Abstract
Objective
Changes in reward-related behavior are an important component of normal adolescent affective development. Understanding the neural underpinnings of these normative changes creates a foundation for investigating adolescence as a period of vulnerability to affective disorders, substance use disorders, and health problems. Studies of reward-related brain function have revealed conflicting findings regarding developmental change in the reactivity of the striatum and medial prefrontal cortex (mPFC) and have not considered puberty. The current study focused on puberty-specific changes in brain function and their association with mood.
Method
A sample of 77 healthy adolescents (26 pre/early pubertal, 51 mid/late pubertal) recruited in a narrow age range (M=11.94 years, SD=.75) were assessed for sexual maturation and circulating testosterone, completed an fMRI guessing task with monetary reward, and underwent experience sampling of mood in natural environments. For comparison, 19 healthy adults completed the fMRI assessment.
Results
Adolescents with more advanced pubertal maturation exhibited less striatal and more mPFC reactivity during reward outcome than similarly aged adolescents with less advanced maturation. Testosterone was positively correlated with striatal reactivity in boys during reward anticipation and negatively correlated with striatal reactivity in girls and boys during reward outcome. Striatal reactivity was positively correlated with real-world subjective positive affect and negatively correlated with depressive symptoms. mPFC reactivity was positively correlated with depressive symptoms.
Conclusions
Reward-related brain function changes with puberty and is associated with adolescents' positive affect and depressive symptoms. Increased reward-seeking behavior at this developmental point could serve to compensate for these changes.
Keywords: reward, brain function, development, depression
Introduction
Adolescent development is marked by changes in several aspects of reward-related behavior.1, 2 These changes include increases in risk-taking and sensation-seeking, which are associated with pubertal maturation.3 A deeper understanding of the neural underpinnings of reward-related behavior during adolescence has public health importance because adolescence is a period of markedly increased risk for problems related to reward processing, including affective disorders, substance use disorders, and a broad range of health problems.4-7 For these reasons, understanding normal developmental changes in reward-related brain function during adolescence—particularly in relation to puberty—is critical to the long-term goals of elucidating the etiology and pathophysiology of adolescent-onset health problems.
Efforts to examine the neural bases of adolescent reward-seeking behavior in terms of the functioning of critical reward-related brain regions such as the dorsal and ventral striatum8, 9 have led to conflicting results regarding the direction of developmental changes. There is both theoretical and empirical support for contrasting perspectives. One argument suggests that a relatively enhanced activation of these reward systems could reflect the increased salience of rewards during adolescence, leading to more powerful responses in reward-related circuits that incline adolescents toward greater reward-seeking behavior.7, 10 An alternative argument is that diminished reward-related brain reactivity during adolescence could reflect, in essence, a blunted response to typical rewards. This blunted response could lead to greater motivation to experience high-intensity rewards in order to activate relevant brain circuits to an optimal level.
Studies of adolescents' reward-related brain function have reported diminished reward-related striatal reactivity,11 enhanced striatal reactivity,12, 13 or no differences in striatal reactivity.14 The developmental comparison groups for these studies have varied, with some including adults but not children,11, 12 and others combining children and adolescents in the same developmental group.14 Of particular note, studies of adolescent reward-related brain function have relied on age rather than pubertal development to determine adolescent status,11-14 and only one has included both child and adult comparison groups.11
Finally, little is known about how adolescents' reward-related brain function in laboratory settings maps onto real-world affective experience. Given the long-term goal of understanding the clinical and health-relevant aspects of maturational changes in reward-system reactivity, it is important to examine measures across domains. A recent study of depressed adolescents found that reward-related striatal function was correlated with measures of subjective positive affect in natural settings,15 but no previous studies have examined brain-affect relationships during pubertal maturation through ecological momentary assessment.
The current study focused on puberty-specific changes in reward-related brain function by studying healthy adolescents in a small age range who varied in pubertal maturation, and by including adults for comparison. Participants completed an fMRI guessing task with monetary reward that reliably elicits striatal and medial prefrontal cortex (mPFC) reactivity during the anticipation and receipt of reward. Puberty-related development in striatal reactivity was examined, based on the interest in adolescent reward processing. In addition, puberty-related development in rostral mPFC reactivity was examined, based on findings of reward-related reactivity in that region in healthy11, 16, 17 and depressed adolescents,15 as well as the region's putative role in self-processing,18 which changes importantly with adolescent development.19 Among adolescents, reward-related brain function was examined in relation to two aspects of subjective experience: positive affect in real-world settings measured through ecological momentary assessment (EMA), and depressive symptoms. We predicted group differences in reward-related brain function but did not have a strong directional hypothesis. Based on previous findings on reward-related brain function in adolescent depression,15, 20 we predicted that striatal reactivity would be positively associated with positive affect and negatively associated with depressive symptoms; we predicted the opposite pattern for mPFC reactivity.
