Abstract
Objectives
In this study, we sought to compare the sterilizing activity of human-equivalent doses of the ‘Denver regimen’ against acute tuberculosis (TB) infection in the standard mouse model and in the guinea pig.
Methods
Pharmacokinetic studies in guinea pigs were used to establish human-equivalent doses for rifampicin, isoniazid and pyrazinamide. Guinea pigs and mice were aerosol-infected with Mycobacterium tuberculosis CDC1551 and treatment was started 2 weeks later with rifampicin/isoniazid/pyrazinamide for up to 6 months. For the first 2 weeks of therapy, the dosing frequency was 5 days/week, and for the remaining period, twice weekly. Treatment was discontinued in groups of 30 mice and 10 guinea pigs at 5 months and at 6 months, and these animals were held for a further 3 months in order to assess relapse rates.
Results
Guinea pig lungs became culture-negative after 3 months of predominantly twice-weekly treatment and relapse rates were 0% (0/10) both after 5 months and after 6 months of treatment. In contrast, all mice remained culture-positive despite 6 months of the same treatment, and 93% (28/30) and 69% (20/29) of mice relapsed after treatment for 5 and 6 months, respectively.
Conclusions
Treatment with rifampicin/isoniazid/pyrazinamide administered at human-equivalent doses is much more potent against acute TB infection in guinea pigs than in mice. Our findings have important implications for the use of alternative animal models in testing novel TB drug regimens and for modelling M. tuberculosis persistence.
Keywords: Mycobacterium tuberculosis, isoniazid, rifampicin, pyrazinamide, chemotherapy, persistence
Introduction
Animal models have played a significant role in the pre-clinical assessment of drug candidates for tuberculosis (TB) treatment.1 Although the guinea pig has historical precedence,2 the mouse has been the preferred model for chemotherapy studies since the 1960s because of its reduced cost, robustness, ease of handling and ability to accurately emulate the activity of anti-TB drugs in humans.1,3 After a large intravenous or aerosol inoculation,4,5 mice contain a peak lung burden of ∼108 bacilli, approaching that of a human lung cavity6 and permitting the evaluation of whether a drug regimen is able to prevent the selection of drug-resistant mutants and achieve culture conversion without relapse upon treatment completion. Because of these features, the mouse model has been considered essential for the development of new anti-TB drugs.1 However, mice infected with Mycobacterium tuberculosis develop TB-related pathology histologically distinct from human TB lesions and mice metabolize drugs differently from humans. In addition, although highly intermittent regimens such as the ‘Denver regimen’7 are clinically effective in humans with non-cavitary TB, these regimens are unable to sterilize the majority of mouse lungs following 6 months of treatment,5 suggesting that the standard mouse model is overly ‘pessimistic’ with respect to predicting the response to anti-TB therapy in humans.
The guinea pig model has been used in the past to demonstrate successfully the anti-TB activity of streptomycin,2 isoniazid8 and ethambutol,9 and to evaluate the respective contributions of isoniazid, rifampicin and ethambutol when the drugs are given in combination.10 However, the guinea pig remains poorly characterized as a model of experimental TB chemotherapy. Previous guinea pig studies have used variable infectious doses, routes of infection, drug doses and incubation periods prior to treatment, rendering direct comparisons between experiments difficult and comparison with the mouse model impossible.1
As a first step, we sought to compare directly the activity of the ‘Denver regimen’ against acute TB infection in the guinea pig and the mouse. This intermittent regimen consisting of 2 weeks of daily therapy followed by 24 weeks of twice-weekly treatment (62 doses) was developed two decades ago by Sbarbaro and colleagues7 in an effort to facilitate supervision of TB treatment and improve treatment completion rates among medically non-adherent patients. Because of the limited data available,11–14 we first characterized the pharmacokinetic (PK) parameters in the guinea pig of the standard anti-TB drugs rifampicin, isoniazid and pyrazinamide to establish human- and mouse-equivalent doses based on the area under the serum concentration–time curve (AUC). Using similar lung bacterial densities and equivalent drug doses, we then evaluated the bactericidal and sterilizing activities of the standard short-course regimen rifampicin/isoniazid/pyrazinamide against acute TB infection in guinea pigs and mice. A high inoculum was used to infect guinea pigs and mice in order to achieve 14 days later, at treatment initiation, a bacillary burden approaching that of human pulmonary cavities.6
Materials and methods
M. tuberculosis strains
Log-phase cultures of a virulent M. tuberculosis wild-type CDC1551 strain15 were used for animal infections, as previously described.16
Animals
Female outbred Hartley guinea pigs (250–300 g) with and without jugular vein vascular catheters and female BALB/c mice (6–8 weeks old) were purchased from Charles River (Wilmington, MA, USA). All procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins.
