Abstract
Background:
Macrophage inhibitory cytokine-1(MIC-1) is a potential modulator of systemic inflammation and nutritional depletion, both of which are adverse prognostic factors in oesophago-gastric cancer (OGC).
Methods:
Plasma MIC-1, systemic inflammation (defined as plasma C-reactive protein (CRP) of ⩾10 mg l–1 or modified Glasgow prognostic score (mGPS) of ⩾1), and nutritional status were assessed in newly diagnosed OGC patients (n=293). Healthy volunteers (n=35) served as controls.
Results:
MIC-1 was elevated in patients (median=1371 pg ml–1; range 141–39 053) when compared with controls (median=377 pg ml–1; range 141–3786; P<0.001). Patients with gastric tumours (median=1592 pg ml–1; range 141–12 643) showed higher MIC-1 concentrations than patients with junctional (median=1337 pg ml–1; range 383–39 053) and oesophageal tumours (median=1180 pg ml–1; range 258–31 184; P=0.015). Patients showed a median weight loss of 6.4% (range 0.0–33.4%), and 42% of patients had an mGPS of ⩾1 or plasma CRP of ⩾10 mg l–1 (median=9 mg l–1; range 1–200). MIC-1 correlated positively with disease stage (r2=0.217; P<0.001), age (r2=0.332; P<0.001), CRP (r2=0.314; P<0.001), and mGPS (r2=0.336; P<0.001), and negatively with Karnofsky Performance Score (r2=−0.269; P<0.001). However, although MIC-1 correlated weakly with dietary intake (r2=0.157; P=0.031), it did not correlate with weight loss, BMI, or anthropometry. Patients with MIC-1 levels in the upper quartile showed reduced survival (median=204 days; 95% CI 157–251) when compared with patients with MIC-1 levels in the lower three quartiles (median=316 days; 95% CI 259–373; P=0.036), but MIC-1 was not an independent prognostic indicator.
Conclusions:
There is no independent link between plasma MIC-1 levels and depleted nutritional status or survival in OGC.
Keywords: macrophage inhibitory cytokine 1, oesophageal cancer, gastric cancer, cachexia, survival, nutrition
Systemic inflammation has been linked with adverse survival in a variety of cancer types (Falconer et al, 1995; Scott et al, 2002; Hefler et al, 2008). This association could be explained by a variety of tumour-related phenomena, including enhanced tumour progression (Hefler et al, 2008), angiogenesis (Krzystek-Korpacka et al, 2008), and metastasis (Weinstein et al, 1984). However, the presence of systemic inflammation has also been linked with both hypermetabolism (Falconer et al, 1994) and reduced food intake (Fearon et al, 2006), two key components of the cachexia syndrome that is known to result in shortened survival in patients with advanced malignancy (Fearon et al, 2006). The mechanisms by which systemic inflammation arises in cancer patients are not established clearly. One hypothesis is that interaction between host and tumour cells within the tumour mass results in activation of peripheral blood mononuclear cells (PBMCs) (O’Riordain et al, 1999). The latter circulate to distant target organs in which enhanced cytokine/mediator production results in the generation of a systemic inflammatory response. Target organs include the liver (production of acute-phase proteins, e.g., C-reactive protein (CRP); O’Riordain et al, 1999), the brain (induction of anorexia; Buchanan and Johnson, 2007), and skeletal muscle (induction of protein degradation and net amino-acid mobilisation; Acharyya et al, 2005; Dejong et al, 2005). We have shown previously increased pro-inflammatory cytokine release by PBMCs in patients with elevated plasma CRP concentrations (O’Riordain et al, 1999). The ability of such PBMCs to induce acute-phase protein production from co-cultured human hepatocytes seemed to be IL-6 dependent (O’Riordain et al, 1999). In patients with oesophago-gastric cancer (OGC; in which systemic inflammation is associated with weight loss and shortened survival), we have previously shown that tumour IL-1β overexpression and chronic inflammatory cell infiltrate are independent factors influencing systemic inflammation (Deans et al, 2006). However, the precise role of various pro-inflammatory cytokines within human tumours in the generation of a systemic response is still incompletely understood.
