Abstract
Objective
Differential item functioning (DIF) assesses the consistency of items on a metric across clinical samples in relation to the attribute being measured. We hypothesized that in older adults with persistent pain, items of the Geriatric Depression Scale (GDS) would evidence DIF based on presence or intensity of pain.
Design
Unidimensionality was determined by factor and item analyses. DIF was tested using Rasch Modeling. We then evaluated the psychometric properties of a revised GDS (GDS-PAIN), comprised of items that did not evidence DIF.
Patient and Settings
A total of 677 community dwelling older adults (age 65–91) participating in observational or treatment studies of low back or knee pain who endorsed at least moderate pain for at least 3 months. A total of 201 pain-free controls were included in the analysis.
Results
Ten of the 30 items displayed significant DIF. These items were: 1) dropping activities and interests; 2) bothered by persistent thoughts; 3) often get fidgety and restless; 4) prefer to stay home; 5) do not feel full of energy; 6) do not enjoy getting up in the morning; 7) mind is not as clear as it was, 8) feel life is empty; 9) feel more problems with memory; and 10) do not find life very exciting. The modified GDS-PAIN scale did not adversely affect the psychometric properties of the scale.
Conclusions
The performance of the GDS is affected by pain. When unstable items are removed, the revised GDS (GDS-PAIN) appears to be psychometrically stable and maintains both internal consistency and similar correlation values with a measure of pain as the original scale.
Keywords: Depression, Geriatric, Chronic Pain, Measurement
Introduction
Depression is common in patients with persistent pain [1]. Rates of depression in mixed age patients with persistent pain are reported to be between 30% and 54% [2]. The co-occurrence of depression within medical populations, such as patients with persistent pain conditions, is associated with increased medical symptom reporting [3,4], worsened functional impairment [5], and increased mortality [6]. Older adults currently constitute more than 12% of the United States’ population. In community-dwelling older adults, the prevalence of pain experienced every day for at least 3–6 months has been estimated to occur at rates of up to 50% [7]. Other studies have estimated that up to 70–85% of people aged 65 and older experience a significant health problem that predisposes them to persistent pain [8,9].
Distinguishing major depression from the symptoms of persistent pain can be challenging in older adults. In addition to the emotional and cognitive symptoms, clues to depression in older adults include the presence of somatic symptoms such as chronic fatigue, disturbed sleep, gastrointestinal disturbance [10,11], and anxiety [12]. These symptoms often are present in persistent pain patients, and may make accurate diagnosis and treatment planning challenging.
Measures of depression that were created for and validated in nonpain samples may perform differently when used with persistent pain patients. This may be especially true for older adults because of the effect persistent pain and other somatic complaints have on depression symptom reporting. In addition to somatic complaints, older adults with persistent pain may also express elevated fears about the future (i.e., not getting pain relief), feel helpless and hopeless about their situation [13,14], evidence impaired cognitive functioning [15-17], and report decreased self-esteem [18,19] and social isolation [20]. Like the somatic complaints of persistent pain, these symptoms overlap with depression.
Differential item functioning (DIF) provides an assessment of the consistency of items on a metric across different clinical samples in relation to the underlying attribute being measured [21,22]. DIF analysis can help to determine if test questions are appropriate for use in various clinical groups. Applying DIF is especially useful when evaluating an assessment tool that taps symptoms common to more than one condition, such as persistent pain and depression. This need for an understanding of how an individual’s clinical state interacts with the measurement process is critical for purposes of case identification, diagnosis, and treatment planning.
We hypothesized that in a sample of older adults with persistent pain, certain items of the Geriatric Depression Scale (GDS) [23] would show evidence of DIF. We predicted that both the 1) presence of persistent pain and 2) intensity of pain would differentially affect the function of a group of items on the GDS.
Methods
Subjects
Subjects were 677 English-speaking community dwelling older adults (age 65–91). Pain was measured with the pain thermometer [24,25] and had to be of at least moderate intensity, more days than not, for at least 3 months of duration. The average length of time subjects were in pain was 12.8 years (N = 476). Subjects who were free of pain also were included in the analysis (N = 201). Pain-free status was defined as no pain or pain occurring less than once per week of little intensity as measured with the pain thermometer.
All subjects received a comprehensive medical, pain, and psychosocial evaluation as part of their participation in either: 1) a research study evaluating psychosocial and functional differences between older adults with and without chronic low back pain (N = 374) [26]; 2) a clinical trial evaluating the efficacy of percutaneous electrical nerve stimulation (PENS) for low back pain in older adults (N = 215) [27]; or 3) a clinical trial evaluating the efficacy of periosteal electroacupuncture (osteopuncture) for knee pain in older adults (N = 88). All assessments were completed before any testing or treatment procedures were begun.
All subjects were cognitively intact, as determined by screening with the Mini Mental State Examination score [28] (mean = 28.5, SD = 1.5). Prior to their participation subjects signed informed consent, which was approved by the University of Pittsburgh Institutional Review Board. Subjects were recruited via newspaper and radio advertisements and fliers placed in senior centers and primary care clinics.
Screening occurred in two phases. The first phase occurred over the telephone (N = 2997), which was followed up on-site by one of the investigators (D.K.W.) with a structured history and physical examination (N = 1025). Exclusion criteria included cognitive impairment (Mini Mental State Examination [MMSE] < 24 adjusted for age and education), severe visual or hearing impairment, and acute illness or pain. The majority of subjects with pain reported the lower back as their primary pain site (73%), followed by knee pain (18%).
Subject demographics are shown in Table 1. The pain-free and persistent pain subjects were not significantly different with respect to age, gender, educational level, ethnicity, or marital status. However, compared with pain-free subjects, the pain subjects were found to have a significantly higher number of medical comorbidities as measured by the Cumulative Illness Rating Scale (CIRS) [29], and modestly lower MMSE scores. The GDS scores were significantly higher for the pain subjects (mean = 5.3, SD = 5.4) compared with the pain-free subjects (mean = 1.9, SD = 2.6).
Table 1.
