75 (12.7%) |
| Status before chemotherapy (n, %) | ||
| newly diagnosed | 514 (30.4%) | 476 (80.4%) |
| complete/partial remission | 905 (53.5%) | 82 (13.9%) |
| relapse | 158 (9.3%) | 25 (4.2%) |
| refractory | 116 (6.9%) | 9 (1.5%) |
| Concomitant disease (n, %) | ||
| cardiovascular | 261 (15.4%) | 107 (18.1%) |
| pulmonary | 265 (15.7%) | 73 (12.3%) |
| Duration of neutropenia (days, interquartile range) | 13 (6–20) | 15 (8–20) |
AML, acute myeloid leukaemia; B-ALL, B-cell acute lymphoblastic leukaemia; B-NHL, B-cell non-Hodgkin lymphoma.
aOnly the first course of each patient in the database was analysed; median number of courses per patient = 2.
In 1117/1693 courses (66.0%) at least one febrile episode occurred. Fever of unknown origin was present in 533/1693 courses (31.5%), pneumonia in 341 (20.1%), microbiologically documented infection in 401 (23.7%) and other clinically documented infection in 119 (7.0%). Antifungal therapy had been applied in 381/1693 courses (22.5%). In-hospital death occurred in 214/592 patients (36.1%). Main reasons of death were infection in 154/214 patients (72%) and underlying disease in 27/214 patients (12.6%).
Courses with IFI
The total number of chemotherapy courses in which an IFI occurred was 149/1693 (8.8%, 95% CI 8%–10%). Seventy-five IFIs were classified as possible (4.4%), 32 as probable (1.9%) and 42 as proven (2.5%). Proven IFI was pulmonary IFI in 16/42 (38.1%) and fungaemia in 20/42 (47.6%) courses. The remaining proven infections included tissue infections of multiple organs (three patients), tissue infections of other organs (two patients) and pulmonary IFI combined with fungaemia (one patient).
Median time to IFI was 18 days after the start of chemotherapy (interquartile range 13–26 days). Systemic antifungal prophylaxis had been administered in 128/149 courses (85.9%) with IFI and in most cases itraconazole was given [119/149 (79.9%)]. The reason to start antifungal therapy was empirical therapy (i.e. absence of radiological findings) in 31/149 courses (20.8%) and definite therapy in 114/149 courses (76.5%). Four patients did not receive antifungal therapy.
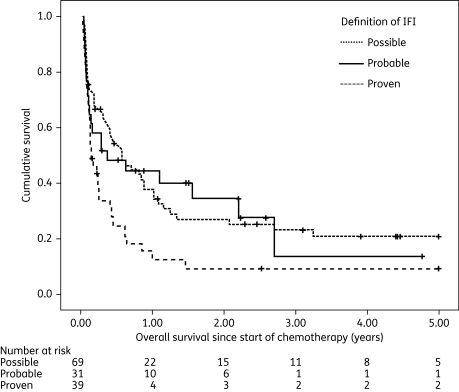
Patients received a median of two antifungal agents for a median treatment duration of 17 days (interquartile range 11–27 days). Combination therapy had been given in 29/149 courses (19.5%), and 15 of these included caspofungin and/or voriconazole. Conventional amphotericin B had been administered as mono or combination therapy in 124 courses (83.2%), liposomal amphotericin B in 13 courses (8.7%), amphotericin B lipid complex in 5 courses (3.4%), flucytosine in 10 courses (6.7%), fluconazole in 6 courses (4.0%), itraconazole in 26 courses (17.4%), voriconazole in 11 courses (7.4%) and caspofungin in 48 courses (32.2%). The success rate with conventional amphotericin B was 42/124 (33.9%, 95% CI 26%–43%), with amphotericin B lipid formulations was 6/18 (33.3%, 95% CI 13%–59%), with itraconazole was 9/26 (34.6%, 95% CI 17%–56%), with voriconazole was 6/11 (54.5%, 95% CI 23%–83%) and with caspofungin was 30/48 (62.5%, 95% CI 47%–76%). IFI-related death occurred in 61/149 courses (40.9%). In-hospital death rate was significantly higher in patients with IFI than in controls (58/149, 38.9% versus 156/1544, 10.1%, P < 0.001). Seventeen patients (11.4%) suffered a further IFI and 10 of these were in our hospital. OS analysis was performed on a patient-based dataset (N = 139). Patients with second episodes of IFI were not censored at the time of the second IFI. Median OS for patients with IFI was 148 days (95% CI 93–203 days), with significant differences for patients with proven, probable or possible IFI (Figure 1): 56 days (95% CI 22–90 days) in proven IFI compared with 138 days (95% CI 0–360 days) in probable and 209 days (95% CI 93–325 days) in possible IFI (P = 0.024).