Method
Participants
The final sample included 77 adolescents—26 pre/early pubertal, 51 mid/late pubertal—and 19 adults (Table 1). Consistent with our previous approach to examining affective development,21 we classified adolescents as pre/early pubertal if they were Tanner breast/genital stage 1 or 2 and as mid/late pubertal if they were stage 3, 4, or 5. Adolescents were recruited for a study of normal pubertal development and have not contributed data to our previous publications on depression and reward-related brain function.
Table 1. Sample Characteristics, by Developmental Group.
| Group | |||
|---|---|---|---|
| Pre/Early Pubertal | Mid/Late Pubertal | Adult | |
| N | 26 | 51 | 19 |
| Demographics | |||
| Age | 11.42 (.58) | 12.20 (.69) | 45.47 (6.59) |
| Gender (% female) | 69.2 | 41.2 | 31.6 |
| Race (%) | |||
| European American | 84.6 | 70.6 | 100 |
| African American | 8 | 6 | |
| Latino | 0 | 0 | |
| Asian | 0 | 2 | |
| Native American | 4 | 0 | |
| Tanner Breast/Genital Score | 1.83 (.37) | 3.61 (.70) | |
| Reaction Time | 1105.23 (265.94) | 1083.96 (363.41) | |
| Testosterone Level | .21 (.09) | 1.01 (1.18) | |
| Mood | |||
| Depressive Symptoms | 5.50 (6.34) | 7.30 (8.31) | |
| Subjective Positive Affect | 3.46 (.73) | 3.18 (.74) | |
Note: Values are M(SD) unless noted. Adults did not complete assessments for Tanner staging, testosterone level, or mood. Reaction time data were missing for adults due to experimenter error. Subjective positive affect was the mean of a 4-day ecological momentary assessment based on the PANAS-C,29 and depressive symptoms are based on self-report on the Mood and Feelings Questionnaire.30
Adolescents were recruited from the community through advertisements, flyers, and demographically targeted phone lists. Adolescents were recruited to be in a narrow age range—11-13 years—but to vary in pubertal development. More specifically, girls were 11-12 years old (M=11.49, SD=.60), and boys were 12-13 years old (M=12.39, SD=.59), based on findings that girls in the United States undergo puberty earlier than boys.22, 23 We thus intended to maximize the pubertal variability within groups of girls and boys and to avoid confounding of puberty by age. Adolescents were free of current and lifetime psychiatric disorders; history of head injury, serious medical illness, psychotropic medication, alcohol use, or illicit drug use; and did not have braces.
The original sample included 125 adolescents. Data for 46 adolescents were excluded for excessive head movement (n=22; 10 female, 6 pre/early pubertal), having siblings in the study (n=9), possible medical or psychiatric conditions (n=3), missing pubertal maturation data (n=12), or withdrawal from the study (n=2). All adults' data were included.
Adults were participants in a larger community study of individual differences in behavioral and biological traits among healthy, middle-aged adults.24, 25 Adults were in good general health and free of the following: medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease; lifetime history of psychotic symptoms; use of psychotropic, glucocorticoid, or hypolipidemic medication; conditions affecting cerebral blood flow and metabolism (e.g., hypertension); and psychiatric diagnoses. Adult participants and parents or guardians of adolescent participants provided informed consent. Adolescents provided assent. The study was approved by the University of Pittsburgh Institutional Review Board.
Materials
Psychiatric health
If the parent or guardian of an adolescent reported that the adolescent had received diagnosis, treatment, or medication for “any mental or behavioral health issues” during a phone screen, the adolescent was excluded from participation. Adults were assessed using the Structured Clinical Interview for DSM-IV, nonpatient edition.26
Pubertal development
Adolescents underwent physical examination by a nurse trained in scientific study of puberty to determine sexual maturation stage using criteria specified by Marshall and Tanner.27 Because breast and genital development reflect changes in levels of gonadal steroids, which influence neural development and affect-related brain function during puberty, participants were classified based on the Tanner breast/genital scale.