Subject demographic and clinical data
| Variable | Group |
P Value | |
|---|---|---|---|
| Pain-Free | Pain | ||
| Sample size | 201 | 476 | — |
| Age | |||
| Mean | 73.6 | 73.4 | 0.64 |
| SD | 4.7 | 5.9 | |
| Gender (%) | |||
| Males | 57 | 51 | 0.22 |
| Females | 43 | 49 | |
| Education (in %) | |||
| High school graduate (or less) | 20.5 | 25.7 | 0.33 |
| Some college (or trade school) | 19.5 | 16.7 | |
| College graduate | 60.0 | 57.6 | |
| Ethnicity (%) | |||
| White | 88.1 | 90.9 | 0.54 |
| African American | 9.7 | 7.6 | |
| Other | 2.2 | 1.5 | |
| Marital status | |||
| Single, never married | 4.3 | 6.9 | 0.62 |
| Separated/divorced | 11.4 | 10.1 | |
| Married | 61.6 | 59.4 | |
| Widowed | 22.7 | 23.6 | |
| Cumulative Illness Rating Scale | |||
| Mean | 6.5 | 9.2 | <0.001 |
| SD | 2.9 | 4.3 | |
| Folstein Mini Mental State Examination | |||
| Mean | 28.7 | 28.4 | 0.008 |
| SD | 1.3 | 1.6 | |
| Geriatric Depression Scale total score | |||
| Mean | 1.9 | 5.3 | <0.001 |
| Median | 1.0 | 3.0 | |
| SD | 2.6 | 5.4 | |
| Range | 0–19 | 0–29 | |
| Duration of pain (in years) | — | ||
| Mean | — | 12.8 | |
| SD | — | 14.1 | |
Procedures
Depressed mood was assessed with the GDS [30], a brief self-report questionnaire both developed and normed for use with older adults. Patients are asked to respond yes or no to 30 questions about their mood state on the day of testing. The GDS has been shown to have excellent reliability and validity [31]. The alpha coefficient is 0.94, test–retest reliability 1 week apart is 0.85, and correlations with structured depression interviews are high (e.g., 0.83 with the Hamilton Rating Scale for Depression [30,32]).
Pain intensity was measured with the McGill Pain Questionnaire Short Form (MPQ-SF) [33] that has been validated in community dwelling older adults with chronic low back pain [34,35].
The CIRS and MMSE were completed by one of the investigators (D.K.W.). The CIRS is a well-validated measure of comorbidity and was completed based on data that were collected during the structured history and physical examination. The MMSE [28] is a method widely used to screen for cognitive impairment, and it has been validated and normed for use in older adults [36].
Data Analyses
A requirement when applying item response theory (IRT) methods to data is that the items measure a dominant trait. For example, the items must be unidimensional enough to allow for the unbiased scaling of individuals on a common latent trait. We used three common approaches to evaluate whether the 30 items of the GDS are essentially unidimensional. These were: 1) internal consistency reliability; 2) item–scale correlations; and 3) factor analysis, which evaluated the size of eigenvalues via a scree plot to determine if most of the variance was accounted for by the first factor. IRT item parameters and model fit were evaluated with WINSTEPS [37], using a one-parameter IRT model. WINSTEPS is a psychometric computer program that implements Rasch analysis, a method for obtaining objective, fundamental, linear measures from stochastic observations of ordered category responses. The software is very flexible and calculates item weights, processes a variety of rating scale formats, investigates dimensionality with principal components analysis of residuals, and analyzes item, person, and category model misfit. Item fit was assessed by examining the infit mean square statistic. The infit mean square statistic has an expected value of 1.0. If the infit mean square lies between 0.6 and 1.4 the item was considered to have a good fit [38]. Any item with an infit value <0.6 denotes “over-fit,” which indicates a poorly discriminating item that makes little contribution to the overall scale scores. An infit value >1.4 denotes an item with unexpected variation and does not fit the Rasch model, which is an undesirable characteristic for forming a well-calibrated unidimensional scale. Assuring item fit is a necessary first step before testing for DIF.
After unidimensionality and item fit to the Rasch model were determined, DIF was tested in two ways. First, the GDS item calibrations were compared between respondents who were pain free and those respondents with persistent pain. A second set of DIF analyses for the GDS items were computed for those with lower pain scores on the MPQ-SF vs those with higher MPQ-SF scores. The WINSTEPS program was used for item equating and to test for the invariance of item estimates. The null hypothesis in DIF is that two parameter estimates for an item, estimated from separate samples, are the same, except for measurement error. A t-test was used to test for significant DIF. A P value of less than 0.05, two-tailed, was considered significant.
Results
Evaluating Unidimensionality
Table 2 displays the point–biserial correlations for the 30 GDS items. These correlations are between each item and the total score, with that particular item deleted from the total score. As can be seen, all 30 GDS items were reasonably high and ranged from 0.30 to 0.66, with an average point–biserial correlation of 0.47. The Cronbach alpha (KR-20) reliability index for the 30 GDS items was 0.90, suggesting high internal consistency. Finally, the scree plot of the eigenvalues from the factor analysis of the 30-item GDS indicated that the majority of the variance was accounted for by the first factor (Figure 1). Taken together, these results provide converging evidence that the GDS items are essentially unidimensional.
Table 2.