Figure 1.
Overall survival (years) since start of chemotherapy in patients with proven, probable or possible IFI; P = 0.024. Patients with second episodes of IFI were not censored at the time of the second IFI.
Comparison of the two time periods (1995–2001 versus 2002–06)
When analysing all courses, no differences between the two time periods could be observed regarding dose intensity of chemotherapy (standard, high-dose cytarabine, autologous SCT), duration of neutropenia, neutropenia ≥ 10 days, controlled underlying disease (complete or partial remission) before or after chemotherapy and incidence of pneumonia. In-hospital death was significantly lower in 2002–06 (83/774 courses, 10.7%, 95% CI 9%–13% versus 131/919, 14.3%, 95% CI 12%–17% in 1995–2001, P = 0.033), whereas the incidence of IFI was significantly higher. From 1995 to 2001, an IFI occurred in 65/919 courses (7.1%, 95% CI 5%–9%) as opposed to 84/774 courses (10.9%, 95% CI 9%–13%) with IFI from 2002 to 2006, P = 0.007.
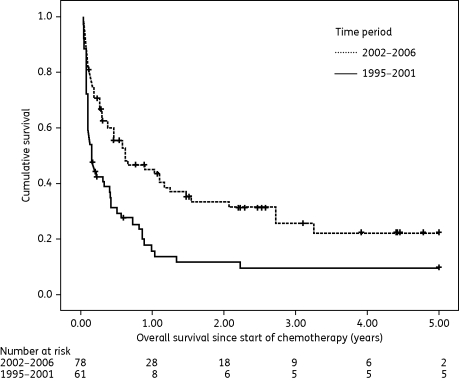
A comparison of variables in courses with IFI in the two time periods is given in Table 2. A significant difference was detected for incidence of proven and probable IFI (P = 0.006 and P < 0.001, respectively), reason for antifungal treatment (P = 0.001), median time to IFI treatment from day of IFI diagnosis (P = 0.047) and overall change in antifungal treatment regime from 2002 onwards, i.e. less frequent use of amphotericin B (P = 0.001) and increased use of caspofungin (P < 0.001) and voriconazole (P = 0.003). Interestingly, a reduction in IFI-related mortality could be found: 37/65 courses with IFI (56.9%, 95% CI 44%–69%) had a fatal outcome in 1995–2001 versus 24/84 courses (28.6%, 95% CI 19%–39%) in 2002–06, P = 0.001. The same result was obtained if IFI-related mortality was analysed per patient (only the first episode analysed): death with continuing signs of IFI was observed in 35/61 patients (57.4%, 95% CI 44%–70%) before 2002 versus 22/78 patients (28.2%, 95% CI 19%–40%) in the period 2002–06, P = 0.001. An increased OS in patients with IFI could be demonstrated for the time period 2002–06 (Figure 2, patient-based analysis, N = 139). Median OS was 54 days (95% CI 26–82 days) before 2002 versus 229 days (95% CI 35–423 days) for the period from 2002 onwards, P = 0.001.
Table 2.
