Abstract
Alzheimer’s disease (AD) is characterized by a progressive loss of cognitive function and constitutes the most common and fatal neurodegenerative disorder.[1] Genetic and clinical evidence supports the hypothesis that accumulation of amyloid deposits in the brain plays an important role in the pathology of the disease. This event is associated with perturbations of biological functions in the surrounding tissue leading to neuronal cell death, thus contributing to the disease process. The deposits are comprised primarily of amyloid (Aβ) peptides, a 39–43 amino acid sequence that self aggregates into a fibrillar β-pleated sheet motif. While the exact three-dimensional structure of the aggregated Aβ peptides is not known, a model structure that sustains the property of aggregation has been proposed.[2] This creates opportunities for in vivo imaging of amyloid deposits that can not only help evaluate the time course and evolution of the disease, but can also allow the timely monitoring of therapeutic treatments.[3]
Keywords: amyloid peptides, fluorescent probes, imaging agents, molecular rotors
Historically, Congo Red (CR) and Thioflavin T (ThT) have provided the starting point for the visualization of amyloid plaques and are still commonly employed in post mortem histological analyses (Figure 1).[4] However, due to their charge these probes are unsuitable for in vivo applications.[5] To address this issue, several laboratories developed probes with noncharged, lipophilic (log P = 0.1–3.5) and low-molecular weight chemical structures (MW <650) that facilitate crossing of the blood–brain barrier.[6] Further functionalization of these compounds with radionuclides led to a new generation of in vivo diagnostic reagents (Figure 1) that target plaques and related structures for imaging with positron emission tomography (PET) and single-photon emission computed tomography (SPECT).[7] Despite these advances, there is a pressing need for the design and development of new amyloid-targeting molecules with improved physical, chemical and biological characteristics.[8] At present, identification of new amyloid sensing molecules is based mainly on modification of existing dyes[9] and/or screening of libraries of dyes.[10]
Figure 1.
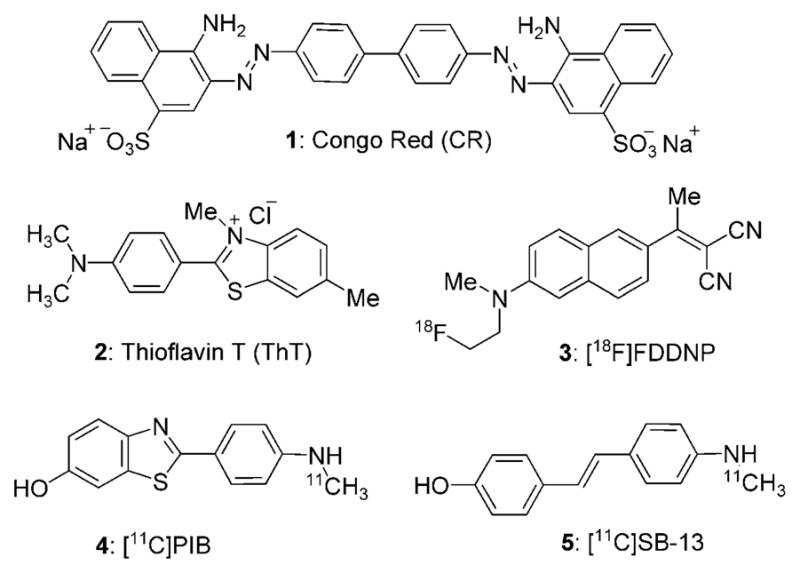
Structures of selected amyloid imaging reagents.