Assessment of Rasch Rating Scale Model to Geriatric Depression Scale (GDS) items, total sample (N = 677)
| GDS Item Number and Question | Measure Parameter | SE | Infit Statistic | Point–BiserialCorrelation | |
|---|---|---|---|---|---|
| 1 | Basically unsatisfied with life* | 0.00 | 0.14 | 0.83 | 0.53 |
| 2 | Dropped activities and interests | −0.56 | 0.12 | 0.94 | 0.54 |
| 3 | Feel life is empty | 0.92 | 0.18 | 0.78 | 0.47 |
| 4 | Often get bored | 0.06 | 0.14 | 1.00 | 0.46 |
| 5 | Not hopeful about the future* | 0.44 | 0.15 | 1.07 | 0.40 |
| 6 | Bothered by thoughts cannot get out of head | −0.26 | 0.13 | 1.07 | 0.45 |
| 7 | Not in good spirits most of the time* | 1.42 | 0.21 | 0.85 | 0.40 |
| 8 | Afraid something bad is going to happen to you | 0.98 | 0.18 | 1.00 | 0.38 |
| 9 | Feel unhappy most of the time* | 0.22 | 0.14 | 0.81 | 0.52 |
| 10 | Often feel helpless | 0.54 | 0.16 | 0.82 | 0.49 |
| 11 | Often get fidgety and restless | −0.73 | 0.12 | 1.08 | 0.48 |
| 12 | Prefer to stay at home | −1.09 | 0.11 | 1.20 | 0.45 |
| 13 | Frequently worry about the future | −0.33 | 0.13 | 1.00 | 0.49 |
| 14 | Feel more problems with memory than most | 0.49 | 0.15 | 1.29 | 0.30 |
| 15 | Do not think it is wonderful to be alive now* | 1.05 | 0.18 | 0.97 | 0.38 |
| 16 | Feel downhearted and blue | 0.10 | 0.14 | 0.71 | 0.57 |
| 17 | Feel pretty worthless the way you are now | 1.08 | 0.18 | 0.89 | 0.43 |
| 18 | Worry a lot about the past | 1.19 | 0.19 | 0.88 | 0.41 |
| 19 | Do not find life very exciting* | −1.71 | 0.10 | 1.02 | 0.57 |
| 20 | Hard to get started on new projects | −1.39 | 0.11 | 0.99 | 0.57 |
| 21 | Do not feel full of energy* | −2.29 | 0.10 | 0.85 | 0.66 |
| 22 | Feel situation is hopeless | 1.81 | 0.23 | 0.84 | 0.36 |
| 23 | Think that most people are better off than you are | 0.80 | 0.17 | 0.88 | 0.45 |
| 24 | Frequently get upset over little things | −0.42 | 0.12 | 1.14 | 0.42 |
| 25 | Frequently feel like crying | 0.77 | 0.17 | 0.90 | 0.44 |
| 26 | Have trouble concentrating | −0.16 | 0.13 | 1.09 | 0.45 |
| 27 | Do not enjoy getting up in the morning* | −0.09 | 0.13 | 1.19 | 0.39 |
| 28 | Prefer to avoid social occasions | −0.09 | 0.13 | 1.02 | 0.46 |
| 29 | Is not easy to make decisions* | −0.53 | 0.12 | 1.09 | 0.46 |
| 30 | Mind is not as clear as it used to be* | −2.25 | 0.10 | 1.32 | 0.46 |
Items have been rephrased using a negative valence to assure a consistent metric for the Measure Parameter.
Figure 1.
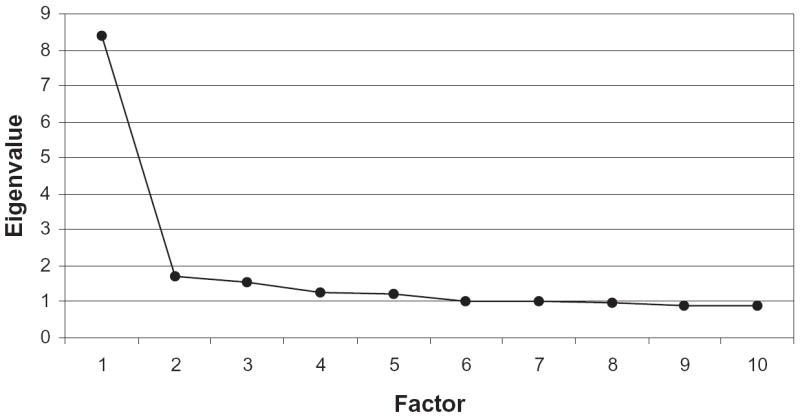
Scree plot for factor analysis of the 30 Geriatric Depression Scale items (N = 677).
Evaluating Item Fit to the Rasch Rating Scale Model
The GDS ratings from all 677 subjects were tested for their goodness-of-fit to the Rasch Rating Scale Model. Table 2 presents the resulting item parameters, their standard error of estimate, and the infit statistic. Using our infit criteria of between 0.6 and 1.4, all 30 GDS items were found to fit the Rasch model. Analogous to the beta weights that result from regression analysis but using nonlinear estimation methods, the Rasch analysis of data produces a parameter for each item in a scale, referred to as a difficulty parameter. These are listed in column 2 of Table 2 and labeled as Measure Parameter. For our present purposes, the item Measure Parameter represents the point on the latent depression dimension, measured in logits, where the probability of a positive response to that particular GDS item is 0.50. Thus, this parameter is a location parameter with respect to the measurement scale. Consistency was maintained among the items in terms of how they were asked. For example, “hopeful about the future” was rephrased as “not hopeful about the future.” Likewise, “think it is wonderful to be alive now” has been changed to “do not think it is wonderful to be alive now.” This adjustment allowed the Measure Parameter of all the items, both positively and negatively worded, to be evaluated along a similar metric.
Those items with a relatively high negative Measure Parameter, such as items #21 (do not feel full of energy; measure value = −2.29) and #30 (mind is not as clear as it used to be; measure value = −2.25), indicate items with less informational value. Those items with relatively high positive measures, such as items #7 (not in good spirits most of the time; measure value = 1.42) and #22 (feel situation is hopeless; measure value = 1.81), suggest greater informational value. A good measurement scale should provide measures with adequate coverage or sensitivity over the full range of the latent construct of interest. For this analysis, a full range of GDS items, including lower, middle, and higher item parameters, were needed to adequately address the full range of depression. We stated that the higher positive items were most informative because endorsing these items is predictive of the respondent more likely endorsing many of the items with a lower parameter value. Our Rasch analysis of the GDS indicates that the items in this scale indeed provide sufficient coverage over the full range of the latent depression scale, as indicated by the wide spread of item measure estimates (Table 2).
DIF Analyses Related to the Presence of Pain
Our first set of DIF analyses tested whether Rasch scale parameter estimates for the 30 GDS items were equivalent (within random estimation error) for subjects without pain and those subjects with persistent pain. These results are presented in Table 3. As can be seen, seven of the 30 items were found to display significant DIF, indicating these items function differently in deriving depression scores on the GDS based on an individual’s pain status. These items were: 1) dropping activities and interests; 2) bothered by thoughts cannot get out of head; 3) often get fidgety and restless; 4) prefer to stay at home; 5) do not feel full of energy; 6) do not enjoy getting up in the morning; and 7) mind is not as clear as it used to be.
Table 3.