PK experiments
Separate groups of three catheterized guinea pigs were given a single dose of 50 mg/kg, 100 mg/kg or 150 mg/kg rifampicin (Bioworld, Dublin, OH, USA) or 200 mg/kg, 300 mg/kg or 400 mg/kg pyrazinamide (Acros Organics, Morris Plains, NJ, USA). Another group of animals received 100 mg/kg rifampicin 1 h prior to concurrent dosing with 60 mg/kg isoniazid15 and 300 mg/kg pyrazinamide. To enhance palatability, doses of each drug were prepared in a homogeneous suspension of 40% sucrose (w/v) in a final volume of 0.5 mL for each individual guinea pig, and delivered in the posterior oropharynx by automatic pipette with disposable tip, as previously described.15 Blood (∼0.3 mL) was removed serially from guinea pigs through the intravenous catheter at the following timepoints after antibiotic dosing: 30 min; 1 h; 2 h; 4 h; 6 h; 8 h; 12 h; and 24 h. Serum was separated and frozen at −80°C before analysis at Dr Peloquin's Infectious Disease Pharmacokinetics Laboratory.15,17,18 Serum rifampicin and isoniazid concentrations were determined using a validated HPLC assay comprising a ThermoFinnegan P4000 HPLC pump (San Jose, CA, USA) with model AS1000 fixed-volume autosampler, a model UV2000 ultraviolet detector, a Gateway Series e computer (Poway, CA, USA) and the Chromquest HPLC data management system. Serum concentrations of pyrazinamide were determined using a validated gas-chromatography assay with mass selective detection (Agilent 6890 GC and 5973 MS; Wilmington, DE, USA). The plasma standard curves for rifampicin, isoniazid and pyrazinamide ranged from 0.5 to 50 mg/L, 0.5 to 20 mg/L and 0.5 to 100 mg/L, respectively. The absolute recoveries of rifampicin, isoniazid and pyrazinamide from plasma were 96%, 61% and 100%, respectively. The within-day precision [percentage coefficient of variation (CV)] of validation quality control (QC) samples for rifampicin was 2.4%–4.6%, and the overall validation precision was 6.3%–7.1%. QC sample concentrations for rifampicin were 26, 8 and 3 mg/L. The within-day precision (%CV) of validation QC samples (13, 6 and 0.8 mg/L) for isoniazid was 1%–6%, and the overall validation precision was 6%–10%. Inter-day and intra-day precision values for QC samples (16, 32 and 64 mg/L) for pyrazinamide were 2.2%–3.2% and 2.8%–3.3% CV, respectively. Serum concentration data were entered into a WinNonlin worksheet (WinNonlin Version 5.2, 2008; Pharsight, Mountain View, CA, USA) and analysed using standard non-compartmental techniques to determine the relevant PK parameters.
Aerosol infections
Log-phase (A600 ∼0.5) cultures of M. tuberculosis CDC1551 were used undiluted for aerosol infection of mice and diluted 5-fold (to ∼107 cfu/mL) in 1× PBS for aerosol infection of guinea pigs. Groups of 77 guinea pigs and 115 mice were aerosol-infected separately with these M. tuberculosis cultures using the Inhalation Exposure System (Glas-Col, Terre Haute, IN, USA), which was calibrated to deliver ∼3.5 log10 cfu and ∼5 log10 cfu into the lungs of mice and guinea pigs, respectively.