Macrophage inhibitory cytokine-1 (MIC-1) is a divergent member of the transforming growth factor-β superfamily that is produced by macrophages in response to activation (Bootcov et al, 1997). MIC-1 is not expressed in most human tissues at basal conditions (except the placenta) but is expressed at high concentrations during inflammation and injury (Fairlie et al, 1999; Schober et al, 2001). MIC-1 is overexpressed by malignant melanoma cells and is associated with tumourigenicity (Boyle et al, 2009). High serum concentrations of MIC-1 have been observed in patients with pancreatic (Koopmann et al, 2004, 2006), gastric (Baek et al, 2009), and breast cancer (Welsh et al, 2003), and have been associated with adverse survival in colorectal cancer (Brown et al, 2003) and glioblastoma (Shnaper et al, 2009). In prostate cancer, high patient serum levels of MIC-1 have been associated with increased disease stage (Selander et al, 2007), docetaxel resistance (Zhao et al, 2009), and adverse survival (Brown et al, 2009). In prostate cancer bone metastases, MIC-1 induced osteoclast activation (Wakchoure et al, 2009). In mice bearing human prostate cancer xenografts, elevated MIC-1 concentrations were associated with marked weight, fat, and lean tissue loss that was mediated by decreased food intake and was reversed by an antibody to MIC-1 (Johnen et al, 2007). In addition, normal mice administered systemic MIC-1, and transgenic mice overexpressing MIC-1, showed hypophagia and reduced body weight (Johnen et al, 2007). In a small group of cachectic prostate cancer patients (n=26), serum MIC-1 concentrations were significantly associated with weight loss and weakly correlated with serum IL-6 (Johnen et al, 2007).
To determine whether MIC-1 is associated with inflammatory and nutritional status, we aimed to measure circulating concentrations of MIC-1 in a large cohort (n>250) of patients with OGC, a disease strongly associated with cachexia, and to analyse the relationships between MIC-1, systemic inflammation, nutritional status, and survival.
Materials and methods
Patients and controls
All patients provided written, informed consent, and the study was approved by the Lothian Research Ethics Committee. Patients with a new histological diagnosis of OGC were recruited (n=293; 198 males and 95 females). Whole blood was taken at diagnosis for plasma analysis of MIC-1 and CRP concentration. Staging was carried out according to the AJCC/UICC system (Sobin and Wittekind, 2002). The majority of patients (n=186) were followed up until death. A healthy control cohort (n=35; 25 males, 10 females, median age 29 years, range 24–85), composed of laboratory and hospital staff (n=28) and hospital patients undergoing minor operative procedures for benign conditions (n=7), was recruited for comparative MIC-1 analysis. Exclusion criteria for controls included recent weight change, ill-health, or underlying inflammatory illness (e.g., rheumatoid arthritis).
Nutritional assessment
At recruitment, pre-illness stable weight was self-reported by patients and percentage weight loss was calculated (Deans et al, 2009). Height was measured using a wall-mounted stadiometer with the patient standing erect without shoes. Patients were weighed on spring balance scales without shoes and wearing light clothing. Mid-arm circumference (MAC) was measured at the mid-point between the acromion and olecranon processes. Triceps skinfold thickness (TSF) was measured with Harpenden skin callipers (Holtain, Crymych, UK). Mid-arm muscle circumference (MAMC) was calculated according to the formula: MAMC=MAC-[π × TSF]. Karnofsky performance score (KPS) was documented in all patients by the recruiting physician. As surrogates of dietary intake, dysphagia score (normal swallowing=0; dysphagia to solids=1; dysphagia to softened foods=2; dysphagia to liquids=3; and total dysphagia=4) and subjective diet score (normal dietary intake=1; reduced dietary intake=2; and poor dietary intake=3) were assessed in a subset of patients (n=188). Cachexia was defined as weight loss of ⩾10% when compared with pre-morbid weight. Control subjects did not undergo nutritional assessment beyond confirmation of weight stability.
Total plasma MIC-1 concentration determination
Samples were examined in duplicate using a well-established sandwich ELISA as previously described (Moore et al, 2000). In brief, the mouse MAb 26G6H6 was used for antigen capture; and the sheep PAb 233-P was used for detection (Moore et al, 2000; Fairlie et al, 2001; Brown et al, 2002). The hMIC-1 plasma concentration was determined by reference to a standard curve constructed using recombinant hMIC-1 as the standard. All samples had duplicate values with a coefficient of variation (CV) of <10%. Assay performance was monitored additionally using standard diagnostic laboratory procedures.