Differential item functioning assessment of Geriatric Depression Scale (GDS) items, no pain sample vs pain sample (N = 677)
| GDS Item Number and Question | Measure Parameter (SE) |
t Value | P Value | ||
|---|---|---|---|---|---|
| No Pain | Pain | ||||
| 1 | Basically unsatisfied with life* | 0.12 (0.37) | 0.00 (0.15) | 0.30 | 0.765 |
| 2 | Dropped activities and interests | 1.81 (0.73) | −0.76 (0.13) | 3.47 | 0.001 |
| 3 | Feel life is empty | 1.81 (0.73) | 0.85 (0.18) | 1.28 | 0.203 |
| 4 | Often get bored | 0.12 (0.37) | 0.07 (0.15) | 0.13 | 0.896 |
| 5 | Not hopeful about the future* | −0.12 (0.33) | 0.57 (0.17) | −1.85 | 0.064 |
| 6 | Bothered by thoughts cannot get out of head | −0.75 (0.27) | −0.12 (0.14) | −2.04 | 0.042 |
| 7 | Not in good spirits most of the time* | 3.75 (1.81) | 1.30 (0.21) | 1.34 | 0.182 |
| 8 | Afraid something bad is going to happen to you | 1.81 (0.73) | 0.92 (0.19) | 1.18 | 0.237 |
| 9 | Feel unhappy most of the time* | 0.60 (0.44) | 0.18 (0.15) | 0.89 | 0.374 |
| 10 | Often feel helpless | 0.80 (0.48) | 0.51 (0.17) | 0.57 | 0.566 |
| 11 | Often get fidgety and restless | −0.01 (0.35) | −0.82 (0.13) | 2.20 | 0.028 |
| 12 | Prefer to stay at home | −1.51 (0.22) | −0.93 (0.12) | −2.29 | 0.022 |
| 13 | Frequently worry about the future | −0.01 (0.35) | −0.36 (0.14) | 0.94 | 0.347 |
| 14 | Feel more problems with memory than most | 0.26 (0.38) | 0.54 (0.17) | −0.67 | 0.502 |
| 15 | Do not think it is wonderful to be alive now* | 0.60 (0.44) | 1.14 (0.2) | −1.13 | 0.260 |
| 16 | Feel downhearted and blue | 1.05 (0.53) | 0.00 (0.15) | 1.92 | 0.055 |
| 17 | Feel pretty worthless the way you are now | 1.37 (0.6) | 1.06 (0.19) | 0.49 | 0.624 |
| 18 | Worry a lot about the past | 1.37 (0.6) | 1.18 (0.2) | 0.30 | 0.761 |
| 19 | Do not find life very exciting* | −1.83 (0.2) | −1.62 (0.12) | −0.87 | 0.385 |
| 20 | Hard to get started on new projects | −1.14 (0.24) | −1.41 (0.12) | 1.01 | 0.313 |
| 21 | Do not feel full of energy* | −1.61 (0.21) | −2.41 (0.11) | 3.32 | 0.001 |
| 22 | Feel situation is hopeless | 1.81 (0.73) | 1.82 (0.25) | −0.02 | 0.987 |
| 23 | Think that most people are better off than you are | 0.60 (0.44) | 0.84 (0.18) | −0.52 | 0.605 |
| 24 | Frequently get upset over little things | 0.12 (0.37) | −0.49 (0.13) | 1.56 | 0.119 |
| 25 | Frequently feel like crying | 0.42 (0.41) | 0.85 (0.18) | −0.96 | 0.336 |
| 26 | Have trouble concentrating | 0.42 (0.41) | −0.22 (0.14) | 1.49 | 0.137 |
| 27 | Do not enjoy getting up in the morning* | −0.60 (0.28) | 0.05 (0.15) | −2.00 | 0.046 |
| 28 | Prefer to avoid social occasions | 0.12 (0.37) | −0.10 (0.14) | 0.57 | 0.569 |
| 29 | Is not easy to make decisions* | −0.96 (0.25) | −0.40 (0.14) | −1.94 | 0.052 |
| 30 | Mind is not as clear as it used to be* | −2.72 (0.18) | −1.98 (0.11) | −3.51 | 0.001 |
Items have been rephrased using a negative valence to assure a consistent metric for the Measure Parameter.
DIF Analyses Related to Pain Severity
Our second set of DIF analyses tested whether Rasch scale parameter estimates for the 30 GDS items were equivalent for subjects with lower vs higher levels of pain severity. A median split on the MPQ-SF (median = 10.0) was used to classify subjects with persistent pain as having lower pain severity (below the median) or higher pain severity (above the median). The results of these DIF analyses are presented in Table 4. As displayed in Table 4, seven of the 30 items were found to display significant DIF, indicating these items function differently in deriving depression scores on the GDS based on an individual’s level of pain severity. These items were: 1) dropped activities and interests; 2) feel life is empty; 3) bothered by thoughts cannot get out of head; 4) often get fidgety and restless; 5) prefer to stay at home; 6) feel more problems with memory than most; and 7) do not find life very exciting.
Table 4.