Comparison of variables in courses with IFI during two time periods (1995–2001 versus 2002–06), N = 149
| 1995–2001 | 2002–06 | P | |
|---|---|---|---|
| Median age (years, min.–max.) | 59 (19–76) | 60 (24–81) | 0.195 |
| Median duration of neutropenia (days, IQR) | 22 (17–29) | 21 (17–27) | 0.612 |
| Neutropenia ≥ 10 days (n/N, %) | 60/64 (93.8%) | 79/82 (96.3%) | 0.699 |
| Courses in patients with AML (n/N, %) | 53/65 (81.5%) | 63/84 (75.0%) | 0.427 |
| Treatment with high-dose AraC (n/N, %) | 27/65 (41.5%) | 26/84 (31.0%) | 0.227 |
| Courses with controlled disease before chemotherapy (n/N, %) | 23/65 (35.4%) | 32/84 (38.1%) | 0.864 |
| Courses with controlled disease after chemotherapy (n/N, %) | 38/65 (58.5%) | 56/84 (66.7%) | 0.311 |
| Systemic antifungal prophylaxis (n/N, %) | 59/65 (90.8%) | 69/84 (82.1%) | 0.159 |
| Incidence of IFI (n/N, %) | 65/919 (7.1%) | 84/774 (10.9%) | 0.007 |
| proven IFI | 26/65 (40.0%) | 16/84 (19.0%) | 0.006 |
| probable IFI | 5/65 (7.7%) | 27/84 (32.1%) | <0.001 |
| possible IFI | 34/65 (52.3%) | 41/84 (48.8%) | 0.742 |
| IFI-related mortality (n/N, %) | 37/65 (56.9%) | 24/84 (28.6%) | 0.001 |
| Pathogens in proven IFI | 0.063 | ||
| Aspergillus spp. | 11/26 (42.3%) | 5/16 (31.3%) | |
| Candida albicans | 1/26 (3.8%) | 3/16 (18.8%) | |
| Candida non-albicansa | 8/26 (30.8%) | 8/16 (50.0%) | |
| Aspergillus spp. + Candida spp. | 3/26 (11.5%) | 0 | |
| Mucor sp. | 1/26 (3.8%) | 0 | |
| Blastoschizomyces capitatus | 1/26 (3.8%) | 0 | |
| B. capitatus + Aspergillus spp. | 1/26 (3.8%) | 0 | |
| Reason for starting antifungal treatment | 0.001 | ||
| empirical (n/N, %) | 22/65 (33.8%) | 9/80 (11.3%) | |
| definitive (n/N, %) | 43/65 (66.2%) | 71/80 (88.8%) | |
| Median time to IFI treatment from date of IFI diagnosis (days, IQR) | 0 (−2 to 1) | 0 (0–1) | 0.047 |
| Initial antifungal treatmentb (n/N, %) | |||
| amphotericin B | 52/64 (81.3%) | 57/84 (67.9%) | 0.090 |
| caspofungin | 3/64 (4.7%) | 10/84 (11.9%) | 0.151 |
| voriconazole | 0 | 5/84 (6.0%) | 0.070 |
| itraconazole | 3/64 (4.7%) | 2/84 (2.4%) | 0.652 |
| fluconazole | 0 | 1/84 (1.2%) | 1.000 |
| liposomal amphotericin B | 1/64 (1.6%) | 0 | 0.432 |
| combination therapy | 5/64 (7.8%) | 5/84 (6.0%) | 0.746 |
| no antifungal treatment | 0 | 4/84 (4.8%) | 0.134 |
| Any antifungal treatmentb,c (n/N, %) | |||
| amphotericin B | 61/64 (95.3%) | 63/84 (75.0%) | 0.001 |
| caspofungin | 8/64 (12.5%) | 40/84 (47.6%) | <0.001 |
| voriconazole | 0 | 11/84 (13.1%) | 0.003 |
| itraconazoled | 11/63 (17.5%) | 15/84 (17.9%) | 1.000 |
| fluconazoled | 4/63 (6.3%) | 2/84 (2.4%) | 0.402 |
| liposomal amphotericin Bd | 12/63 (19.0%) | 1/84 (1.2%) | <0.001 |
| combination therapyd | 14/63 (22.2%) | 15/84 (17.9%) | 0.536 |
IQR, interquartile range; AraC, cytarabine.