Antibiotic treatment
Chemotherapy was initiated 14 days after aerosol infection (on Day 0) in each species. For the first 2 months of treatment, both species were given rifampicin/isoniazid/pyrazinamide and for the remaining 4 months, only rifampicin/isoniazid. Dosing frequency was daily (5 days/week) for the first 2 weeks and then twice weekly for the remaining period without modification in dose size. Mice received 10 mg/kg rifampicin, 25 mg/kg isoniazid and 150 mg/kg pyrazinamide by oesophageal cannula; guinea pigs received 100 mg/kg rifampicin, 60 mg/kg isoniazid and 300 mg/kg pyrazinamide. In each species, the rifampicin dose preceded that of the other drugs by at least 1 h to limit drug interactions.19 Groups of five mice and four guinea pigs were sacrificed at 2 weeks and monthly thereafter up to 6 months after treatment initiation to assess the bactericidal activity of the regimen. Treatment was discontinued for groups of 30 mice and 10 guinea pigs at 5 months and at 6 months, and each group of animals was sacrificed 3 months after discontinuation of treatment in order to assess the relapse rate, i.e. the sterilizing activity of the regimen. Relapse was defined as positive culture upon plating entire undiluted lung homogenates. Mouse and guinea pig lungs were homogenized as previously described.15 Diluted and undiluted lung homogenates were plated on Middlebrook 7H11 plates containing cycloheximide (50 mg/L), carbenicillin (100 mg/L), polymyxin B (200 U/mL) and trimethoprim (20 mg/L) for cfu enumeration. Log-transformed cfu values were used to calculate averages and standard errors for graphing purposes.
Results
Identification of the human-equivalent doses of rifampicin and pyrazinamide in guinea pigs
Based on AUC/MIC, which appears to be the parameter most closely correlated with the bactericidal activity of isoniazid,20 the human-equivalent dose of isoniazid in the guinea pig was determined recently to be 60 mg/kg.15 In the current study, we evaluated the PK parameters of the companion drugs rifampicin and pyrazinamide in the guinea pig (Table 1). After oral administration of rifampicin in guinea pigs, the mean peak serum concentrations (Cmax) were 2.9 ± 2.0, 15.9 ± 2.9 and 40.5 ± 5.2 mg/L after 50 mg/kg, 100 mg/kg and 150 mg/kg rifampicin, respectively. Corresponding AUC0→∞ values were 13.4 ± 5.8, 133.9 ± 35.9 and 417.1 ± 56.2 mg·h/L for 50 mg/kg, 100 mg/kg and 150 mg/kg rifampicin, respectively.
Table 1.
Comparison of anti-TB drug PK in guinea pigs, mice and humans
| Test species | Drug dosage (mg/kg) | Cmax (mg/L) | Tmax (h) | t1/2 (h) | AUC0→∞ (mg·h/L) |
|---|---|---|---|---|---|
| Isoniazid | |||||
| guinea piga | 30 | 9.0 ± 3.2 | 0.9 ± 0.1 | 1.4 ± 0.3 | 22.9 ± 5.5 |
| guinea piga | 60 | 16.8 ± 3.5 | 0.8 ± 0.03 | 0.9 ± 0.2 | 34.1 ± 4.9 |
| guinea pig | 60b | 16.4 ± 15.7 | 2.4 ± 0.3 | 2.1 ± 0.9 | 45.5 ± 35.2 |
| mousec | 25 | 19.4 ± 2.0 | 0.5 | 1.6 ± 0.2 | 29.4 ± 0.5 |
| humand | 6.2 ± 6 | ||||
| rapid acetylators | 5.4 ± 20 | 1.1 ± 0.5 | 1.5 ± 0.31 | 19.9 ± 6.1 | |
| slow acetylators | 7.1 ± 1.9 | 1.1 ± 0.6 | 3.7 ± 0.59 | 48.2 ± 1.5 | |
| Rifampicin | |||||
| guinea pig | 50 | 2.9 ± 2.0 | 2.0 ± 0.01 | 2.4 ± 1.8 | 13.4 ± 5.8 |
| guinea pig | 100 | 15.9 ± 2.9 | 2.9 ± 1.1 | 5.2 ± 1.0 | 133.9 ± 35.9 |
| guinea pig | 100b | 14.6 ± 4.4 | 1.9 ± 1.0 | 4.9 ± 4.5 | 141.4 ± 127.7 |
| guinea pig | 150 | 40.5 ± 5.2 | 2.7 ± 1.2 | 5.9 ± 0.3 | 417.1 ± 56.2 |
| mousec | 10 | 16.2 ± 3.5 | 1–4 | 5.2 ± 3.2 | 165 ± 37.0 |
| humand | 10–15 | 14.9 | 2.8 | 2.5 | 117.9 |
| Pyrazinamide | |||||
| guinea pig | 200 | 80.4 ± 11.1 | 0.7 ± 0.3 | 0.7 ± 0.1 | 143 ± 26.6 |
| guinea pig | 300 | 165.0 ± 43.6 | 0.9 ± 0.2 | 1.0 ± 0.01 | 378.1 ± 19.7 |
| guinea pig | 300b | 145.3 ± 65.0 | 2.5 ± 0.02 | 1.2 ± 0.1 | 399.5 ± 190.5 |
| guinea pig | 400 | 268.7 ± 93 | 0.63 ± 0.1 | 0.7 ± 0.03 | 607 ± 209.7 |
| mousec | 150 | 153 | 0.5 | 1.3 | 350 |
| humand | 27 ± 4 | 38.7 ± 5.9 | 1 ± 0 | 9.6 ± 1.8 | 520 ± 101 |
aDenotes data from reference 15.