Differential item functioning assessment of Geriatric Depression Scale (GDS) items, lower pain sample vs higher pain sample (N = 476)
| GDS Item Number and Question | Measure Parameter (SE) |
t Value | P Value | ||
|---|---|---|---|---|---|
| Lower Pain | Higher Pain | ||||
| 1 | Basically unsatisfied with life* | 0.25 (0.25) | −0.15 (0.19) | 1.32 | 0.187 |
| 2 | Dropped activities and interests | −0.40 (0.21) | −1.02 (0.17) | 2.37 | 0.018 |
| 3 | Feel life is empty | 1.50 (0.38) | 0.56 (0.22) | 2.15 | 0.032 |
| 4 | Often get bored | 0.45 (0.26) | −0.15 (0.19) | 1.87 | 0.062 |
| 5 | Not hopeful about the future* | 0.32 (0.25) | 0.76 (0.23) | −1.31 | 0.191 |
| 6 | Bothered by thoughts cannot get out of head | 0.25 (0.25) | −0.36 (0.18) | 2.01 | 0.046 |
| 7 | Not in good spirits most of the time* | 1.36 (0.36) | 1.28 (0.26) | 0.19 | 0.847 |
| 8 | Afraid something bad is going to happen to you | 0.75 (0.29) | 1.03 (0.24) | −0.75 | 0.454 |
| 9 | Feel unhappy most of the time* | 0.38 (0.26) | 0.06 (0.19) | 0.98 | 0.326 |
| 10 | Often feel helpless | 0.52 (0.27) | 0.52 (0.21) | −0.01 | 0.996 |
| 11 | Often get fidgety and restless | −0.35 (0.21) | −1.16 (0.17) | 3.05 | 0.002 |
| 12 | Prefer to stay at home | −1.21 (0.17) | −0.67 (0.17) | −2.16 | 0.031 |
| 13 | Frequently worry about the future | −0.26 (0.21) | −0.42 (0.18) | 0.57 | 0.567 |
| 14 | Feel more problems with memory than most | 0.08 (0.23) | 0.92 (0.23) | −2.52 | 0.012 |
| 15 | Do not think it is wonderful to be alive now* | 0.75 (0.29) | 1.41 (0.27) | −1.70 | 0.090 |
| 16 | Feel downhearted and blue | 0.32 (0.25) | −0.19 (0.19) | 1.62 | 0.106 |
| 17 | Feel pretty worthless the way you are now | 1.02 (0.32) | 1.09 (0.24) | −0.17 | 0.867 |
| 18 | Worry a lot about the past | 1.65 (0.4) | 0.97 (0.24) | 1.45 | 0.146 |
| 19 | Do not find life very exciting* | −1.89 (0.16) | −1.33 (0.16) | −2.44 | 0.015 |
| 20 | Hard to get started on new projects | −1.50 (0.17) | −1.33 (0.16) | −0.73 | 0.468 |
| 21 | Do not feel full of energy* | −2.29 (0.16) | −2.53 (0.16) | 1.07 | 0.283 |
| 22 | Feel situation is hopeless | 2.27 (0.52) | 1.64 (0.29) | 1.06 | 0.291 |
| 23 | Think that most people are better off than you are | 1.11 (0.33) | 0.71 (0.22) | 1.01 | 0.314 |
| 24 | Frequently get upset over little things | −0.63 (0.19) | −0.36 (0.18) | −1.04 | 0.300 |
| 25 | Frequently feel like crying | 1.02 (0.32) | 0.76 (0.23) | 0.68 | 0.500 |
| 26 | Have trouble concentrating | −0.17 (0.22) | −0.26 (0.18) | 0.30 | 0.762 |
| 27 | Do not enjoy getting up in the morning* | −0.26 (0.21) | 0.30 (0.20) | −1.92 | 0.055 |
| 28 | Prefer to avoid social occasions | −0.40 (0.21) | 0.14 (0.20) | −1.88 | 0.061 |
| 29 | Is not easy to make decisions* | −0.48 (0.20) | −0.33 (0.18) | −0.56 | 0.575 |
| 30 | Mind is not as clear as it used to be* | −2.17 (0.16) | −1.77 (0.16) | −1.75 | 0.080 |
Items have been rephrased using a negative valence to assure a consistent metric for the Measure Parameter.
Testing a GDS Scale Unbiased by Pain
Inspection of the results presented in Tables 3 and 4 indicated that a total of 10 GDS items displayed significant DIF (there were four items that displayed significant DIF for both the presence of pain and pain severity). The 10 DIF items were eliminated and a new revised GDS scale comprised of the remaining 20 items was created and evaluated to determine if its psychometric properties were similar to the full 30-item scale. As expected, the full and revised GDS scales (GDS-PAIN) were highly correlated, r = 0.96. The correlations between the MPQ-SF and GDS-PAIN and full GDS scale were very similar, r = 0.32 and r = 0.34, respectively. The GDS-PAIN scale had a Cronbach alpha of 0.87, compared with a Cronbach alpha value of 0.89 for the full scale. Rudy et al. [26] found a significant difference on the full GDS scale between older adults with and without chronic low back pain, with an effect size of 0.83 (i.e., large) between these two groups. Using the GDS-PAIN scale, this significant difference remained, and the effect size was found to be very similar (0.80).
An important psychometric question not addressed by the above analyses is what happens to the diagnostic sensitivity of the GDS when the GDS-PAIN scale is used instead of the full scale. To evaluate this we used a cutoff score of 10 on the GDS to indicate depression [39]. As the GDS-PAIN scale contained only 20 items, we rescaled the raw scores to have the same range as the full GDS scale (int(((30*GDS-PAIN)/20) + 0.5)). The results of this analysis are presented in Table 5. These two scales resulted in significantly different classification results (χ2(1) = 444.1, P < 0.0001). Using the full scale and a cutoff score of 10 resulted in 86 or 12.7% of this sample being classified as depressed. However, 27 of these 86 subjects (31.4%) were not classified as depressed when the GDS-PAIN scale was used. Overall, the number of depressed subjects dropped to 59 or 8.7% when the GDS-PAIN scale was used.
Table 5.
Comparison of subjects classified as depressed (Geriatric Depression Scale [GDS] score ≥ 10) using the full GDS scale and the revised GDS (GDS-PAIN) (N = 677)
| GDS-PAIN Scale |
Total | |||
|---|---|---|---|---|
| Non-Depressed | Depressed | |||
| Full GDS scale | Non-depressed | 591 (87.3%) | 0 (0.0%) | 591 (87.3%) |
| Depressed | 27 (3.9%) | 59 (8.7%) | 86 (12.7%) | |
| Total | 618 (91.2%) | 59 (8.7%) | 677 | |
Discussion
Our findings support the aims of this analysis that were to 1) evaluate if the presence of pain, regardless of its intensity, created DIF in at least some of the depression items of the GDS and 2) describe if, as pain severity increased, DIF would be more widespread, perhaps because of the increased life disruption and disability that has been shown to be correlated with pain intensity [40-43]. Specifically, several items appear to behave differently as a result of the presence or severity of pain. When these items that displayed DIF are removed, the revised GDS (GDS-PAIN) appears to be psychometrically stable and maintains both internal consistency and similar correlation values with a measure of pain as the original scale. Four of the 10 items (40%) were biased by both presence and severity of pain. The clinical salience of our findings is supported as both of these characteristics of pain (presence and severity) resulted in overlapping differential item bias.