Examination of the chemical structures shown in Figure 1 reveals that the majority of these probes contain an electrondonor unit in conjugation with an electron acceptor (D-π-A motif). This motif is a typical feature in molecular rotors, a family of fluorescent probes known to form twisted intramolecular charge-transfer (TICT) complexes in the excited state producing a fluorescence quantum yield that is dependent on the surrounding environment.[11] Following photoexcitation, this motif has the unique ability to relax either via fluorescence emission or via an internal nonradiative molecular rotation. This internal rotation occurs around the σ-bonds that connect the electron-rich π-system with the donor and acceptor groups, and can be modified by altering the chemical structure and microenvironment of the probe.[12] Hindrance of the internal molecular rotation of the probe by increasing the surrounding media rigidity, or by reducing the available free volume needed for relaxation, leads to a decrease in the nonradiative decay rate and consequently an increase in fluorescence. In contrast, relaxation proceeds mainly via nonradiative pathways in environments of low viscosity or of high free volume. Due to these properties, molecular rotors have been used to study polarity, free volume and viscosity changes in solvents and organized assemblies,[13] such as liposomes,[14] cells[15] and polymers.[16]
Intrigued by the above observations, we asked whether we could design amyloid-binding agents based on the molecular rotor motif. We envisioned that π-conjugation of a dialkyl amino group, as the electron donor (D), with a 2-cyano acrylate unit, as the electron acceptor (A), would produce Aβ-binding molecules with inherent fluorescence properties.[17] Interestingly, the fluorescence properties of such a motif could be fine-tuned by modifying the electronic density and extent of conjugation between the donor and acceptor units. The solubility of these amyloid-binding agents in aqueous media can be achieved by the introduction of water solubilizing groups (WSG), such as esters of triethylene glycol monomethyl ether (TEGME) or of glycerol. The design concept is shown in Figure 2.
Figure 2.
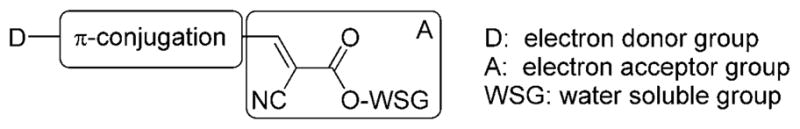
Design of amyloid-binding agents based on the structure of a molecular rotor (D-π-A motif).
Key to the synthesis of all probes was a Knoevenagel condensation of the appropriate aldehyde, for example, 6, with the appropriate malonic acid derivative, for example, 7, (Scheme 1). This reaction was catalyzed by piperidine and was completed within 21 h in refluxing THF.[18] After a standard chromatographic purification on silica gel, the desired product 8 was isolated in excellent yields (Table 1).
Scheme 1.
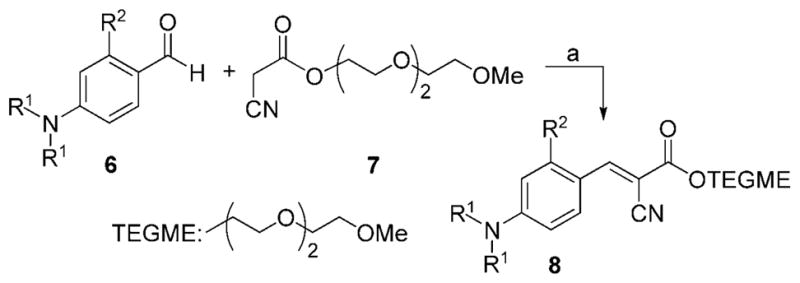
Reagents and conditions: a) 1.0 equiv 6, 1.1 equiv 7, 0.1 equiv piperidine, THF, 50 °C, 21 h.
Table 1.
Structures and yields of probes 8a–8d.
| Compd | R1 | R2 | Yield (%) |
|---|---|---|---|
| 8a | Me | H | 98 |
| 8b | Me | OMe | 98 |
| 8c | Et | H | 90 |
| 8d | nBu | H | 78 |
Naphthalene-based probe 11 was synthesized by treatment of commercially available methoxy naphthaldehyde 9 with lithiated piperidine[19] and Knoevenagel condensation of the resulting aldehyde 10 with cyano ester 7 (Scheme 2, 29 % combined yield).
Scheme 2.
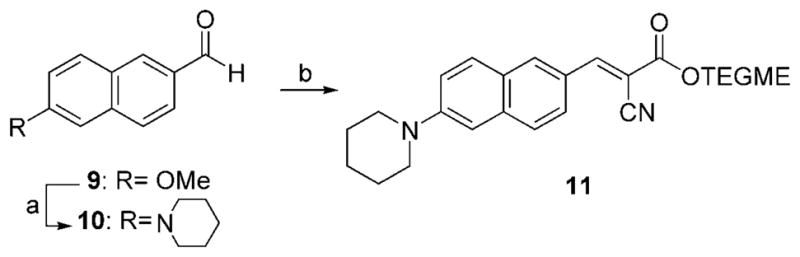
Reagents and conditions: a) 8.0 equiv piperidine in benzene/HMPA: 1/1, 0 °C, 8.0 equiv nBuLi, 0 °C, 15 min, then 1.0 equiv 9, 25 °C, 12 h, 35 %; b) 1.0 equiv 10, 1.1 equiv 7, 0.1 equiv piperidine, THF, 50 °C, 21 h, 82 %.