aCandida glabrata in 10 courses, Candida tropicalis in 2 courses, Candida krusei in 2 courses, Candida guilliermondii in 1 course and Candida sp. in 1 course.
bIndependent of the duration of treatment; use of caspofungin in years before 2002 in clinical trials only.
cMultiple antifungal agents possible.
dAntifungal treatment was not completely evaluable in one course.
Figure 2.
Overall survival (years) since start of chemotherapy in patients with IFI in the years 1995–2001 and 2002–06; HR = 0.535 (95% CI 0.363–0.788), P = 0.001. Patients with second episodes of IFI were not censored at the time of the second IFI.
No differences between the two time periods in courses with IFI could be shown for age, duration of neutropenia, patients with AML, controlled disease before or after chemotherapy, systemic antimycotic prophylaxis, dose intensity of chemotherapy, fungaemia/tissue infection, pathogens in proven IFI and hepatic or renal dysfunction before start of antifungal treatment.
Multivariate analysis for OS in patients with IFI
More than 20 variables that might have an influence on OS in patients with IFI were tested in univariate analysis, i.e. age, sex, underlying disease, kind of chemotherapy, status of haematological disease before and after chemotherapy, duration of neutropenia, concomitant diseases, renal and/or hepatic dysfunction, year of IFI diagnosis, classification, and treatment of IFI. The following factors had a significant influence on OS (P < 0.1): age <60 years; controlled disease before chemotherapy; controlled disease after chemotherapy; proven IFI; possible IFI; therapy with novel antifungal agents (i.e. caspofungin and/or voriconazole); and time period (1995–2001 versus 2002–06). Including all these variables in a multivariate Cox regression analysis, controlled disease after chemotherapy (HR 0.228, 95% CI 0.149–0.351, P < 0.001), classification as possible IFI (HR 0.537, 95% CI 0.349–0.828, P = 0.005), age <60 years (HR 0.583, 95% CI 0.392–0.869, P = 0.008), time period 2002–06 (HR 0.612, 95% CI 0.403–0.930, P = 0.021) and use of novel antifungal agents (HR 0.589, 95% CI 0.362–0.959, P = 0.033) were associated with significantly improved OS (Table 3).
Table 3.
Multivariate analysis in patients with IFI
| HR | 95% CI | P | |
|---|---|---|---|
| Controlled disease after chemotherapy | 0.228 | 0.149–0.351 | <0.001 |
| Certainty of IFI diagnosis (possible) | 0.537 | 0.349–0.828 | 0.005 |
| Age < 60 years | 0.583 | 0.392–0.869 | 0.008 |
| Time period 2002–06 | 0.612 | 0.403–0.930 | 0.021 |
| Use of novel antifungal agents | 0.589 | 0.362–0.959 | 0.033 |
| Controlled disease before chemotherapy | 1.236 | 0.735–2.080 | 0.425 |
| Certainty of IFI diagnosis (proven) | 1.225 | 0.674–2.228 | 0.505 |
Discussion
In our study of a large consecutive single-centre cohort of patients in whom treatment of underlying disease and prophylaxis strategy did not change over 12 years, we could show that the detection rate of IFI increased, whereas IFI-related mortality decreased and OS in patients with IFI increased during the years 2002–06. We also identified factors influencing OS in patients with IFI. By multivariate analysis, controlled disease after chemotherapy, classification as possible IFI (in contrast to diagnosis of probable or proven IFI), age <60 years, time period 2002–06 and use of novel antifungal agents were significantly associated with improved OS. Because there is collinearity between the time period and the use of novel antifungal agents, which might have an impact on multivariate analysis, we repeated the analysis and excluded the time period from the Cox regression model. In this second model (data not shown), all previously significant variables remained significant as independent prognostic factors.