bDenotes dose given as part of rifampicin/isoniazid/pyrazinamide combination; in each case, rifampicin was given 60 min prior to the administration of the two companion drugs.
cDenotes single-dose PK data obtained as part of rifampicin/isoniazid/pyrazinamide combination in BALB/c mice;32 standard deviations are not provided for pyrazinamide values in this reference.
dDenotes data from reference 29; standard deviations are not provided for rifampicin values in this reference.
The mean Cmax values of pyrazinamide were 80.4 ± 11.1, 165.0 ± 43.6 and 268.7 ± 93 mg/L following dosing with 200 mg/kg, 300 mg/kg and 400 mg/kg pyrazinamide, respectively. Corresponding AUC0→∞ values for pyrazinamide were 143 ± 26.6, 378.1 ± 19.7 and 607 ± 209.7 mg·h/L for 200 mg/kg, 300 mg/kg and 400 mg/kg pyrazinamide, respectively (Table 1).
In the absence of extensive pharmacodynamic data for rifampicin and pyrazinamide, we chose AUC as the PK parameter on which to model human drug exposure in the current study. Therefore 100 mg/kg rifampicin and 300 mg/kg pyrazinamide were chosen to accompany 60 mg/kg isoniazid20 for treatment of guinea pigs. It should be noted that the Cmax as well as AUC values for 100 mg/kg rifampicin in guinea pigs closely approximate the corresponding values in humans and mice (Table 1). Although the Cmax of 300 mg/kg pyrazinamide in guinea pigs is ∼4-fold higher than the corresponding value in humans, it very closely approximates the Cmax of 150 mg/kg pyrazinamide in the mouse, and the AUC of 300 mg/kg pyrazinamide in guinea pigs is very similar to the corresponding values in mice (Table 1), thus permitting a direct comparison of the sterilizing activity of 100 mg/kg rifampicin + 60 mg/kg isoniazid + 300 mg/kg pyrazinamide in guinea pigs with that of the standard regimen 10 mg/kg rifampicin + 25 mg/kg isoniazid + 150 mg/kg pyrazinamide in mice.
In order to determine if sequential administration of rifampicin followed by isoniazid/pyrazinamide could avoid an effect on the PK parameters of isoniazid and pyrazinamide, as in mice,19 guinea pigs received concurrent dosing with 60 mg/kg isoniazid and 300 mg/kg pyrazinamide 1 h after dosing with 100 mg/kg rifampicin. As shown in Table 1, under these sequential dosing conditions, rifampicin did not have a significant effect on the Cmax of isoniazid or pyrazinamide, and the AUC0→∞ of each of these drugs was very mildly increased.
Bactericidal activity of rifampicin/isoniazid/pyrazinamide in guinea pigs versus mice
On the day after aerosol infection (Day −13 of treatment), 3.4 ± 0.3 log10 cfu and 4.7 ± 0.4 log10 cfu of wild-type M. tuberculosis CDC1551 were present in the lungs of mice and guinea pigs, respectively. In each species, the bacilli grew exponentially to 7.4 ± 0.1 log10 cfu and 8.7 ± 0.2 log10 cfu on Day 0 of treatment in mice and guinea pigs, respectively. Despite the higher bacillary burden in guinea pigs, the bacillary density in the lungs of each species was very similar (7.9 ± 0.1 log10 cfu/g of lung tissue in mice and 7.7 ± 0.2 log10 cfu/g of lung tissue in guinea pigs).