Compound 14 was prepared by condensation of aldehyde 6 a with α-cyano ester 12, followed by an acid-catalyzed deprotection of the acetonide unit (Scheme 3, 68 % combined yield).[20]
Scheme 3.
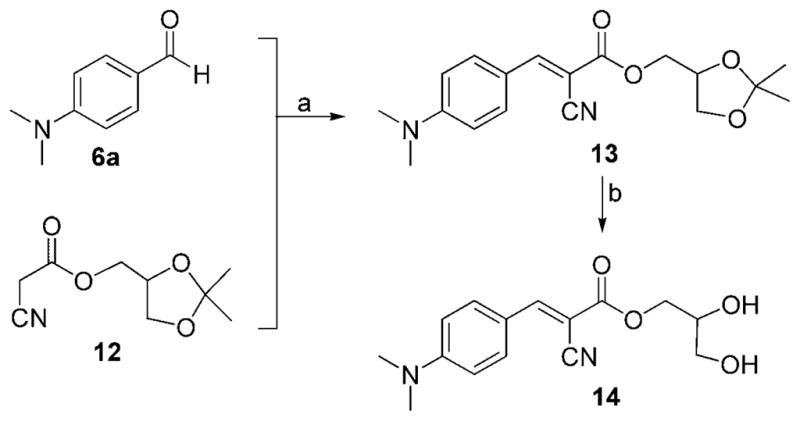
Reagents and conditions: a) 1.0 equiv 6a, 1.1 equiv 12, 0.1 equiv piperidine, THF, 50 °C, 21 h, 91 %; b) 1.5 mmol 13, 0.10 g DOWEX-H+, THF/CH3OH (1:1), 25 °C, 20 h, 75 %.
Stilbene-based probe 19 was synthesized in four steps that included: a) conversion of benzyl bromide 15 to phosphonate 16;[21]; b) Horner–Emmons olefination of 16 with aldehyde 6 a to form 17; c) lithiation of bromide 17 and formylation to produce aldehyde 18; d) Knoevenagel condensation of the resulting aldehyde 18 with cyano ester 7 (Scheme 4, 42 % combined yield).
Scheme 4.
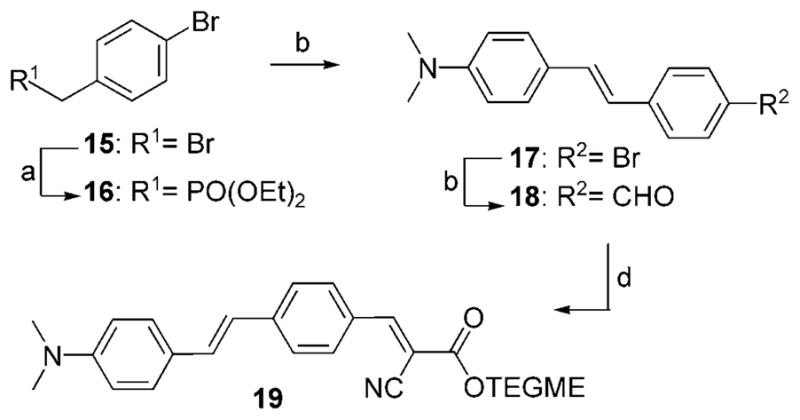
Reagents and conditions: a) 1.0 equiv 15, 15 equiv triethyl phosphite, 90 °C, 19 h, 98 %; b) 1.0 equiv 16, 1.0 equiv NaOMe, 1.0 equiv 6a, excess DMF, 25 °C, 24 h, 74 %; c) 1.0 equiv 17, 1.0 equiv nBuLi, 1.33 equiv DMF, THF, −78 °C, 60 %; d) 1.0 equiv 18, 1.1 equiv 7, 0.1 equiv piperidine, THF, 50 °C, 21 h, 97 %.