Whereas antileukaemic treatment has not changed significantly during the past 10 years, supportive therapy has improved considerably.17 With the approval of newer azoles as well as echinocandins, alternatives to amphotericin B, its lipid formulations or pyrimidine derivates have become available. In our multivariate analysis, we show for the first time that in patients with haematological malignancies and myelosuppressive chemotherapy or autologous SCT, the use of novel antifungal agents was an independent factor for improved OS in patients with IFI. In a study by Upton et al.25 a similar evaluation was undertaken, and risk factors for death and fungal infection-attributable death were analysed. In contrast to our study, they only examined patients with allogeneic SCT and showed that a number of transplantation-related variables were associated with mortality. Allogeneic transplantations were not performed at our centre and, thus, we determined a different range of factors influencing survival in our patients. Interestingly, in a subanalysis of the study of Upton et al.,25 administration of voriconazole was associated with a lower risk for death due to invasive aspergillosis but not for all-cause mortality. Nivoix et al.26 analysed factors associated with overall mortality in patients with cancer and invasive aspergillosis, and found an improved OS in patients who received voriconazole as first-line therapy. Because, here, multivariate analysis was stratified by first-line antifungal therapy, voriconazole as an independent prognostic factor for OS could not be analysed. Interestingly, in this heterogeneous population, the authors also found that the degree of certainty of diagnosis is an independent prognostic factor, with an improved OS in patients with possible aspergillosis. In contrast, in the study by Auberger et al.,7 whose analysis was similar to ours, only proven IFI was predictive for death in a multivariate analysis. A possible reason why they could not identify more risk factors might be the inhomogeneous population analysed, including patients with intensive chemotherapy, and autologous and allogeneic SCT.
Besides improved OS in patients with IFI, we also found a decrease in IFI-related mortality from 56.9% to 28.6% in the later period. The IFI-related mortality rate of 40.9% for the whole observation period was similar compared with earlier reports.7,25,27,28 A reduction in IFI-related mortality was also described in the recent literature,7,8,25 despite a significant increase in the incidence of IFI,7 comparing well with our results. Our multivariate analysis showed a strong impact of the use of novel antifungal agents on OS in patients with IFI, independent of the inclusion of time period in the model. Thus, improvement in therapeutic agents is probably the most important cause for this finding.29 An additional important factor most likely to be associated with improved OS may be the earlier start of antifungal treatment because of improved diagnostics. In the later time period, newer diagnostic methods have been implemented and the time period 2002–06 remained an independent prognostic factor in our multivariate analysis, leading us to assume a causal relationship. Unfortunately, in our study reliable evidence for an earlier start of treatment due to improved diagnostics cannot be achieved, because the true date of occurrence of IFI is unknown. Prospective studies are needed to solve this question.