Assessment of systemic inflammation
Systemic inflammation was determined in two ways. In all patients, the presence of a plasma CRP concentration of ⩾10 mg l–1 was used to define an acute-phase protein response (APPR). In a subset of patients (n=197), plasma albumin concentration was determined, and thus calculation of a systemic inflammation-based score, the modified Glasgow Prognostic Score (mGPS) (McMillan, 2009), was performed. The mGPS was calculated as follows: patients with an elevated level of CRP (>10 mg l–1) were allocated a mGPS of 1 or 2, depending on the absence or presence of hypoalbuminaemia (<35 g l–1), whereas patients showing no elevated level of CRP (⩽10 mg l–1) were allocated a mGPS of 0. Plasma CRP and albumin concentrations were assayed using automated methods on Olympus AU 2700 and Olympus 640 analysers, respectively (Olympus Diagnostica GmbH (Irish Branch), Lismeehan, Ireland), in the Department of Clinical Chemistry, Royal Infirmary of Edinburgh (fully accredited by Clinical Pathology Accreditation (UK) Ltd). Appropriate IQC were included, with CVs typically 3.4% at concentrations of <15 mg l–1and 1.6% at 80 mg l–1 for CRP, and <3.0% at all concentrations for albumin.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Services version 13.0 (SPSS 13.0; Chicago, IL, USA). Plasma MIC-1 data are presented as box plots. Mild outliers (MIC-1 concentration more than 1.5 times the interquartile range (IQR) above the third quartile) are represented as circles and extreme outliers (MIC-1 concentration more than 3 times the IQR above the third quartile) are presented as stars. For visual clarity, the y axes are limited to a maximum MIC-1 concentration of 5000 or 8000 pg ml–1. Differences between the distribution functions of data for three or more subject groups were determined using Kruskal–Wallis test (shown in text and figure legends). Subsequent analysis for determining differences between any two groups was determined using Mann–Whitney U test (shown in figures). All quoted P-values are two tailed. Correlation analysis was performed using non-parametric Spearman's rank correlation coefficient. Linear regression was used for analysing the relationship between CRP and MIC-1. Significance levels for the explanatory variables of interest were 0.10 to enter stepwise into the model. Survival analyses were performed on those patients followed until death using Kaplan–Meier plots and Cox's proportional hazards models. For construction of the latter model, treatment regimen was defined as either surgery with curative intent, radical chemo/radiotherapy with curative intent, chemo/radiotherapy with palliative intent, or nil. Statistical significance was set at P<0.05 level.
Results
Patient demographics
Oesophago-gastric cancer patients showed a median weight loss of 6.4% (range 0.0–33.4% Table 1), and 34% of patients had lost ⩾10% body weight, consistent with significant cachexia. Furthermore, median BMI, MAMC, and TSF measures were lower than those reported in healthy elderly populations (Corish and Kennedy, 2003). In all, 42% of patients (n=123) showed plasma CRP concentrations of ⩾10 mg l–1 consistent with the presence of an APPR. Of the assessed patients, 29.9% (59 out of 197) had a mGPS of 1, and 13.7% (27 out of 197) had a mGPS of 2.
Table 1. Demographics, nutritional status, and plasma concentrations of inflammatory mediators of the oesophago-gastric cancer patients. Data are presented as medians with ranges.
| Patients (n=293) | |
|---|---|
| Male:female | 198 : 95 |
| Age (years) | 70 (26–91) |
| Tumour site | |
| Oesophageal | 139 |
| OGJ | 51 |
| Gastric | 103 |
| Histology | |
| Adenocarcinoma | 242 |
| Squamous | 43 |
| Undifferentiated | 6 |
| Neuroendocrine | 2 |
| Disease stage | |
| I | 36 |
| II | 45 |
| III | 106 |
| IV | 97 |
| Unknown | 46 |
| Body mass index (kg m–2) | 24.6 (13.9–46.7) |
| Weight loss (% loss of pre-morbid weight) | 6.4 (0.0–33.4) |
| Mid-arm muscle circumference (cm) | 23.8 (15.6–32.10) |
| Triceps skin-fold thickness (mm) | 12.0 (3.0–52.0) |
| KPS | 90 (30–100) |
| CRP (mg l–1) | 9.0 (1.0–200.0) |
| MIC-1 (pg ml–1) | 1246.5 (140.7–39 052.9) |
Abbreviations: CRP=C-reactive protein; KPS=Karnofsky performance score; MIC-1=macrophage inhibitory cytokine-1.