In 41 adolescents, circulating levels of testosterone were assessed in the morning through bloodspot sampling using a minimally invasive finger-stick procedure developed by Worthman and collegues.28 Testosterone assays were a modification of a commercially available serum/plasma radioimmunoassay kit (Pantex, Santa Monica, CA). None of the participants were below the minimal detectable dose sensitivity criterion (14.2 ng/dL for males and 14.0 ng/dL for females), indicating that the assay was sensitive to testosterone levels in female and prepubertal male participants. Sensitivity and inter-assay coefficients of variation were acceptable. Adults did not provide hormone data.
Reward processing
The fMRI paradigm was a slow event-related card-guessing game15 that allows the probing of striatal response to the anticipation and receipt of monetary reward feedback. Participants received win, loss, or no-change feedback for each trial. Participants were told that their performance would determine a monetary reward after the scan, with $1 for each win and 50 cents deducted for each loss. Trials were presented in pseudorandom order with predetermined outcomes. Earnings totaled $6. Trials were presented in 4 runs, with 12 trials per run and a balanced number of trial types within runs (i.e., 6 possible-win and 6 possible-loss trials in each). During each trial, participants guessed via button press whether the value of a visually presented card was high or low (3s), learned the trial type (possible-win or possible-loss) and anticipated feedback (12s), and received outcome feedback (1s plus 11s inter-trial interval). Participants were unaware of fixed outcome probabilities, and their engagement and motivation were maintained by verbal encouragement between runs. Because participants practiced the task before the scan and did not exhibit a change in reaction time across task runs, it is likely that they understood the anticipation period. During debriefing, all participants stated that they understood the task, thought that outcomes were due to chance, and found the task engaging.
Subjective positive affect
The PANAS-C,29 a mood questionnaire with good psychometric properties, was administered by trained staff to assess adolescents' positive affect. For each PANAS-C item (e.g., happy) participants rated their experience “at the moment the phone rang” using a 5-point scale. All 20 items were administered once per day, and a subset of 8 items (happy, joyful, energetic, excited for positive affect) was administered at all other EMA calls to reduce time burden on participants. Because positive affect within day was not excessively variable, mean positive affect across the weekend (i.e., mean of the 12 call-specific mean PA scores) was included in analyses.
Depressive symptoms
Adolescents completed the Mood and Feelings Questionnaire (MFQ),30 a psychometrically sound measure of depressive symptoms. Total self-report score was included in analyses. Four participants did not complete the questionnaire and were excluded from related analyses. Adults did not report depressive symptoms.
Procedure
Adolescents completed a midweek fMRI scan, a 4-day EMA protocol on the closest weekend to the scan (79% underwent scanning first; M=4.2 days between scan and EMA), and the MFQ. Adults completed an fMRI scan only.
fMRI acquisition, processing, and analysis
Each participant was scanned using a Siemens 3T Allegra scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 34 axial slices (3mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25ms, FOV=20cm, matrix=64×64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems.
Whole-brain image analysis was completed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, images for each participant were realigned to to correct for head motion. Data sets were then selected for quality based on our standard small-motion correction (<2mm for adults, <4mm for adolescents).15 Realigned images were spatially normalized into standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model. Normalized images were smoothed with a 6mm full-width at half-maximum Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Preprocessed data sets were then analyzed using second-level random effect models that account for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses. For each participant and scan, predetermined condition effects (i.e., main effects of task) at each voxel were calculated using a t-statistic, producing a statistical image for the 2 contrasts of interest: (a) reward anticipation > baseline and (b) reward outcome (i.e., win) > baseline. Baseline was defined as the last 3s of each inter-trial interval. Because the study focused on reward-related brain function, analyses included trials involving reward anticipation and reward outcome. Based on our hypotheses and previous strategy with this task,15 analyses focused on the first run, since these data are less likely to reflect fatigue, boredom, frustration with task length, and habituation. Habituation is a particular concern because striatal response tends to diminish with repeated experience of a reward.31
Individual contrast images were then included in region of interest (ROI) analyses to determine group-level main effects of task within striatal and mPFC ROIs using within-group t-tests, thresholded at a voxel level of p<.01 and a minimum extent of 10 contiguous voxels. The striatal region of interest (ROI) was constructed using the WFU PickAtlas Tool (v1.04) and defined as a 3642-voxel sphere with 20mm radius, centered on the ventral striatum using Talairach coordinates x=0, y=10, and z=-10, and encompassing the head of the caudate nucleus and ventral areas. The mPFC ROI was constructed using the PickAtlas and defined as a 5393-voxel sphere including medial Brodmann Area (BA)10 and BA32, which are part of the anterior rostral mPFC region implicated in social cognition and self systems.18
EMA Protocol
Adolescents provided information on their momentary affect during 12 10-min calls on receive-only cell phones at consistent intervals over 4 days, as in our previous depression study.15 The weekend was chosen for EMA because it allows for flexibility in adolescents' choice of activities and companions. Data were missing for 7.1% of calls, and 2 participants did not complete the protocol.