We found an overall incidence (based on courses of myelosuppressive chemotherapy) of 8.8% for IFI, which is within the range reported in recent literature,2,6,7 and a significant difference for the rate of proven and probable IFI in the two time periods, with less proven and more probable infections in years 2002–06. Improved diagnostics might at least partly explain the increased detection rate of IFI. Interestingly, there was no difference between the earlier and the later cohort in known risk factors for IFI development,1,30 such as the duration of neutropenia, intensity of chemotherapy or the disease status before or after chemotherapy, which could account for the increased incidence of IFI. Also, the antimicrobial prophylaxis was not changed for the whole observation period. Therefore, improved diagnostics probably lead to an overall higher sensitivity of diagnosing IFI, but the actual incidence of IFI may not have been changed. It is important to note that no major building works were performed during the entire study period. The higher rate of probable fungal infections might be attributable to the routine use of the Aspergillus galactomannan antigen test and PCR performed in BAL samples. Although PCR is not a microbiological criterion in the 2002 IFI diagnosis criteria,18 it is an important tool in clinical practice for the early diagnosis of IFI and was often positive in our study without further accepted microbiological criteria. One should keep in mind that diagnosis of a probable IFI was not based on PCR alone, but that a CT suspicious of IFI was also present. If original EORTC criteria were applied, the incidence of probable IFI in the period 2002–06 decreased to 17.9%, with a P value of 0.091, compared with the period 1995–2001 (7.7%). Possible IFI remained an independent prognostic factor for improved OS in the multivariate analysis. Interestingly, the difference in OS for certainty of diagnosis (Figure 1) remained significant too. The higher rate of proven infections in the earlier years is possibly due to the fact that probable infections could not be detected early enough. After 2000, when Aspergillus antigen was monitored twice weekly, a probable IFI was assumed and treated before becoming a proven infection. Another explanation might be that autopsies were less frequently performed in the later years, as has been shown by Donhuijsen et al.8
In addition to an increase of mould infection, an increase in candidaemia by non-albicans Candida species has been reported.31 Interestingly, Pagano et al.2 found a pathogen pattern similar to ours, as in their cohort 90% of mould infections were Aspergillus species and 91% of yeast infections were Candida species, with non-albicans species responsible for >50% of the episodes of candidaemia. Similarly, in our cohort, the rate of non-albicans Candida infections increased from 30% in 1995–2001 to 50% in 2002–06. We would like to point out that the rate of Candida infections did not differ between the two time periods (13.8% of all IFIs in 1995–2001 versus 13.1% in 2002–06, P = 1.000) and, thus, the improved OS in the later period was not caused by a higher rate of Candida infections.
In conclusion, the results of our study show that the outcomes of patients with IFI, i.e. IFI-related mortality and OS, have improved significantly in the past years. This is partly due to the administration of novel antifungal agents. Other factors improving OS were controlled underlying disease after chemotherapy, possible IFI (in contrast to proven or probable IFI) and age <60 years.
Funding
This study was supported by MSD Sharp & Dohme GmbH, Haar, Germany and the Leukämie-Initiative Bonn e.V. The sponsors had no influence on the evaluation of data, the preparation of the manuscript and the decision to submit it for publication.
Transparency declarations
C. H.-A. has received research support from Pfizer Pharma GmbH. M. v. L.-T. has received research support from MSD Sharp & Dohme GmbH and is a member of the advisory board of MSD Sharp & Dohme GmbH. All other authors: none to declare.
Acknowledgements
Presented as an oral presentation at the Gemeinsame Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaft für Hämatologie/Onkologie, Mannheim, Germany, 2009 (Abstract in Onkologie 2009; 32 Suppl 4: 160 No. V581).
We would like to thank M. Ruhnke's laboratory in Berlin for performing the real-time PCR analysis.
References
- 1.Martino R, Subira M. Invasive fungal infections in hematology: new trends. Ann Hematol. 2002;81:233–43. doi: 10.1007/s00277-002-0466-3. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–75. [PubMed] [Google Scholar]
- 3.Böhme A, Ruhnke M, Buchheidt D, et al. Treatment of invasive fungal infections in cancer patients. Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2009;88:97–110. doi: 10.1007/s00277-008-0622-5. [DOI] [PubMed] [Google Scholar]