Plasma MIC-1 concentrations are elevated in oesophago-gastric cancer
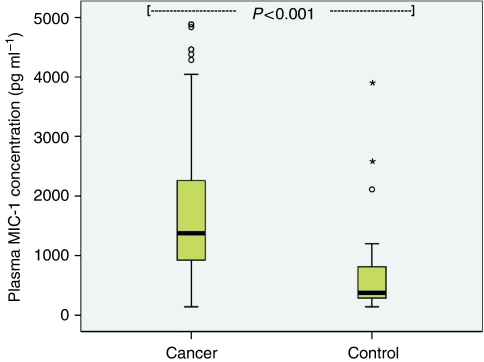
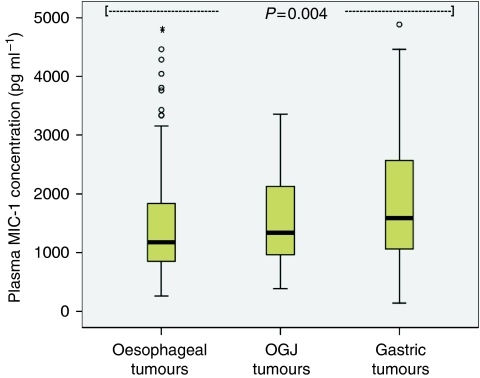
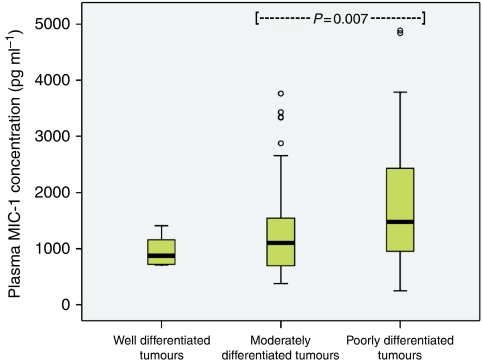
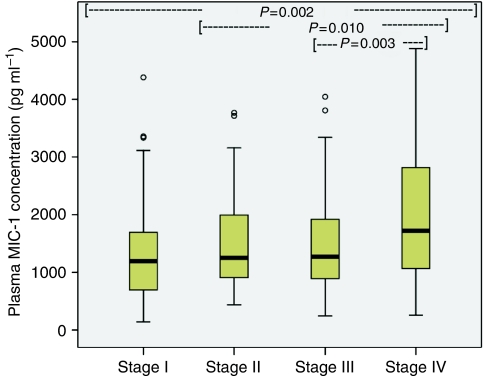
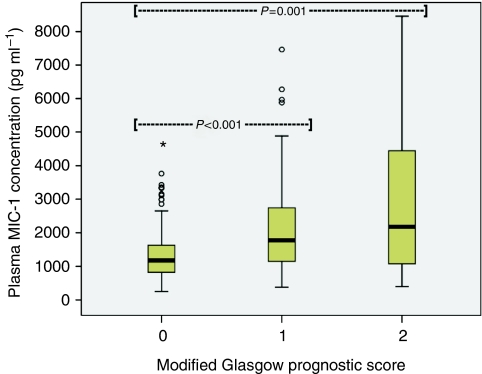
Plasma MIC-1 was elevated in OGC patients (median 1371 pg ml–1; range 141–39 053) when compared with controls (median 377 pg ml–1; range 141–3786; P<0.001; Figure 1). Patients with gastric tumours (median 1592 pg ml–1; range 141–12 643) showed higher MIC-1 concentrations than patients with oesophago-gastric junction (OGJ; median 1337 pg ml–1; range 383–39 053) and oesophageal tumours (median 1180 pg ml–1; range 258–31 184; P=0.015; Figure 2). Patients with poorly differentiated tumours (median 1480 pg ml–1; range 245–9000) showed higher MIC-1 concentrations than patients with moderately differentiated (median 1103 pg ml–1; range 378–6646) and well-differentiated tumours (median 875 pg ml–1; range 710–1407; P=0.010; Figure 3). Plasma MIC-1 concentration also increased with worsening disease stage (Figure 4) and increasing mGPS (Figure 5).