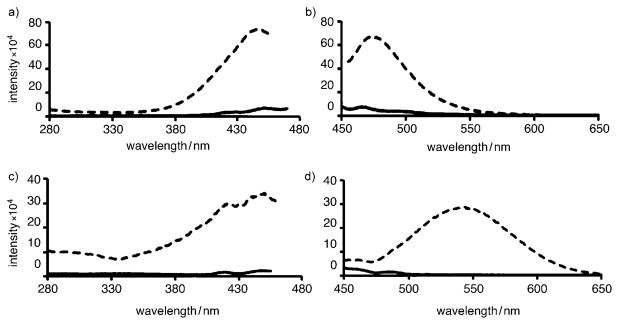
An initial study to determine whether a probe can associate with aggregated Aβ is to compare its fluorescence spectra before and after mixing with the Aβ aggregates.[9, 10] Typically, a fluorescent amyloid-binding agent displays a significant fluorescence intensity increase after binding to Aβ aggregates as compared to its native fluorescence in solution.[22] Along these lines, we measured the fluorescent properties of each probe at 4 μM before and after mixing with preaggregated Aβ(1–42) peptides (5 μM, aggregated in PBS buffer for 3 days at 25 °C). In all cases, a 1.3- to 9.4-fold fluorescence intensity increase was observed in the presence of aggregated Aβ, indicating that these compounds bind to the peptide (Table 2). In most cases a modest blue shift (6–20 nm) was observed upon binding. Only in the case of the naphthalene-based probe 11 was a significant red shift of 76 nm observed upon binding to preaggregated Aβ (Figure 3c and d). Interestingly, this binding was accompanied with a 9.3-fold intensity increase. A similar intensity increase has been observed with FDDNP[23] and may be explained by the ability of the naphthalene motif to create excimers upon binding to its target.[24] Probes 8 a and 8b exhibited similar fluorescence characteristics suggesting that addition of a methoxy group on the phenyl group does not alter the binding properties of the probe. On the other hand, it is worth noting that increasing the size of the alkyl groups of the nitrogen leads to a significant increase in the fluorescence intensity after binding (Table 2, 8 a, 8c, 8 d). This is likely a result of the decreased rotational freedom of the molecules upon binding to the aggregated forms of Aβ peptide.[25] Interestingly, no increase of fluorescence intensity was observed upon mixing of these probes with monomeric Aβ peptide (see Supporting Information). This supports the notion that these probes bind selectively to aggregated forms of Aβ. The fluorescence profile of 8 d (excitation and emission) is shown in Figure 3a and b.
Table 2.
Fluorescence profile, Kd, IC50 and related values for the interaction of the synthesized probes with aggregated Aβ(1–42) peptides.
| Compd | Excitation Maximuma [nm] | Emission Maximuma [nm] | Fold increase | Kd [μM] | R2 | Imaxb [%] | IC50b [μM] | Log P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | |||||||
| 8a | 439 | 435 | 476 | 470 | 1.8 | 2.6 | 0.93 | 81 | 129.0 | 1.74 |
| 8b | 442 | 444 | 478 | 469 | 1.3 | 5.3 | 0.99 | 92 | 1.2 | 1.54 |
| 8c | 445 | 442 | 478 | 470 | 4.2 | 4.8 | 0.96 | 98 | 11.4 | 2.49 |
| 8d | 432 | 440 | 466 | 468 | 9.4 | 4.4 | 0.95 | 91 | 90.6 | 4.62 |
| 11 | 445 | 440 | 462 | 538 | 9.3 | 2.5 | 0.98 | 58 | 74.3 | 3.81 |
| 14 | 437 | 434 | 476 | 467 | 2.2 | 3.3 | 0.99 | 79 | 82.1 | 1.07 |
| 19 | 312 | 319 | 658 | 638 | 2.3 | 1.4 | 0.98 | 40 | 33.6 | 4.30 |
Before or after binding.
Maximum percent inhibition (Imax) and IC50 values were determined by ELISA assay.
Figure 3.
Fluorescence excitation (a, c) and emission spectra (b, d) of probes 8d (a, b) and 11 (c, d) in aqueous PBS solution (—) and in the presence of aggregated Aβ peptide (---).
We also measured the apparent binding constants (Kd) of the probes (in concentrations of 10, 5, 2.5 and 1.25 μM) to 5.0 μM pre-aggregated Aβ(1–42) peptide. The Kd can be measured from the double reciprocal of the fluorescence maximum (Fmax) and the concentration of the probe.[22] All Kd values were measured between 1.4 and 5.3 μM (Table 2). It is remarkable that, despite the structural differences, these probes display similar Kd values suggesting that they bind in a similar fashion to aggregated Aβ. Moreover, these values are similar to the reported Kd values for ThT (2 μM).[22, 26] The double reciprocal plot of fluorescence intensity versus concentration of probes 8 d and 11 are shown in Figure 4. The Kd corresponds to the −1/(x-intercept) of the linear regression.[22]
Figure 4.