Data Analysis
Pubertal effects were examined using one-way analyses of covariance (ANCOVAs) focused on reactivity of the striatum and mPFC. We define reactivity as task-related BOLD signal change, and do not imply the construct of affective reactivity (vs. regulation). BOLD signal values for striatal clusters or mPFC clusters reflecting reward anticipation or reward outcome were entered as dependent variables in SPM, with developmental group (pre/early pubertal, mid/late pubertal, adult) as the independent variable. If a group effect was detected, pairwise post hoc comparisons were computed (e.g., pre/early vs. mid/late pubertal group) within the clusters exhibiting an overall effect. Regression analyses in SPM tested associations between reward-related brain function and testosterone level, subjective PA, and depressive symptoms. For all analyses, a threshold of p<.01 and minimum extent of 10 contiguous voxels were applied. All results were then corrected for multiple comparisons by masking with the main effects of task (e.g., response to reward outcome) within each ROI and applying the false discovery rate to resulting clusters. Adults did not complete bloodspot, mood, and symptom measures and were not included in the regressions. Because testosterone level was only available for a subset of adolescents, analyses with testosterone were exploratory. Models were also computed in SPSS using extracted data to determine overall model fit.
Because the pre/early pubertal group was slightly younger than the mid/late group (F=23.83, p<.001), and had a higher proportion of female participants (X2=5.42, p<.05), and the adult group had a higher proportion of European American participants than the mid/late adolescent group (X2=27.61, p<.001), age, gender, and race were included as covariates in between-group analyses. Despite findings of; gender differences in brain development32 and adolescent-onset disorders,33, 34 preliminary analyses indicated no gender X development interaction effects. Thus, we did not conduct group analyses separately by gender. Analyses with testosterone were computed separately for male and female groups, however, because of differences in typical circulating levels. Results of analyses for group differences below were largely consistent when pubertal development was treated as a continuous variable.
Results
Behavior
Pre/early and mid/late pubertal groups did not differ in mean subjective positive affect or depressive symptoms, but age was moderately and inversely correlated with positive affect (r=-.31, p<.01). Reaction time did not differ between pubertal groups and was not correlated with striatal response, mPFC response, positive affect, or depressive symptoms. Depressive symptoms and subjective positive affect were weakly and inversely correlated (r=-.19, p=.10).
Striatal Reactivity and Sexual Maturation
As in our previous study with a different sample of adolescents, the guessing task elicited reactivity in the striatum and other reward-related areas in all three developmental groups (see Supplemental Tables 1 and 2). ANCOVAs to test developmental effects yielded findings in 2 caudate clusters during reward outcome (Figure 1a; 253 voxels, [Talairach coordinates: 6,-5,17; includes thalamus], df =92, t=3.50, p<.001; 17 voxels, [6,18,3], df=92, t=2.41, p<.01). Within the first cluster, post hoc pairwise tests indicated that mid/late pubertal adolescents exhibited less caudate reactivity than pre/early adolescents (160 voxels, [10,-5,15], t=3.12, p<.005). Groups did not differ during reward anticipation. Mid/late and pre/early pubertal adolescents did not differ from adults during reward outcome. Mid/late pubertal adolescents did not exhibit more reactivity than pre/early in any striatal region.
Figure 1.