Figure 1.
Box plot showing increased plasma MIC-1 concentration in oesophago-gastric cancer patients when compared with the controls (P<0.001).
Figure 2.
Box plot showing increasing plasma MIC-1 concentration with increasing distal situation of the primary tumour (P=0.015).
Figure 3.
Box plot showing increasing plasma MIC-1 concentration with worsening tumour grade (P=0.010).
Figure 4.
Box plot showing increasing plasma MIC-1 concentration with worsening disease stage (P=0.002).
Figure 5.
Box plot showing increasing plasma MIC-1 concentration with increasing modified Glasgow Prognostic Score (P<0.001).
Relationship of MIC-1 with systemic inflammation and nutritional status
Plasma MIC-1 concentration correlated positively with disease stage (r2=0.217; P<0.001), patient age (r2=0.332; P<0.001), CRP (r2=0.314; P<0.001), and mGPS (r2=0.336; P<0.001), and correlated negatively with KPS (r2=−0.269; P<0.001; Table 2). Plasma MIC-1 also correlated weakly with diet score (r2=0.157; P=0.031) but did not correlate with dysphagia score or any of the nutritional and anthropometric parameters measured. However, there was a small but significant increase (18.9%) in plasma MIC-1 between patients who had lost ⩾10% weight (median 1493 pg ml–1; range 258–31 184) compared with those who had not (median 1256 pg ml–1; range 141–39 053; P=0.036). In contrast, both CRP (r2=0.247; P<0.001) and mGPS (r2=0.280; P<0.001) correlated with weight loss, and mGPS also correlated negatively with MAMC (r2=−0.318; P<0.001; Table 2). Furthermore, there was a highly significant increase in CRP and mGPS between patients who had lost ⩾10% weight compared with those who had not (median CRP 16.5 vs 6.0 mg l–1; median mGPS 1 vs 0; P=0.001 for both tests).
Table 2. Correlations between inflammatory mediators and nutritional status.
| Inflammatory mediator | Positive correlates | r 2 | P-value | Negative correlates | r 2 | P-value |
|---|---|---|---|---|---|---|
| MIC-1 | CRP | 0.314 | <0.001 | Albumin | −0.316 | <0.001 |
| mGPS | 0.336 | <0.001 | KPS | −0.269 | <0.001 | |
| Age | 0.332 | <0.001 | ||||
| Tumour grade | 0.234 | 0.002 | ||||
| Stage | 0.217 | <0.001 | ||||
| Diet score | 0.157 | 0.031 | ||||
| CRP | MIC-1 | 0.314 | <0.001 | Albumin | −0.489 | <0.001 |
| Tumour grade | 0.341 | <0.001 | KPS | −0.257 | <0.001 | |
| Stage | 0.220 | <0.001 | ||||
| % Weight loss | 0.247 | <0.001 | ||||
| Diet score | 0.265 | <0.001 | ||||
| mGPS | MIC-1 | 0.336 | <0.001 | MAMC | −0.318 | <0.001 |
| Tumour grade | 0.268 | 0.001 | KPS | −0.362 | <0.001 | |
| Stage | 0.234 | 0.001 | ||||
| % Weight loss | 0.280 | <0.001 | ||||
| Diet score | 0.262 | <0.001 |
Abbreviations: CRP=C-reactive protein; KPS=Karnofsky performance score; MAMC=mid-arm muscle circumference; mGPS=modified Glasgow Prognostic Score; MIC-1=macrophage inhibitory cytokine-1.