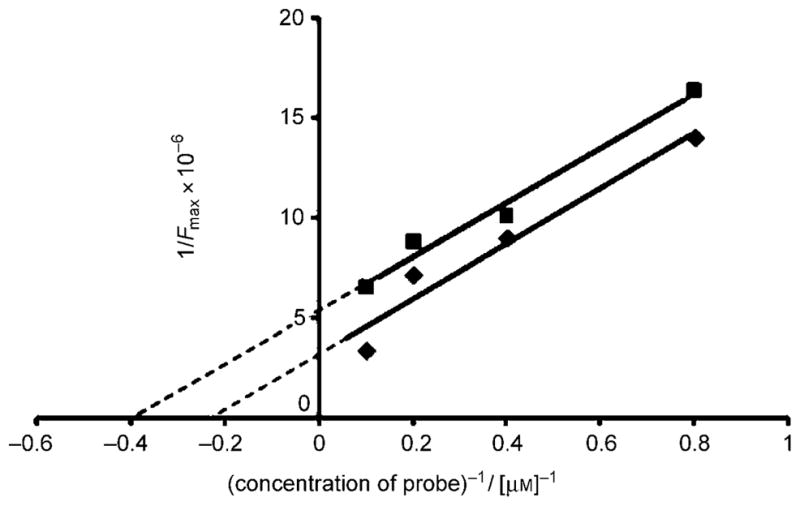
Determination of the apparent binding constant (Kd) of probes 8d (◆; R2 = 0.95) and 11 (■; R2 = 0.98) to preaggregated Aβ peptide.
The association of the synthesized compounds with aggregated Aβ peptides was tested using a semi-quantitative ELISA-based assay developed by Yang and co-workers.[27] The assay is based on screening for molecules that inhibit the interaction of the aggregated Aβ peptide with a monoclonal anti-Aβ IgG raised against residues 1–17 of Aβ. Table 2 shows the concentrations of the probes corresponding to 50 % inhibition (IC50) of the IgG-Aβ interactions as well as the maximal percentage of IgG inhibited from binding to the aggregated peptide. All probes exhibited IC50 values at micromolar levels, the lowest value being measured for compound 8b (IC50 = 1.2 μM). The maximum inhibition (Imax), a measure of the extent of surface coating of the aggregated peptide by the probes,[27] was determined to be between 40–98 % (Table 2). Comparison of these data indicates that the surface coating increases by decreasing the size of the probe or the extent of the π system. Specifically, while the maximum inhibition is between 81–98 % for the phenyl compounds, it decreases to 58 % for the longer naphthalene compound 11 and to 40 % for the more conjugated stilbene 19. Representative graphs for 8 d and 11 are shown in Figure 5.
Figure 5.
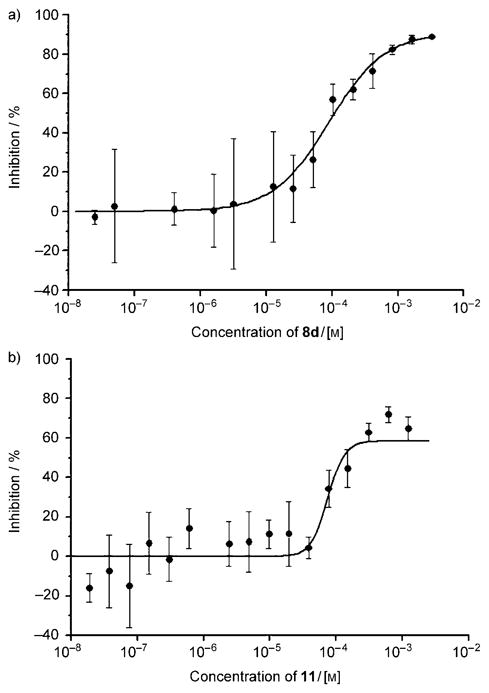
Inhibition of IgG-Aβ interactions with probes a) 8d (Imax = 91 %, IC50 = 91 μM) and b) 11 (Imax = 58 %, IC50 = 74 μM).