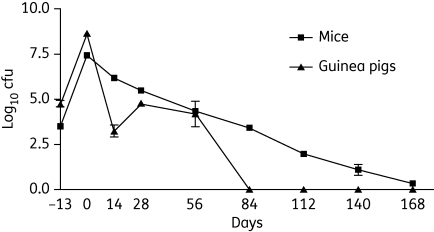
The combination of 100 mg/kg rifampicin + 60 mg/kg isoniazid + 300 mg/kg pyrazinamide administered five times weekly had very potent bactericidal activity during the first 14 days of treatment in guinea pigs, as lung cfu declined by an average of 5.4 log10 cfu (Figure 1). In contrast, 10 mg/kg rifampicin + 25 mg/kg isoniazid + 150 mg/kg pyrazinamide given five times weekly had much more modest bactericidal activity in mouse lungs during that time period, as lung bacillary counts declined by an average of only 1.3 log10 cfu. At Month 1, 14 days after the dosing regimen was switched to twice weekly, guinea pig lung cfu increased slightly and remained relatively stable after 2 months of treatment (4.2 ± 1.4 log10). Surprisingly, guinea pig lungs became culture-negative after 3 months of treatment and remained culture-negative at Month 4, Month 5 and Month 6 of treatment (Figure 1).
Figure 1.
Human-equivalent dosing of rifampicin/isoniazid/pyrazinamide given predominantly twice weekly has greater bactericidal activity against M. tuberculosis in guinea pig lungs relative to mouse lungs. Chemotherapy with the combination regimen was initiated on Day 14 after aerosol infection in each species, and was administered daily (5 days/week) for the first 14 days of treatment, and then twice weekly for the remainder of treatment. Animals received human-equivalent doses of rifampicin and isoniazid during the continuation phase of chemotherapy. In guinea pigs, the following doses were used: 100 mg/kg rifampicin; 60 mg/kg isoniazid; and 300 mg/kg pyrazinamide. In mice, the following doses were used: 10 mg/kg rifampicin; 25 mg/kg isoniazid; and 150 mg/kg pyrazinamide.
Guinea pig lung weights increased from 2.2 ± 0.2 g on the day after infection (Day −13 of treatment) to 10.4 ± 0.8 g at treatment initiation, although there was no gross evidence of inflammation. By Day 28 of treatment, gross examination of guinea pig lungs revealed foci of inflammation without discrete tubercles and normalized lung weights were 2.4 ± 0.2 g. By Month 4 of treatment, guinea pig lung weights were 2.2 ± 0.4 g and there was gradual improvement in lung inflammation grossly (data not shown).
In mice, although the rifampicin/isoniazid/pyrazinamide regimen given twice weekly continued to have activity, it was significantly less potent than in guinea pig lungs. Lung cfu declined by ∼1 log10 cfu per month and all five mouse lungs tested remained culture-positive after 6 months of treatment with an average bacillary burden of 0.3 ± 0.1 log10 cfu (Figure 1). These results are very similar to our previous experience with this regimen in mice.5
Sterilizing activity of rifampicin/pyrazinamide/isoniazid in guinea pigs versus mice
Groups of 10 guinea pigs and 30 mice were held following 5 months and 6 months of rifampicin/isoniazid/pyrazinamide treatment in order to assess culture-positive relapse rates. When assessed 3 months after completion of treatment, not a single guinea pig exhibited culture-positive relapse after treatment was stopped at Month 5 and Month 6 (Table 2). In contrast, 93% (28/30) and 69% (20/29) of mice were still culture-positive 3 months after treatment was stopped at Month 5 and Month 6, respectively, of equivalent rifampicin/isoniazid/pyrazinamide treatment.
Table 2.
Sterilizing activity of a predominantly twice-weekly regimen of rifampicin/isoniazid/pyrazinamide in guinea pig and mouse lungs
| Animal species | Culture positivity at 5 (+3)a months, no. of animals (%) | Culture positivity at 6 (+3)a months, no. of animals (%) |
|---|---|---|
| Guinea pig | 0/10 (0) | 0/10 (0) |
| Mouse | 28/30 (93) | 20/29 (69) |
a5 (+3) and 6 (+3) indicate that animals were sacrificed 3 months after completing 5 and 6 months, respectively, of rifampicin/isoniazid/pyrazinamide treatment.