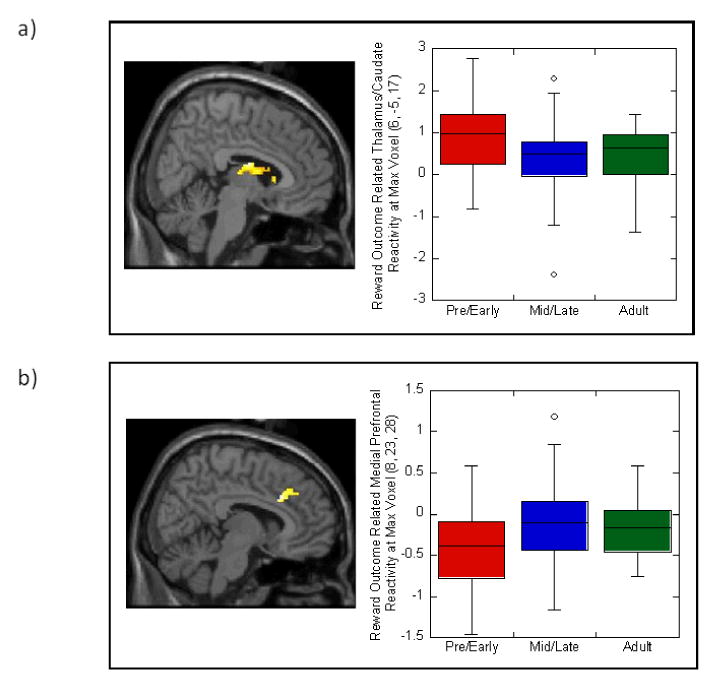
Developmental group differences, based on sexual maturation, in blood oxygen-level-dependent response of a) striatum and b) medial prefrontal cortex to reward outcome. Boxplots provide descriptive information on reactivity for all three developmental groups (pre/early adolescent, mid/late adolescent, and adult) at the maximum voxel for the cluster in each image. Results remained significant when outliers were removed from analyses.
mPFC Reactivity and Sexual Maturation
Also as in our previous study, the task elicited reward-related mPFC activation in all three developmental groups (Supplemental Table 1). ANCOVAs indicated developmental effects in BA32 during reward outcome (Figure 1b; 129 voxels, [8,23,28], df=92, t=2.78, p<.005). Within this cluster, post hoc tests indicated that mid/late adolescents exhibited greater reactivity than pre/early pubertal adolescents (113 voxels, [10,23,28], t=3.08, p<.005). Groups did not differ during reward anticipation. Mid/late and pre/early pubertal adolescents did not differ from adults during reward outcome. Mid/late pubertal adolescents did not exhibit less mPFC reactivity than pre/early.
Reward-Related Reactivity and Testosterone Level
In the subsample of adolescents with hormone data, regressions conducted within gender groups indicated that testosterone was correlated with striatal reactivity. During reward anticipation, testosterone was positively correlated with caudate reactivity in boys (34 voxels, [4, 16, 5], df=15, t=3.35, p < .005). During reward outcome, testosterone was negatively correlated with caudate reactivity in both girls (90 voxels, [6, 6, 13], df=18, t=4.01, p < .001) and boys (1149 voxels, [12, -4, 2; includes globus pallidus], df=15, t=4.81, p < .001).
Reward-Related Reactivity and Subjective Positive Affect and Depressive Symptoms
Within adolescents, striatal reactivity during reward anticipation and reward outcome was positively correlated with subjective positive affect (Table 2; Figure 2). Striatal reactivity during reward outcome was negatively correlated with depressive symptoms, and mPFC reactivity during reward anticipation was modestly, positively correlated with depressive symptoms.
Table 2. Relation of Reward-Related Striatal and Rostral Medial Prefrontal Activation to Adolescents' Subjective Positive Affect in Natural Settings and Depressive Symptoms.
| Region | Hemisphere | Talairach coordinates of maximum voxel in cluster | Cluster Size | t | pFDR < | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Subjective Positive Affect | |||||||
| Reward Anticipation – Positive Correlation | |||||||
| Thalamus/Caudate | R | 8 | -9 | 13 | 238 | 3.34 | 0.05 |
| Reward Outcome – Positive Correlation | |||||||
| Thalamus/Caudate | R | 10 | -2 | 10 | 693 | 2.94 | 0.05 |
| Depressive Symptoms | |||||||
| Reward Anticipation – Positive Correlation | |||||||
| Medial Frontal Gyrus, BA10 | R | 6 | 62 | 1 | 91 | 3.45 | 0.05 |
| Anterior Cingulate, BA32 | L | -6 | 19 | 27 | 82 | 2.45 | 0.05 |
| Reward Outcome – Negative Correlation | |||||||
| Ventral Striatum | L | -14 | 2 | -7 | 402 | 3.09 | 0.05 |
Note: Results are from regressions focusing on the striatum and the rostral medial prefrontal cortex. df=75 for subjective positive affect and 74 for depressive symptoms. For clusters labeled Thalamus/Caudate, the maximum voxel for the cluster was located in the thalamus, but most of the cluster was located in the caudate. BA: Brodmann Area. FDR: False discovery rate-corrected. When excluding cases with depressive symptoms >30, the correlations with BA32 during reward anticipation (63 voxels, [-4,34,4], t=2.69, p<.005) and striatum during reward outcome (caudate: 103 voxels, [-2,12,6], t=2.95, p<.005) remained significant.