The log P values for all the compounds were calculated to be between 1.07 and 4.62 (Table 2)[28] indicating that most of these probes meet the solubility criteria and should be able to cross the blood–brain barrier.[27, 29] Finally, all compounds showed little or no cytotoxicity against human neuroblastoma cells at concentrations up to 100 μM (see Supporting Information). These properties represent significant advantages for further in vivo evaluation.
In conclusion, inspired by the structures of the currently used amyloid-binding agents we have evaluated the possibility to design new Aβ binding fluorescent probes based on the molecular rotor motif. We found that the molecular rotors, designed based on the concept shown in Figure 2, bind to the aggregated Aβ peptide with low micromolar affinity. We hypothesize that this binding is a result of hydrophobic interactions between the rotor and the amyloid peptide. This binding reduces the free volume around the rotor resulting in an increased fluorescence emission.[30] A similar effect has been reported for the binding of molecular rotors to actin, albumin and other proteins.[31] We have demonstrated that these molecules can be readily synthesized and have no significant cytotoxicity. In addition, we have shown that both the physical properties and fluorescence profile of these probes can be fine tuned by modifying their chemical structure. Notably, substituent changes in the electron donor group can affect the intensity of fluorescence emission, while changes in the π-system can affect the emission wavelength. These effects can be implemented for the construction of multicolored dyes and can lead to potential applications for in vitro and in vivo imaging.[32] Interestingly, a recent report describes the identification of CRANAD-2,[33] a small molecule containing two electron-donating groups connected simultaneously via π-conjugation to a single difluoroboronate acceptor. This probe has a high affinity for Aβ aggregates (Kd = 38.0 nM) and suitable near-infrared fluorescence properties for in vivo imaging, further validating our proposed concept of exploring the molecular rotor motif for the development of new amyloid-imaging probes. These findings demonstrate that the D-π-A motif of molecular rotors, presented in Figure 2, is a privileged scaffold and represents an important first step for the rational design of new diagnostic tools for Alzheimer’s disease and related amyloid-based neurodegenerative disorders.
Supplementary Material
Acknowledgments
Financial support by the NSF (CMMI-0652476) and the NIH (grant 1R21RR025358) are gratefully acknowledged. C.C.C. and J.Y. acknowledge support from the Wallace H. Coulter Foundation, the Alzheimer’s Disease Research Center (NIH 3P50 AG005131), and the Alzheimer’s Association (NIRG 08–91651).
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cmdc.200900440.
Contributor Information
Jerry Yang, Email: jerryyang@ucsd.edu.
Emmanuel A. Theodorakis, Email: etheodor@ucsd.edu.
References
- 1.a) Minati L, Edginton T, Bruzzone MG, Giaccone G. Am J Alzheimers Dis. 2009;24:95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schott JM, Kennedy J, Fox NC. Curr Opin Neurol. 2006;19:552–558. doi: 10.1097/01.wco.0000247611.44106.76. [DOI] [PubMed] [Google Scholar]
- 2.a) Mathis CA, Wang Y, Klunk WE. Curr Pharm Des. 2004;10:1469–1492. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]; b) Kim YS, Lee JH, Ryu J, Kim DJ. Curr Pharm Des. 2009;15:637–658. doi: 10.2174/138161209787315648. [DOI] [PubMed] [Google Scholar]