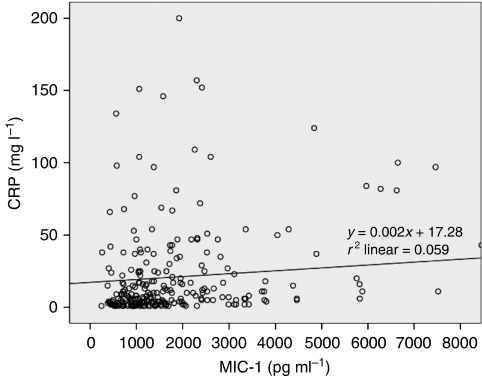
The relationship between MIC-1 and CRP was not linear, as dot-plots showed wide variance of CRP with increasing MIC-1 (Figure 6). However, assuming linearity, regression analysis showed that MIC-1 accounted for 5.9% of the variation in plasma CRP (P<0.001). In a regression model of CRP (incorporating MIC-1, disease stage, tumour grade, percentage weight loss, diet score and KPS, i.e., those factors found to correlate with CRP), plasma MIC-1 concentration (P=0.003), diet score (P=0.001), and tumour grade (P=0.033) were significant determinants (r2=0.179; Table 3). In a regression model of percentage weight loss (incorporating disease stage, mGPS, diet score, and dysphagia score), all four factors were significant determinants (r2=0.259; Table 3). Plasma MIC-1 concentration did not qualify to enter the model.
Figure 6.
Dot plot of MIC-1 vs CRP (linear r2=0.059).
Table 3. Results of the regression models of CRP (r2=0.179) and percentage weight loss (r2=0.259) demonstrating significant determinants.
|
Unstandardised coefficients
|
Standardised coefficients |
95% CI for B
|
||||||
|---|---|---|---|---|---|---|---|---|
| Model | Factor | B | Standard error | β | t | P-value | Lower boundary | Upper boundary |
| CRP | (Constant) | −35.759 | 13.387 | −2.671 | 0.008 | −62.213 | −9.305 | |
| MIC-1 | 0.005 | 0.002 | 0.231 | 3.001 | 0.003 | 0.002 | 0.008 | |
| Diet score | 12.613 | 3.670 | 0.258 | 3.437 | 0.001 | 5.361 | 19.864 | |
| Tumour grade | 9.977 | 4.628 | 0.165 | 2.156 | 0.033 | 0.832 | 19.123 | |
| % Weight loss | (Constant) | −3.788 | 2.107 | −1.798 | 0.074 | −7.946 | 0.370 | |
| Diet score | 2.871 | 1.066 | 0.221 | 2.693 | 0.008 | 0.767 | 4.975 | |
| mGPS | 2.491 | 0.835 | 0.205 | 2.983 | 0.003 | 0.843 | 4.140 | |
| Stage | 1.459 | 0.639 | 0.161 | 2.284 | 0.024 | 0.198 | 2.719 | |
| Dysphagia score | 1.182 | 0.583 | 0.155 | 2.028 | 0.044 | 0.031 | 2.333 | |
Abbreviations: CI=confidence interval; CRP=C-reactive protein; mGPS=modified Glasgow Prognostic Score; MIC-1=macrophage inhibitory cytokine-1.
Relationship of MIC-1 with survival
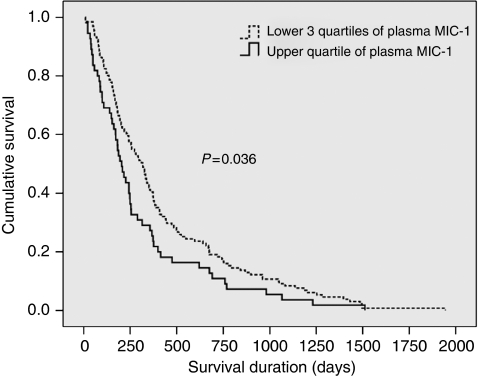
Patients with plasma MIC-1 concentrations in the upper quartile (>2270 pg ml–1) showed worsened survival (median 204 days; 95% CI 157–251) when compared with patients with MIC-1 concentrations in the lower three quartiles (median 316 days; 95% CI 259–373; P=0.036; log-rank test; Figure 7). In Cox's proportional hazards model (incorporating MIC-1, CRP, percentage weight loss, disease stage, tumour grade, patient age, dysphagia score, diet score, and treatment regimen), disease stage (P<0.001), treatment regimen (P=0.003), CRP (P=0.034), and percentage weight loss (P=0.002), but not plasma MIC-1 concentration, were significant determinants of survival (Table 4). Substitution of mGPS for CRP within the model revealed stage, treatment regimen, and weight loss as the only significant determinants.
Figure 7.
Kaplan–Meier plot of survival of oesophago-gastric cancer patients according to plasma MIC-1 concentration. Patients with MIC-1 concentrations in the upper quartile showed worsened survival (median 204 days; 95% CI 157–251) when compared with patients with MIC-1 concentrations in the lower three quartiles (median 316 days; 95% CI 259–373; P=0.036, log-rank test).