The 10 items removed from the GDS include 1) dropping many activities and interests; 2) bothered by thoughts cannot get out of head; 3) often get fidgety and restless; 4) prefer to stay at home; 5) do not feel full of energy; 6) do not enjoy getting up in the morning; 7) mind is not as clear as it used to be; 8) feel life is empty; 9) feel more problems with memory than most; and 10) do not find life very exciting. These items can be grouped into preliminary groups, which we have labeled 1) low energy/isolation; 2) anxious; and 3) cognitive. Table 6 lists the items which comprise each of these groups.
Table 6.
Differential item functioning items removed from the revised Geriatric Depression Scale (GDS-PAIN)
| Low Energy/Isolation | Anxiety | Cognitive |
|---|---|---|
|
|
|
None of the items that functioned differently because of either the presence or intensity of pain dealt explicitly with depressed mood, anhedonia, or hopelessness. Indeed, the six items that were grouped as low energy/isolation (which are most closely related to depression) perhaps reflect a fatigue, sense of feeling “slowed down,” and decreased “zest” for life that may be independent of depression. This is apparent in the 1) dropping activities and interests; 2) feeling life is empty and not exciting; 3) preferring to stay at home; 4) lacking energy; and 5) not enjoying getting up in the morning. A recent article by Parmelee et al. [44] supports these findings, in particular dropping activities and interests, and suggests that having and retaining favorite pastimes are associated with reduced levels of depression and should be considered when evaluating the effects of pain upon quality of life.
One explanation for these potentially linked items is that pain is exhausting for older adults. While they might not be depressed (evidenced by the low GDS scores for the group as a whole), they might be fatigued by their persistent pain. It has been reported that fatigue (defined as tiredness in daily activities) among nondisabled older adults is a risk factor for subsequent disability [45]. This finding is supported by other work reported by Wijeratne et al. who used exploratory factor analysis to determine the structure underlying the responses of 10,662 ambulatory primary care patients, aged 60 years and over, who completed the 34-item SPHERE (Somatic and Psychological HEalth REport) questionnaire of somatic and psychological symptoms [46]. They derived a clinically interpretable four-factor solution consisting of mood, cognitive, musculoskeletal, and fatigue symptoms. An explanation why the item “do not enjoy getting up in the morning” showed evidence of DIF is because for many patients with persistent pain, in particular osteoarthritis and fibromyalgia, morning stiffness is a prominent complaint. Thus, morning may be a time of worse pain.
Another potential explanation is that these items represent a latent variable known as learned helplessness [47]. Learned helplessness occurs when individuals come to expect that either negative outcomes will occur or positive outcomes will not occur and simultaneously feel a lack of control over the occurrence of these outcomes [48]. Perhaps these items tap a loss of pleasure seeking (e.g., going out with friends, spending time on hobbies) that is negatively affected by the presence of pain, which is independent of depression in older adults.
The differential functioning of cognitive items builds upon work with older adults by our group that reported the effect of pain on both memory and executive functioning [16,17]. The presence or severity of pain differentially affected the following two items related to cognition: 1) feel more problems with memory than most; and 2) mind not as clear as it used to be. In earlier studies we reported that pain severity was associated with decreased performance on a test of number–letter switching, indicating a relationship between pain and mental flexibility [17]. We also reported that older adults with chronic low back pain demonstrated impaired neuropsychological performance as compared with pain-free older adults. Further, pain severity was inversely correlated with neuro-psychological performance [16].
While the sample in this report had relatively low scores on the GDS, suggesting minimal depression, it has been reported that approximately 45% of patients with depression in old age exhibit cognitive impairments in two or more cognitive domains. These impairments are most commonly characterized by deficits in information-processing speed, executive functioning, and visuospatial construction [49-51]. These reports of cognitive dysfunction, however, are often incidental findings in patients identified as having Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)-defined major depressive disorder. As the subjects in this report have on average mild depression scores yet still reported subjective cognitive changes, perhaps the behavior of these subjective descriptions of cognition may be more affected by the presence of pain than depression.
The anxiety items that exhibited differential item functioning, feeling fidgety and restless and bothered by thoughts cannot get out of head, are similar to symptoms of generalized anxiety disorder (GAD). Older adults with GAD report more restlessness, irritability, fatigue, and muscle tension relative to those without GAD. Wetherell and colleagues found that older adults with GAD reported greater worry, worry more days than not, worry about a greater range of topics (e.g., family, finance, personal health, social/interpersonal matters), and had more difficulty controlling their worry compared with a group of seniors with subsyndromal anxiety and asymptomatic controls [52]. It is known that GAD is a frequent cotraveler with depression [53,54]. However, in the presence of pain, these anxiety items perform differently and do not appear to contribute to a precise assessment of depressive symptoms.
These analyses are limited by several factors. First, our sample had relatively low scores on the GDS. Although this may reflect recruitment bias (i.e., more depressed individuals may not have responded to flyers or other advertisement), we feel the relatively low rates of depression is consistent with reports that older adults living with persistent pain may have lower rates of affective illness than mixed age samples [16,17,55]. Nonetheless, the fact that 10 unique items showed evidence of DIF supports the need for improved mood assessments for older persistent pain patients. Additionally, using a case–control design to evaluate DIF, that is, comparing two extreme groups (one with the condition, one control), does not necessarily ensure that the condition of interest (in our application, pain) is completely responsible for significant DIF. Other potential confounders to the observed DIF are the differences between the pain and control groups in terms of medical comorbidities and MMSE scores. Of note, while the MMSE mean scores were statistically significant between the two groups, this difference of less than 0.5 was not clinically significant. It is unavoidable to find differences in level of medical comorbidity between pain patients and normal controls. For example, individuals with pain are often less physically active, which results in weight gain, hypertension, hypercholesterolemia, and diabetes. As comorbid medical burden may also affect some of the items that evidenced DIF, future work with more medically burdened controls is warranted.