- 3.a) Matthews B, Siemers ER, Mozley PD. Am J Geriatr Psychiatry. 2003;11:146–159. [PubMed] [Google Scholar]; b) Nichols L, Pike VW, Cai LS, Innis RB. Biol Psychiatry. 2006;59:940–947. doi: 10.1016/j.biopsych.2005.12.004. [DOI] [PubMed] [Google Scholar]; c) Golde TE, Bacskai BJ. Nat Biotechnol. 2005;23:552–554. doi: 10.1038/nbt0505-552. [DOI] [PubMed] [Google Scholar]
- 4.a) Kitts CC, Bout DAV. J Phys Chem B. 2009;113:12090–12095. doi: 10.1021/jp903509u. [DOI] [PubMed] [Google Scholar]; b) Schmidt ML, Schuck T, Sheridan S, Kung MP, Kung H, Zhuang ZP, Bergeron C, Lamarche JS, Skovronsky D, Giasson BI, Lee VM, Trojanowski JQ. Am J Pathol. 2001;159:937–943. doi: 10.1016/s0002-9440(10)61769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang YM, Huang GF, Debnath ML, Klunk WE. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 6.Ryu EK, Chen XY. Front Biosci. 2008;13:777–789. doi: 10.2741/2719. [DOI] [PubMed] [Google Scholar]
- 7.a) Stephenson KA, Chandra R, Zhuang ZP, Hou C, Oya S, Kung MP, Kung HF. Bioconjugate Chem. 2007;18:238–246. doi: 10.1021/bc060239q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nordberg A. Curr Opin Neurol. 2007;17:398–402. doi: 10.1097/WCO.0b013e3281a47744. [DOI] [PubMed] [Google Scholar]; c) Garcia-Alloza M, Bacskai BJ. Neuro-Mol Med. 2004;6:65–78. doi: 10.1385/NMM:6:1:065. [DOI] [PubMed] [Google Scholar]
- 8.a) Bacskai BJ, Klunk WE, Mathis CA, Hyman BT. J Cereb Blood Flow Metab. 2002;22:1035–1041. doi: 10.1097/00004647-200209000-00001. [DOI] [PubMed] [Google Scholar]; b) Burn DJ, O’Brien JT. Mov Disord. 2003;18(Suppl 6):S88–95. doi: 10.1002/mds.10568. [DOI] [PubMed] [Google Scholar]
- 9.a) Nesterov EE, Skoch J, Hyman BT, Klunk WE, Bacskai BJ, Swager TM. Angew Chem Int Ed. 2005;44:5452–5456. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]; b) Zhuang ZP, Kung MP, Kung HF. J Med Chem. 2006;49:2841–2844. doi: 10.1021/jm051020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Li QA, Lee JS, Ha C, Park CB, Yang G, Gan WB, Chang YT. Angew Chem. 2004;116:6491–6495. doi: 10.1002/anie.200461600. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:6331–6335. doi: 10.1002/anie.200461600. [DOI] [PubMed] [Google Scholar]; b) Kung HF, Lee CW, Zhuang ZP, Kung MP, Hou C, Plossl K. J Am Chem Soc. 2001;123:12740–12741. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- 11.a) Grabowski ZR, Rotkiewicz K, Rettig W. Chem Rev. 2003;103:3899–4031. doi: 10.1021/cr940745l. [DOI] [PubMed] [Google Scholar]; b) Haidekker MA, Theodorakis EA. Org Biomol Chem. 2007;5:1669–1678. doi: 10.1039/b618415d. [DOI] [PubMed] [Google Scholar]
- 12.Loutfy RO. Pure Appl Chem. 1986;58:1239–1248. [Google Scholar]
- 13.a) Viriot ML, Carré MC, Geoffroy-Chapotot C, Brembilla A, Muller S, Stoltz JF. Clin Hemorheol Microcirc. 1998;19:151–160. [PubMed] [Google Scholar]; b) Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. Bioorg Chem. 2005;33:415–425. doi: 10.1016/j.bioorg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Nipper ME, Majd S, Mayer M, Lee JCM, Theodorakis EA, Haidekker MA. Biochim Biophys Acta, Biomembr. 2008;1778:1148–1153. doi: 10.1016/j.bbamem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.a) Haidekker MA, Ling TT, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. Chem Biol. 2001;8:123–131. doi: 10.1016/s1074-5521(00)90061-9. [DOI] [PubMed] [Google Scholar]; b) Haidekker MA, Tsai AG, Brady T, Stevens HY, Frangos JA, Theodorakis E, Intaglietta M. Am J Physiol Heart Circ Physiol. 2002;282:1609–1614. doi: 10.1152/ajpheart.00712.2001. [DOI] [PubMed] [Google Scholar]; c) Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. Bioorg Chem. 2005;33:415–425. doi: 10.1016/j.bioorg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.a) Frochot C, Muller C, Brembilla A, Carre MC, Lochon P, Viriot ML. Int J Polym Anal Charact. 2000;6:109–122. [Google Scholar]; b) Damas C, Adibnejad M, Benjelloun A, Brembilla A, Carre MC, Viriot ML, Lochon P. Colloid Polym Sci. 1997;275:364–371. [Google Scholar]