Figure 2.
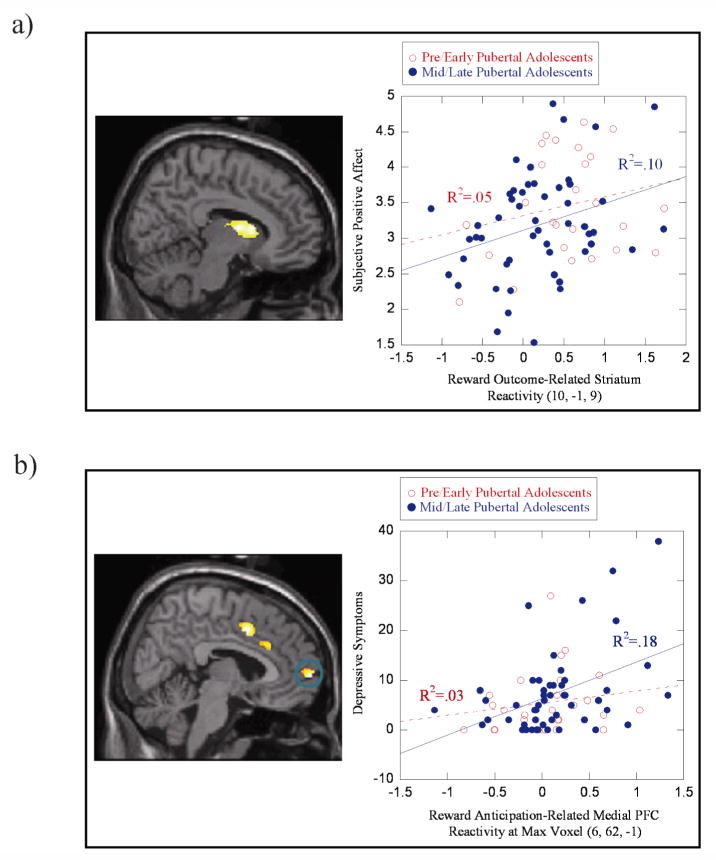
Examples of associations of reward-related brain function with subjective positive affect in natural settings and with depressive symptoms. (a) Positive correlation between subjective positive affect in natural environments and caudate reactivity during reward outcome; (b) positive correlation between depressive symptoms and medial Brodmann Area 10 reactivity during reward anticipation.
Discussion
This study provides new data on key aspects of normal affective development that can help to frame clinical findings on adolescents' difficulties with reward-related mood and behavior. Healthy mid/late pubertal adolescents exhibited less striatal reactivity and more mPFC reactivity during reward outcome than healthy pre/early pubertal adolescents. Mid/late adolescents' striatal and mPFC function did not differ from adults. Within adolescents, reward-related striatal function was associated with subjective positive affect in natural settings, indicating a link between reward-related brain function and real-world affective experience that is relevant to reward. Depressive symptoms, another indicator of clinically meaningful changes in adolescents' experience, were associated with low striatal reactivity and high mPFC reactivity. Together, these results indicate that responsiveness in basic reward-related circuits and social- and self-cognition circuits can be valuable for examining normal and abnormal mood.