- 4.Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 5.Mühlemann K, Wenger C, Zenhäusern R, et al. Risk factors for invasive aspergillosis in neutropenic patients with hematologic malignancies. Leukemia. 2005;19:545–50. doi: 10.1038/sj.leu.2403674. [DOI] [PubMed] [Google Scholar]
- 6.Cuenca-Estrella M, Bernal-Martinez L, Buitrago MJ, et al. Update on the epidemiology and diagnosis of invasive fungal infection. Int J Antimicrob Agents. 2008;32(Suppl 2):S143–7. doi: 10.1016/S0924-8579(08)70016-5. [DOI] [PubMed] [Google Scholar]
- 7.Auberger J, Lass-Flörl C, Ulmer H, et al. Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2008;88:508–15. doi: 10.1007/s12185-008-0184-2. [DOI] [PubMed] [Google Scholar]
- 8.Donhuijsen K, Petersen P, Schmid KW. Trend reversal in the frequency of mycoses in hematological neoplasias. Dtsch Ärztebl Int. 2008;105:501–6. doi: 10.3238/arztebl.2008.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maschmeyer G, Link H, Hiddemann W, et al. Pulmonary infiltrations in febrile patients with neutropenia: risk factors and outcome under empirical antimicrobial therapy in a randomized multicenter study. Cancer. 1994;73:2296–304. doi: 10.1002/1097-0142(19940501)73:9<2296::aid-cncr2820730910>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Brakhage AA. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr Drug Targets. 2005;6:875–86. doi: 10.2174/138945005774912717. [DOI] [PubMed] [Google Scholar]
- 11.Chaoui D, Legrand O, Roche N, et al. Incidence and prognostic value of respiratory events in acute leukemia. Leukemia. 2004;18:670–5. doi: 10.1038/sj.leu.2403270. [DOI] [PubMed] [Google Scholar]
- 12.Maschmeyer G, Beinert T, Buchheidt D, et al. Diagnosis and antimicrobial therapy of pulmonary infiltrates in febrile neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S118–26. doi: 10.1007/s00277-003-0765-3. [DOI] [PubMed] [Google Scholar]
- 13.Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis. 2004;39(Suppl 1):S38–43. doi: 10.1086/383052. [DOI] [PubMed] [Google Scholar]
- 14.Ullmann AJ, Sanz MA, Tramarin A, et al. Prospective study of amphotericin B formulations in immunocompromised patients in 4 European countries. Clin Infect Dis. 2006;43:e29–38. doi: 10.1086/505969. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 16.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 17.Gahrton G, Svensson H, Cavo M, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–16. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 18.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 19.de Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Büchner T, Hiddemann W, Wörmann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93:4116–24. [PubMed] [Google Scholar]
- 21.Schlenk RF, Fröhling S, Hartmann F, et al. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–803. doi: 10.1038/sj.leu.2403528. [DOI] [PubMed] [Google Scholar]
- 22.Glasmacher A, Hahn C, Molitor E, et al. Itraconazole trough concentrations in antifungal prophylaxis with six different dosing regimens using hydroxypropyl-β-cyclodextrin oral solution or coated-pellet capsules. Mycoses. 1999;42:591–600. doi: 10.1046/j.1439-0507.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 23.Maertens J, Verhaegen J, Lagrou K, et al. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001;97:1604–10. doi: 10.1182/blood.v97.6.1604. [DOI] [PubMed] [Google Scholar]
- 24.Reinicke A, Scheer C, Häfner J, et al. Rapid diagnostics of aspergillosis from bronchoalveolar lavage samples using realtime-PCR. Abstracts of the Forty-first Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001; Chicago, IL. Washington, DC, USA.: American Society for Microbiology; p. 386. Abstract J-843. [Google Scholar]
- 25.Upton A, Kirby KA, Carpenter P, et al. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 26.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–84. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 27.Martino R, Subira M, Rovira M, et al. Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol. 2002;116:475–82. doi: 10.1046/j.1365-2141.2002.03259.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi R, Kaneda M, Sato T, et al. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol. 2008;30:886–90. doi: 10.1097/MPH.0b013e3181864a80. [DOI] [PubMed] [Google Scholar]
- 29.Metcalf SC, Dockrell DH. Improved outcomes associated with advances in therapy for invasive fungal infections in immunocompromised hosts. J Infect. 2007;55:287–99. doi: 10.1016/j.jinf.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Post MJ, Lass-Floerl C, Gastl G, et al. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl Infect Dis. 2007;9:189–95. doi: 10.1111/j.1399-3062.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 31.Hachem R, Hanna H, Kontoyiannis D, et al. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer. 2008;112:2493–9. doi: 10.1002/cncr.23466. [DOI] [PubMed] [Google Scholar]