- 17.a) Lord SJ, Conley NR, Lee HLD, Samuel R, Liu N, Twieg RJ, Moerner WE. J Am Chem Soc. 2008;130:9204–9205. doi: 10.1021/ja802883k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lord SJ, Conley NR, Lee HLD, Nishimura SY, Pomerantz AK, Willets KA, Lu ZK, Wang H, Liu N, Samuel R, Weber R, Semyonov A, He M, Twieg RJ, Moerner WE. ChemPhysChem. 2009;10:55–65. doi: 10.1002/cphc.200800581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Chen XH, Zhao ZJ, Liu Y, Lu P, Wang YG. Chem Lett. 2008;37:570–571. [Google Scholar]; b) Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. J Am Chem Soc. 2006;128:398–399. doi: 10.1021/ja056370a. [DOI] [PubMed] [Google Scholar]
- 19.Guo HM, Tanaka F. J Org Chem. 2009;74:2417–2424. doi: 10.1021/jo900013w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haidekker MA, Brady TP, Chalian SH, Akers W, Lichlyter D, Theodorakis EA. Bioorg Chem. 2004;32:274–289. doi: 10.1016/j.bioorg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.a) Meier H, Karpuk E, Holst HC. Eur J Org Chem. 2006:2609–2617. [Google Scholar]; b) Viau L, Maury O, Le Bozec H. Tetrahedron Lett. 2004;45:125–128. [Google Scholar]
- 22.LeVine H., III Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. J Neurosci. 2001;21:189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Abad S, Vaya I, Jimenez MC, Pischel U, Miranda MA. ChemPhysChem. 2006;7:2175–2183. doi: 10.1002/cphc.200600337. [DOI] [PubMed] [Google Scholar]; b) Spies C, Gehrke R. J Phys Chem A. 2002;106:5348–5352. [Google Scholar]
- 25.a) Schuddeboom W, Jonker SA, Warman JM, Leinhos U, Kuehnle W, Zachariasse KA. J Phys Chem. 1992;96:10809–10819. [Google Scholar]; b) Il’chev YV, Kühnle W, Zachariasse KA. J Phys Chem A. 1998;102:5670–5680. [Google Scholar]
- 26.a) Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, Morgenstern JL, Hong GZ, Gee AD, Brown J. J Biol Chem. 2004;280:7677–7684. doi: 10.1074/jbc.M412056200. [DOI] [PubMed] [Google Scholar]; b) Biancalana M, Makabe K, Koide A, Koide S. J Mol Biol. 2008;383:205–213. doi: 10.1016/j.jmb.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Biancalana M, Makabe K, Koide A, Koide S. J Mol Biol. 2009;385:1052–1063. doi: 10.1016/j.jmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Inbar P, Yang J. Bioorg Med Chem Lett. 2006;16:1076–1079. doi: 10.1016/j.bmcl.2005.10.067. [DOI] [PubMed] [Google Scholar]; b) Inbar P, Li CQ, Takayama SA, Bautista MR, Yang J. ChemBioChem. 2006;7:1563–1566. doi: 10.1002/cbic.200600119. [DOI] [PubMed] [Google Scholar]; c) Inbar P, Bautista MR, Takayama SA, Yang J. Anal Chem. 2008;80:3502–3506. doi: 10.1021/ac702592f. [DOI] [PubMed] [Google Scholar]
- 28. [Lastaccessed, November 20, 2010];Log P values were calculated using the Molinspiration Cheminformatics software. http://www.molinspiration.com/
- 29.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 30.a) Loutfy RO, Arnold BA. J Phys Chem. 1982;86:4205–4211. [Google Scholar]; b) Doolittle AK. J Appl Phys. 1952;23:236–239. [Google Scholar]
- 31.a) Iio T, Takahashi S, Sawada S. J Biochem. 1993;113:196–199. doi: 10.1093/oxfordjournals.jbchem.a124025. [DOI] [PubMed] [Google Scholar]; b) Iwaki T, Torigoe C, Noji M, Nakanishi M. Biochemistry. 1993;32:7589–7592. doi: 10.1021/bi00080a034. [DOI] [PubMed] [Google Scholar]
- 32.Sigurdson CJ, Peter K, Nilsson R, Hornemann S, Manco G, Polymenidou M, Schwarz P, Leclerc M, Hammarstrom P, Wuthrich K, Aguzzi A. Nat Methods. 2007;4:1023–1030. doi: 10.1038/nmeth1131. [DOI] [PubMed] [Google Scholar]
- 33.Ran C, Xu X, Raymond SB, Ferrara BJ, Neal K, Bacskai BJ, Medarova Z, Moore A. J Am Chem Soc. 2009;131:15257–15261. doi: 10.1021/ja9047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.