The general pattern of adolescents' striatal and mPFC reactivity replicates our previous findings, using the same fMRI task, from a study of adolescent depression.15 As in previous studies using this paradigm,15 striatal reactivity findings centered on the caudate, a dorsal region implicated in affect regulation35 and learning rewarding outcomes of actions.8 The presence of developmental effects during reward outcome suggests that pubertal development may be particularly linked to changes in consummatory or “liking” aspects of reward processing, which reflect pleasure and are considered to have different neural underpinnings from the “wanting” aspects of reward processing.36
While the pattern of reduced striatal reactivity in mid/late pubertal adolescents appears to contrast with the results of two other studies, it is also consistent with some aspects of those studies' findings. In those studies, adolescents exhibited greater ventral striatal reactivity than adults,12, 13 children,13 or younger adolescents,12 but only during high-magnitude reward conditions. The findings also differ from reports that striatal reactivity to reward is associated with impulsivity in adults.25 Given evidence that sensation-seeking but not impulsivity increases with puberty,3, 37 perhaps changes in striatal function with puberty have implications for sensation-seeking rather than impulsivity.
A possible interpretation of the pattern of results across studies is that adolescents are less reactive to low-intensity rewards but more reactive to high-intensity rewards. We could not test this hypothesis because our task did not include varying reward magnitude. However, this inclination toward high-magnitude rewards could contribute to adolescents' tendency to seek intense, risky rewards, a pattern that fits well with findings on pubertal changes in sensation-seeking.1, 38 The current pattern of findings is also consistent with the literature on adolescent mood and behavior, which indicates lower subjective positive affect in humans39 and lower physiological arousal in animals40 during adolescence compared with childhood, combined with increased tendency to engage in reward-seeking behaviors.
It is striking that the mid/late pubertal group did not exhibit either a level of reward-related brain function that was somewhere between the levels exhibited by pre/early pubertal and adult groups or a level that differed from the levels of both groups (e.g., reduced striatal reactivity relative to both). The mid/late pubertal group appeared more similar to adults, unlike a previous study's findings on striatal reactivity,11 than to their same-age but less mature peers. This is surprising because increased sensation-seeking, decreased mood valence, and enhanced self-awareness—relative to both childhood and adulthood—are widely documented in adolescence.4, 41 The current reward paradigm, while sensitive to pubertal changes, may not capture some differences between adults and adolescents in reward function. Another possibility—suggested by the nonsignificant differences between the pre/early pubertal group and the adults—is that the small sample of adults reduced the statistical power to detect differences between mid/late adolescents and adults, and that, in fact, the mid/late group differs from both other developmental groups in reward-related striatal and mPFC reactivity.
Findings on mPFC reactivity were opposite in direction to those reported in other studies of adolescence.17 The location of mPFC findings was in a more rostral region of mPFC than found in one study,11 but the current mPFC ROIwas based on an interest in the development of social cognition and self-awareness.18, 42 Also, another study reported decreased mPFC in adolescents relative to adults during reward decision-making,16 which was not the focus of the current study. In addition, while conceptual models of adolescent development have emphasized differences in rostral mPFC function during adolescence,37 those models have often focused on social processing. The current study indicates that, more specifically, pubertal maturation plays a role in rostral mPFC function during reward.
Associations between testosterone level and reward-related striatal function in a subsample of the adolescents followed a similar pattern to findings with sexual maturation: higher testosterone was associated with reduced outcome-related caudate reactivity. This was the case for both girls and boys, which suggests a similar role for testosterone in reward responding despite sex differences in mean levels. Testosterone was also positively correlated with striatal reactivity during reward anticipation in boys and was unrelated to mPFC function. Thus, different aspects of reward-related brain function may increase and decrease with puberty, and testosterone may influence both motivational and consummatory aspects of reward processing.
The current findings for striatal reactivity suggest that adolescents may compensate for their low reward reactivity—at least to certain types of rewards—with an inclination to seek high-intensity and, at times, risky rewards. The mPFC findings suggest that the self- and social-cognition systems are also increasingly engaged by reward processing during pubertal development, possibly because adolescents are responding to reward in the context of their performance relative to others'. Because the mPFC is conceptualized as part of the brain's default mode network43—a brain system active during alert rest and postulated to underlie self-referential mental states—it is also possible that pubertal development leads to challenges in disengaging from baseline cognitive and affective states in response to reward.
Finally, it is important to note that mid/late adolescents' pattern of decreased striatal reactivity and increased mPFC reactivity to reward—along with the association between depressive symptoms and reduced striatal reactivity to reward—could form the foundation for changes in brain function that occur with depression. This pattern of brain function is similar to that observed in depressed adults and adolescents during reward processing.15, 44 The enhanced mPFC reactivity associated with depressive symptoms was more anterior than that associated with puberty but was consistent with findings in adult depression.45 Given the role of the mPFC in affect regulation,46 the current and extant findings together suggest that depression could involve over-regulation of reward responding.