Toxicity of daily rifampicin/isoniazid/pyrazinamide in guinea pigs
Soon after initiation of treatment with rifampicin/isoniazid/pyrazinamide given five times weekly, the majority of guinea pigs showed signs of toxicity, including weight loss, raised fur and reduced activity. By Day 14 of treatment, 7 of 61 treated guinea pigs had died, each showing evidence of colonic distension at necropsy. In contrast, all mice survived without evidence of toxicity during the same time period. Several faecal samples from three affected guinea pigs were submitted to the Clinical Microbiology Laboratory of Johns Hopkins Hospital. All tests, including Clostridium difficile antigen and toxin, and anaerobic cultures were negative. During the third week of treatment (the week after dosing frequency was reduced to twice weekly), three additional guinea pigs died, but by the fourth week of treatment all animals began to gain weight and did not further succumb to drug-related morbidity during the remainder of the experiment. Mean guinea pig weights increased from 372.2 ± 46.5 g to 554.8 ± 82.5 g between Month 1 and Month 3 of treatment. Daily administration of rifampicin/isoniazid/pyrazinamide did not result in mortality or clinically apparent morbidity in mice.
Discussion
The main finding of this study is that rifampicin/isoniazid/pyrazinamide treatment administered at human-equivalent doses was much more potent against acute TB infection in guinea pigs than in mice. The difference in treatment efficacy cannot be explained by differences in drug exposure or by the bacillary burden at treatment initiation in the two animal models. In each model, drug doses were equivalent and the bacillary burden/g of tissue was the same in both models.
The dosing regimen in our study mirrors that of the ‘Denver regimen’, in which 6 month treatment consisting of rifampicin/isoniazid/pyrazinamide with streptomycin or ethambutol is given for 2 months (daily dosing for the first 2 weeks and twice weekly thereafter) followed by twice-weekly rifampicin/isoniazid for an additional 4 months.7 In the original clinical study using this regimen, only two relapses were noted among 101 patients enrolled. However, more recent data suggest that intermittent dosing during the initial phase of treatment may lead to unacceptably high relapse rates, especially in the presence of cavitation.21 Several trials have been performed to evaluate the efficacy of shortening the duration of treatment to 3, 4 and 5 months using more intensive treatment with rifampicin, isoniazid and pyrazinamide.22 Although the initial response to therapy vis-à-vis culture conversion was excellent in these studies, especially for regimens containing rifampicin and pyrazinamide, all regimens were associated with unacceptably high relapse rates. Compared with these clinical data, it would seem that the guinea pig model of TB chemotherapy is overly ‘optimistic’ in predicting the response to TB treatment in humans. However, since patients with cavitary disease are known to have higher sputum bacillary loads than those with non-cavitary disease,23 it is possible that the bacillary burden in most human TB cavities at the time of clinical presentation is significantly greater than that of the guinea pig non-cavitary lesions in our studies, necessitating a longer duration of antibiotic treatment to achieve stable cure in humans. In support of this hypothesis, several studies have shown that a shorter duration of treatment may be sufficient to achieve acceptably low relapse rates for smear- and culture-negative adult TB patients.24–26 However, contrary to the findings of these studies, a recent randomized study showed that adults with non-cavitary pulmonary TB and negative sputum cultures after 2 months of treatment experienced significantly more relapses when treatment was shortened from 6 months to 4 months.27 Another possible explanation for the absence of relapse in guinea pigs is that this species may be able to mount a more vigorous and effective immune response to M. tuberculosis infection than humans, leading to enhanced bacillary killing in the face of systemic TB chemotherapy. For example, the inability to select katG-deficient isoniazid-resistant mutants following isoniazid monotherapy in guinea pigs,15 whereas such mutants are readily detectable from the sputum of infected patients, suggests the possibility that guinea pig phagocytes mount a more vigorous oxidative burst than do their human counterparts. It should be noted also that our data do not allow us to conclude that guinea pigs were truly ‘cured’ of TB infection since bacillary burden was not assessed in extrapulmonary organs such as spleen or liver, and because of the relatively limited numbers of animals used to assess relapse rates.