Our work adds to the substantial literature examining the complex relationship between depression and persistent pain [56-63]. In addition to describing how both conditions are risk factors for each other and are mutually exacerbating, a common theme in this research area has been how to best distinguish depression among patients with persistent pain [58]. In our comparison of the revised GDS (GDS-PAIN) with the full scale, the psychometric properties appeared to be stable and provide preliminary support that the GDS-PAIN scale that removes items biased by pain does not appear to adversely affect the psychometric properties of the scale. Although the GDS-PAIN scale appears to be more specific in its screening for depression, future work should compare structured clinical interviews for depression, such as the Structured Clinical Interview for Axis I DSM-IV Disorders [64], with the GDS-PAIN to ascertain its diagnostic precision. Future comparisons of older patients with comorbid depression and persistent pain of the GDS-PAIN with other measures of depression severity such as the Hamilton Rating Scale for Depression [32] and the Montgomery Asberg Rating Scale for Depression [65] will also further support its validity and specificity.
According to the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) [66], for authors of a new or adapted measure to recommend it over relevant existing instruments, it is incumbent to demonstrate whether a newly developed measure has incremental advantages, including decreased participant burden or increased reliability or validity. Given 1) the sound psychometric properties of the GDS-PAIN; 2) the fact that it is shorter by 10 items; and 3) the apparently improved specificity for assessing depression when used in older adults with persistent pain, we feel it warrants further testing.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 AG018299, R01 AT000985, P30 MH71944, KL2 RR024154, and by the John A. Hartford Center of Excellence in Geriatric Psychiatry at the University of Pittsburgh School of Medicine.
References
- 1.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 2.Banks S, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 3.Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology. Ann Intern Med. 2001;134:917–25. doi: 10.7326/0003-4819-134-9_part_2-200105011-00017. [DOI] [PubMed] [Google Scholar]
- 4.Katon W, Sullivan MD. Depression and chronic medical illness. J Clin Psychiatry. 1990;51(suppl):3–11. discussion 2–4. [PubMed] [Google Scholar]
- 5.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the medical outcomes study [See comment] JAMA. 1989;262(7):914–9. [PubMed] [Google Scholar]
- 6.Penninx BW, Geerlings SW, Deeg DJ, et al. Minor and major depression and the risk of death in older persons [See comment] Arch Gen Psychiatry. 1999;56(10):889–95. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 7.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–31. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 8.Ross MM, Crook J. Elderly recipients of home nursing services: Pain, disability and functional competence. J Adv Nurs. 1998;27(6):1117–26. doi: 10.1046/j.1365-2648.1998.00620.x. [DOI] [PubMed] [Google Scholar]
- 9.Mobily P, Herr K, Clark M, Wallace R. An epidemiologic analysis of pain in the elderly: The Iowa 65+ Rural Health Study. J Aging Health. 1994;6:139–54. [Google Scholar]
- 10.Kim Y, Pilkonis PA, Frank E, Thase ME, Reynolds CF. Differential functioning of the Beck depression inventory in late-life patients: Use of item response theory. Psychol Aging. 2002;17(3):379–91. doi: 10.1037//0882-7974.17.3.379. [DOI] [PubMed] [Google Scholar]
- 11.Talley J. Geriatric depression: Avoiding the pitfalls of primary care. Geriatrics. 1987;42(4):53–60. [PubMed] [Google Scholar]
- 12.Casten RJ, Parmelee PA, Kleban MH, Lawton MP, Katz IR. The relationships among anxiety, depression, and pain in a geriatric institutionalized sample. Pain. 1995;61(2):271–6. doi: 10.1016/0304-3959(94)00185-H. [DOI] [PubMed] [Google Scholar]
- 13.Varma VK, Chaturvedi SK, Malhotra A, Chari P. Psychiatric symptoms in patients with non-organic chronic intractable pain. Indian J Med Res. 1991;94:60–3. [PubMed] [Google Scholar]
- 14.Koleck M, Mazaux JM, Rascle N, Bruchon-Schweitzer M. Psycho-social factors and coping strategies as predictors of chronic evolution and quality of life in patients with low back pain: A prospective study. Eur J Pain. 2006;10(1):1–11. doi: 10.1016/j.ejpain.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil. 2005;86(6):1147–54. doi: 10.1016/j.apmr.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 17.Karp JF, Reynolds CF, Butters MA, et al. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006;7(5):444–52. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold DT. The clinical impact of vertebral fractures: Quality of life in women with osteoporosis. Bone. 1996;18(3 suppl):185S–9S. doi: 10.1016/8756-3282(95)00500-5. [DOI] [PubMed] [Google Scholar]
- 19.Krol B, Sanderman R, Suurmeijer T, et al. Disease characteristics, level of self-esteem and psychological well-being in rheumatoid arthritis patients. Scand J Rheumatol. 1994;23(1):8–12. doi: 10.3109/03009749409102127. [DOI] [PubMed] [Google Scholar]
- 20.Barkin RL, Barkin SJ, Barkin DS. Perception, assessment, treatment, and management of pain in the elderly. Clin Geriatr Med. 2005;21(3):465–90. doi: 10.1016/j.cger.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Holland P, Wainer H. Differential Item Functioning. Hillsdale: Lawrence Erlbaum Associates; 1993. [Google Scholar]
- 22.Camilli G, Shepard L. Methods for Identifying Biased Test Items. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Herr KA, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med. 2001;17(3):457–78. doi: 10.1016/s0749-0690(05)70080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor LJ, Harris J, Epps CD, Herr K. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil Nurs. 2005;30(2):55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 26.Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR. The impact of chronic low back pain on older adults: A comparative study of patients and controls. Pain. 2007;131:293–301. doi: 10.1016/j.pain.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner DK, Rudy TE, Glick RM, et al. Efficacy of percutaneous electrical nerve stimulation for the treatment of chronic low back pain in older adults. J Am Geriatr Soc. 2003;51(5):599–608. doi: 10.1034/j.1600-0579.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 28.Folstein M, Folstein, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Burke WJ, Roccaforte WH, Wengel SP, Conley DM, Potter JF. The reliability and validity of the geriatric depression rating scale administered by telephone. J Am Geriatr Soc. 1995;43(6):674–9. doi: 10.1111/j.1532-5415.1995.tb07205.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 34.Weiner D, Pieper C, McConnell E, Martinez S, Keefe F. Pain measurement in elders with chronic low back pain: Traditional and alternative approaches. Pain. 1996;67(2–3):461–7. doi: 10.1016/0304-3959(96)03150-8. [DOI] [PubMed] [Google Scholar]