This study's limitations include its cross-sectional design, lack of data on post-scan mood, reliance on single measures of mood and depressive symptoms, and the age range and size of the adult group. The age range of the sample overall did not allow us to examine reward reactivity in detail from adolescence through middle adulthood. Although the restricted age range allowed us to examine pubertal maturation somewhat independently of age, it also reduced our ability to examine mid-adolescence, a point at which other studies have reported enhanced reward responding.13 Also, the application of different movement thresholds for adolescents and adults, while developmentally motivated and based on previous work, could have influenced the results.
In sum, the current study contributes to knowledge about reward-related brain function in adolescent development. By employing a large sample of adolescents within a narrow age range, measuring pubertal development, and including an adult comparison group, the study extends the literature on reward-related brain function during a developmental period critical to the onset of psychiatric disorders. Future studies extending this line of investigation can provide an important foundation for understanding developmental vulnerability to reward-related disorders and developing effective interventions.
Supplementary Material
Supplemental Table 1
Reward-related Brain Function during a Monetary Reward Paradigm for Each Developmental Group, by Task Condition
Supplemental Table 2
Whole-Brain Results for Response to Monetary Reward in Entire Sample of Adolescents and Adults, by Task Condition
Acknowledgments
We thank Kelsey Ronan for help with creating tables and preparing figures. We are very grateful to the adolescents and adults who participated.
This study was supported by NIH R01 DA018910 (Ronald E. Dahl, PI), K01 MH74769 (Erika E. Forbes, PI), and a NARSAD Young Investigator Award (Erika E. Forbes, PI).
Footnotes
Study statistical expert: Satish Iyengar, Ph.D.
Clinical Trials Registry: none.
References
- 1.Steinberg L. A neurobehavioral perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005 Feb;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- 4.Dahl RE, Spear LP. Adolescent brain development. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 5.Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression. Dev Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006 Mar;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007 Aug 1;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 10.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008 Mar;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. J Neurosci. 2004 Feb 25;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005 May 1;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analysis of probability estimation and reward evaluation. Developmental Neuropsychology. 2008;33(2):179–196. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry. 2009 Jan;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 19.Sebastian C, Burnett S, Blakemore S. Development of the self-concept during adolescence. Trends in Cognitive Sciences. 2008;12:441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008 Jan;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 21.Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006 Jan 1;59(1):24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Research in Office Settings network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 23.Karpati AM, Rubin CH, Kieszak SM, Marcus M, Troiano RP. Pubertal stage assessment in American boys: the 1988-1994 Third National Health and Nutrition Examination Survey. Adolescent Health. 2002;30(3):205–212. doi: 10.1016/s1054-139x(01)00320-2. [DOI] [PubMed] [Google Scholar]
- 24.Bleil ME, Gianaros PJ, Jennings JR, Flory JD, Manuck SB. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosom Med. 2008 Apr;70(3):328–337. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- 25.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009 Jan;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders - patient edition (SCID-I/D, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 27.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 28.Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997 Sep;104(1):1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Laurent J, Catanzaro SJ, Joiner TE, Jr, et al. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11(3):326–338. [Google Scholar]
- 30.Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8-17-year-olds: effects of age and gender. J Child Psychol Psychiatry. 2002 Nov;43(8):1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007 Jul 15;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence. Nat Rev Neurosci. 2008 Dec;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 35.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005 Mar 15;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003 Sep;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 37.Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007 Mar;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin CA, Kelly TH, Rayens MK, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002 Dec;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Larson R, Lampman-Petraitis C. Daily emotional states as reported by children and adolescents. Child Dev. 1989;60:1250–1260. doi: 10.1111/j.1467-8624.1989.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 40.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg L. Risk-taking in adolescence: What changes, and why. Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 42.Sebastian C, Burnett S, Blakemore SJ. Development of the self-concept during adolescence. Trends Cogn Sci. 2008 Nov;12(11):441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005 Sep 15;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999 May;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 46.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008 May 15;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1
Reward-related Brain Function during a Monetary Reward Paradigm for Each Developmental Group, by Task Condition
Supplemental Table 2
Whole-Brain Results for Response to Monetary Reward in Entire Sample of Adolescents and Adults, by Task Condition