Another possible explanation for the potent activity of rifampicin/isoniazid/pyrazinamide in guinea pigs observed in this study may be that the PK parameters chosen to model human drug exposure were incorrect. For example, it has been suggested that Cmax, rather than AUC, may be the PK parameter most closely related to isoniazid activity in humans.28 Although the Cmax of 60 mg/kg isoniazid used for guinea pigs in this study is more than double that of human slow acetylators,29 this value is lower than that of 25 mg/kg isoniazid used in the mouse comparison arm, and thus cannot explain the superior activity of rifampicin/isoniazid/pyrazinamide in guinea pigs compared with mice. On the other hand, the Cmax values of the rifampicin and pyrazinamide doses chosen for guinea pigs in this study match well with the corresponding values of the rifampicin and pyrazinamide doses used in the mouse group. The dramatic efficacy of rifampicin/isoniazid/pyrazinamide in guinea pigs may also be partially explained by the acute form of our experimental disease that may have rendered bacilli more susceptible to killing by antibiotics. In support of this hypothesis, a recent study reported that guinea pigs aerosol-infected with ∼20–30 bacilli and receiving rifampicin/isoniazid/pyrazinamide treatment after a 30 day incubation period showed only a 1.7 log10 reduction in lung cfu after 6 weeks of treatment.30 However, the limited activity of rifampicin/isoniazid/pyrazinamide reported in that study may be related at least in part to the lower doses of drugs used (12 mg/kg rifampicin, 10 mg/kg isoniazid and 25 mg/kg pyrazinamide). To clarify this issue we are currently studying the activity of human-equivalent doses of rifampicin, isoniazid and pyrazinamide in a chronic, low-dose aerosol infection model of guinea pig TB.
The gastrointestinal toxicity in guinea pigs associated with human-equivalent dosing of rifampicin/isoniazid/pyrazinamide presents a significant challenge for future TB chemotherapy studies in this species. Although numerous tests did not identify it in stool specimens of affected animals in the current study, C. difficile has been reported to cause similar colitis in guinea pigs following treatment with broad-spectrum antibiotics.31 An alternative explanation is that isoniazid, pyrazinamide or the combination rifampicin/isoniazid/pyrazinamide may have a direct toxic effect on the gastrointestinal tract of guinea pigs. Future long-term combination chemotherapy studies in guinea pigs will probably require intermittent dosing of the control regimen rifampicin/isoniazid/pyrazinamide, reduction in the mg/kg dose of one or more of the drugs in this regimen or concomitant administration of probiotic agents.
Our findings have important implications for the use of alternative animal models in assessing the potential sterilizing activity of new anti-TB drugs and drug combinations and for modelling M. tuberculosis persistence. Any potential advantages of such models must be weighed against practical considerations, including increased costs and drug-related toxicity.
Funding
This work was supported by the Bill & Melinda Gates Foundation (TB Accelerator grant #42851 to J. H. G., E. L. N. and P. C. K.) and the National Institutes of Health (AI064229 to P. C. K.).
Transparency declarations
None to declare.
References
- 1.Nuermberger E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 2008;29:542–51. doi: 10.1055/s-0028-1085705. [DOI] [PubMed] [Google Scholar]
- 2.Schatz A, Bugie E, Waksman SA. Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Proc Soc Exp Biol Med. 1944;55:66–9. doi: 10.1097/01.blo.0000175887.98112.fe. [DOI] [PubMed] [Google Scholar]
- 3.McCune RM, Jr, McDermott W, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169:421–6. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal IM, Williams K, Tyagi S, et al. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am J Respir Crit Care Med. 2005;172:1457–62. doi: 10.1164/rccm.200507-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 7.Cohn DL, Catlin BJ, Peterson KL, et al. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis. A twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med. 1990;112:407–15. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 8.Karlson AG, Feldman WH. Isoniazid in experimental tuberculosis of guinea pigs infected with tubercle bacilli resistant to streptomycin and to para-aminosalicylic acid. Am Rev Tuberc. 1952;66:477–85. doi: 10.1164/art.1952.66.4.477. [DOI] [PubMed] [Google Scholar]