- 35.Gagliese L, Melzack R. Chronic pain in elderly people. Pain. 1997;70(1):3–14. doi: 10.1016/s0304-3959(96)03266-6. [DOI] [PubMed] [Google Scholar]
- 36.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level [See comment] JAMA. 1993;269(18):2386–91. [PubMed] [Google Scholar]
- 37.Linacre J, Wright B. WINSTEPS: Multiple-Choice, Rating Scale, and Partial Credit Rasch Analysis (Computer Software) Chicago, IL: Mesa Press; 2002. [Google Scholar]
- 38.Bond T, Fox C. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. Mahwah, NJ: Lawrence Erlbaum; 2001. [Google Scholar]
- 39.Brink T, Yesavage J, Lum O, et al. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–44. [Google Scholar]
- 40.Gesztelyi G, Bereczki D. Determinants of disability in everyday activities differ in primary and cervicogenic headaches and in low back pain. Psychiatry Clin Neurosci. 2006;60(3):271–6. doi: 10.1111/j.1440-1819.2006.01501.x. [DOI] [PubMed] [Google Scholar]
- 41.Horng Y-S, Hwang Y-H, Wu H-C, et al. Predicting health-related quality of life in patients with low back pain. Spine. 2005;30(5):551–5. doi: 10.1097/01.brs.0000154623.20778.f0. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenstein MJ, Dhanda R, Cornell JE, Escalante A, Hazuda HP. Disaggregating pain and its effect on physical functional limitations. J Gerontol A Biol Sci Med Sci. 1998;53(5):M361–71. doi: 10.1093/gerona/53a.5.m361. [DOI] [PubMed] [Google Scholar]
- 43.Reid MC, Guo Z, Towle VR, Kerns RD, Concato J. Pain-related disability among older male veterans receiving primary care. J Gerontol A Biol Sci Med Sci. 2002;57(11):M727–32. doi: 10.1093/gerona/57.11.m727. [DOI] [PubMed] [Google Scholar]
- 44.Parmelee P, Harralson T, Smith L, Schumacher H. Necessary and discretionary activities in arthritis: Do they mediate the pain–depression relationship. Pain Med. 2007;8(5):449–61. doi: 10.1111/j.1526-4637.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 45.Avlund K, Rantanen T, Schroll M. Tiredness and subsequent disability in older adults: The role of walking limitations. J Gerontol A Biol Sci Med Sci. 2006;61(11):1201–5. doi: 10.1093/gerona/61.11.1201. [DOI] [PubMed] [Google Scholar]
- 46.Wijeratne C, Hickie I, Davenport T. Is there an independent somatic symptom dimension in older people? J Psychosom Res. 2006;61(2):197–204. doi: 10.1016/j.jpsychores.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychol Rev. 1989;96(2):358–72. [Google Scholar]
- 48.Smith TW, Peck JR, Ward JR. Helplessness and depression in rheumatoid arthritis. Health Psychol. 1990;9(4):377–89. doi: 10.1037//0278-6133.9.4.377. [DOI] [PubMed] [Google Scholar]
- 49.Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: Is there a relationship? Am J Geriatr Psychiatry. 2004;12(4):387–94. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]
- 50.Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18(5):529–49. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 51.Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry. 2000;8(3):201–8. [PubMed] [Google Scholar]
- 52.Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: Distinguishing the worried from the well. Psychol Aging. 2003;18(3):622–7. doi: 10.1037/0882-7974.18.3.622. [DOI] [PubMed] [Google Scholar]
- 53.Lenze EJ, Mulsant BH, Mohlman J, et al. Generalized anxiety disorder in late life: Lifetime course and comorbidity with major depressive disorder. Am J Geriatr Psychiatry. 2005;13(1):77–80. doi: 10.1176/appi.ajgp.13.1.77. [DOI] [PubMed] [Google Scholar]
- 54.DeLuca AK, Lenze EJ, Mulsant BH, et al. Comorbid anxiety disorder in late life depression: Association with memory decline over four years. Int J Geriatr Psychiatry. 2005;20(9):848–54. doi: 10.1002/gps.1366. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese SK, Lyness JM, Sorensen S, Duberstein PR. Personality and the association of pain and depression. Am J Geriatr Psychiatry. 2006;14(6):546–9. doi: 10.1097/01.JGP.0000218323.20981.10. [DOI] [PubMed] [Google Scholar]
- 56.Romano JM, Turner JA. Chronic pain and depression: Does the evidence support a relationship? Psychol Bull. 1985;97(1):18–34. [PubMed] [Google Scholar]
- 57.Ranga Rama Krishnan K, France RD, Pelton SZ. Chronic pain and depression: I. classification of depression in chronic low back pain patients. Pain. 1985;22(3):279–87. doi: 10.1016/0304-3959(85)90028-4. [DOI] [PubMed] [Google Scholar]
- 58.Wilson KG, Mikail SF, D’Eon JL, Minns JE. Alternative diagnostic criteria for major depressive disorder in patients with chronic pain. Pain. 2001;91(3):227–34. doi: 10.1016/S0304-3959(00)00440-1. [DOI] [PubMed] [Google Scholar]
- 59.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–8. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 60.Giesecke T, Gracely RH, Williams DA, et al. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52(5):1577–84. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 61.Schatzberg AF. The relationship of chronic pain and depression. J Clin Psychiatry. 2004;65(suppl 12):3–4. [PubMed] [Google Scholar]
- 62.Taylor R, Lovibond PF, Nicholas MK, Cayley C, Wilson PH. The utility of somatic items in the assessment of depression in patients with chronic pain: A comparison of the Zung self-rating depression scale and the depression anxiety stress scales in chronic pain and clinical and community samples. Clin J Pain. 2005;21(1):91–100. doi: 10.1097/00002508-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Turner JA, Ersek M, Kemp C. Self-efficacy for managing pain is associated with disability, depression, and pain coping among retirement community residents with chronic pain. J Pain. 2005;6(7):471–9. doi: 10.1016/j.jpain.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington, DC: American Psychiatric Association Press; 1995. [Google Scholar]
- 65.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 66.Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125(3):208–15. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]