- 9.Karlson AG. Therapeutic effect of ethambutol (dextro-2,2′-(ethylenediimino)-di-1-butanol) on experimental tuberculosis in guinea pigs. Am Rev Respir Dis. 1961;84:902–4. [Google Scholar]
- 10.Dickinson JM, Mitchison DA. Bactericidal activity in vitro and in the guinea-pig of isoniazid, rifampicin and ethambutol. Tubercle. 1976;57:251–8. doi: 10.1016/s0041-3879(76)80002-5. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson JM, Mitchison DA. Suitability of rifampicin for intermittent administration in the treatment of tuberculosis. Tubercle. 1970;51:82–94. doi: 10.1016/0041-3879(70)90131-5. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson JM, Aber VR, Mitchison DA. Studies on the treatment of experimental tuberculosis of the guinea pig with intermittent doses of isoniazid. Tubercle. 1973;54:211–24. doi: 10.1016/0041-3879(73)90026-3. [DOI] [PubMed] [Google Scholar]
- 13.Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis (Edinb) 2005;85:227–34. doi: 10.1016/j.tube.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Zahoor A, Sharma S, Khuller GK. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 2005;26:298–303. doi: 10.1016/j.ijantimicag.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad Z, Klinkenberg LG, Pinn ML, et al. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis. 2009;200:1136–43. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 16.Karakousis PC, Williams EP, Bishai WR. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother. 2008;61:323–31. doi: 10.1093/jac/dkm485. [DOI] [PubMed] [Google Scholar]
- 17.Perlman DC, Segal Y, Rosenkranz S, et al. The clinical pharmacokinetics of rifampin and ethambutol in HIV-infected persons with tuberculosis. Clin Infect Dis. 2005;41:1638–47. doi: 10.1086/498024. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Starke JR, Burman WJ, et al. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy. 2002;22:686–95. doi: 10.1592/phco.22.9.686.34067. [DOI] [PubMed] [Google Scholar]
- 19.Grosset J, Truffot-Pernot C, Lacroix C, et al. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992;36:548–51. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaram R, Shandil RK, Gaonkar S, et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2004;48:2951–7. doi: 10.1128/AAC.48.8.2951-2957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang KC, Leung CC, Yew WW, et al. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med. 2004;170:1124–30. doi: 10.1164/rccm.200407-905OC. [DOI] [PubMed] [Google Scholar]
- 22.Leibert E, Rom W. Principles of tuberculosis management. In: Rom W, Garay S, editors. Tuberculosis. 2nd edn. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 713–28. [Google Scholar]
- 23.Palaci M, Dietze R, Hadad DJ, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol. 2007;45:4064–6. doi: 10.1128/JCM.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A controlled trial of a 2-month, 3-month, and 12-month regimens of chemotherapy for sputum smear-negative pulmonary tuberculosis: the results at 30 months. Am Rev Respir Dis. 1981;124:138–42. doi: 10.1164/arrd.1981.124.2.138. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. [DOI] [PubMed] [Google Scholar]
- 25.Dutt AK, Moers D, Stead WW. Smear- and culture-negative pulmonary tuberculosis: four-month short-course chemotherapy. Am Rev Respir Dis. 1989;139:867–70. doi: 10.1164/ajrccm/139.4.867. [DOI] [PubMed] [Google Scholar]
- 26.Teo SK, Tan KK, Khoo TK. Four-month chemotherapy in the treatment of smear-negative pulmonary tuberculosis: results at 30 to 60 months. Ann Acad Med Singapore. 2002;31:175–81. [PubMed] [Google Scholar]
- 27.Johnson JL, Hadad DJ, Dietze R, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180:558–63. doi: 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchison DA. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin Respir Crit Care Med. 2004;25:307–15. doi: 10.1055/s-2004-829503. [DOI] [PubMed] [Google Scholar]
- 29.Grosset JH, Ji B. Experimental chemotherapy of mycobacterial diseases. In: Gangadharam PRJ, Jenkins PA, editors. Mycobacteria: Chemotherapy. New York: Chapman and Hall; 1998. pp. 51–97. [Google Scholar]
- 30.Lenaerts AJ, Hoff D, Aly S, et al. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother. 2007;51:3338–45. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett JG, Taylor NS, Chang T, et al. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980;33:2521–6. doi: 10.1093/ajcn/33.11.2521. [DOI] [PubMed] [Google Scholar]
- 32.Williams KN, Brickner SJ, Stover CK, et al. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med. 2009;180:371–6. doi: 10.1164/rccm.200904-0611OC. [DOI] [PubMed] [Google Scholar]