Table 4. Results of the Cox's proportional hazards model demonstrating significant determinants of oesophago-gastric cancer patient survival.
|
95% CI
|
||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | d.f. | P-value | Hazard ratio | Lower boundary | Upper boundary | |
| Stage | 0.682 | 0.177 | 14.902 | 1 | <0.001 | 1.979 | 1.399 | 2.798 |
| Treatment regimen | 13.934 | 3 | 0.003 | |||||
| CRP | 0.006 | 0.003 | 4.498 | 1 | 0.034 | 1.006 | 1.000 | 1.012 |
| % Weight loss | 0.038 | 0.013 | 9.232 | 1 | 0.002 | 1.039 | 1.014 | 1.065 |
Abbreviations: CI=confidence interval; CRP=C-reactive protein.
Discussion
This study shows that plasma MIC-1 concentrations are elevated in OGC patients when compared with controls. Furthermore, increasing plasma MIC-1 concentrations are associated with indicators of poor patient prognosis, including tumour grade and stage. Elevated circulating concentrations of MIC-1 have also been associated with poor prognostic indicators in other cancer types, including prostate cancer (Selander et al, 2007; Brown et al, 2009), colorectal cancer (Brown et al, 2003), and glioblastoma (Shnaper et al, 2009).
In this study, plasma MIC-1 also correlated significantly with mGPS and plasma CRP concentration, suggesting that MIC-1 might have a role in the aetiology of systemic inflammation in OGC (although linear regression implies that this may be a minor effect). However, MIC-1 concentrations do not correlate with any measured nutritional or anthropometric parameters, including patient weight loss. In a recent cohort of 220 patients with OGC, we have shown by multiple regression analysis that plasma CRP, stage of disease, and dietary intake are independent variables in determining the degree of weight loss (Deans et al, 2009). These results are confirmed by this study. Given such importance of systemic inflammation, disease stage, and dietary intake in the genesis of weight loss in OGC patients, it is somewhat surprising that although MIC-1 concentrations correlated with all three of these variables in this study, there was no correlation with weight loss. However, MIC-1 levels were slightly elevated in patients with ⩾10% weight loss when compared with patients without, suggesting that MIC-1 may have a role in the maintenance, rather than the initiation, of weight loss.
Studies in mice with xenografted prostate tumours have suggested that the mechanism underlying MIC-1-induced weight loss is hypophagia caused by reduced neuropeptide Y expression and increased pro-opiomelanocortin expression in the hypothalamic arcuate nucleus (Johnen et al, 2007). In patients with OGC, a number of primary and secondary causes of reduced dietary intake are at work simultaneously, including dysphagia (McKernan et al, 2008), early satiety (Davis et al, 2006), chronic nausea (McKernan et al, 2008), alterations in circulating neuroendocrine hormones (e.g., ghrelin (Isomoto et al, 2005) and leptin (Huang et al, 2005; Zhao et al, 2007)), hypogeusia/hyposmia (Plata-Salaman, 2000), and cytokine-induced central anorexia (Plata-Salaman, 2001; Turrin et al, 2004). Assuming that MIC-1 may be a modulator of appetite in humans, the complex constellation of additional factors also controlling food intake may explain why plasma MIC-1 concentrations did not correlate with nutritional status in this study. Future studies of MIC-1 that record accurately calorific intake in OGC patients are required to elucidate the anorectic effect of different factors.
Another proposed mechanism of MIC-1-induced weight loss is through paracrine effects on adipocytes (Ding et al, 2009). Recombinant MIC-1 stimulates adiponectin secretion by human adipocytes, thus potentially negatively regulating body fat mass (Ding et al, 2009). However, in this study, MIC-1 did not correlate with one measure of body fat, namely TSF. Furthermore, previous studies have proven that the relationship between MIC-1 and fat mass is clearly not understood fully, as both obesity and type II diabetes mellitus are associated with increased circulating levels of MIC-1 (Dostálová et al, 2009). However, the latter observation might be consistent with a role for MIC-1 in the aetiology of systemic inflammation.
An alternative hypothesis for explaining the lack of association between plasma MIC-1 and nutritional status in OGC is that circulating MIC-1 concentrations are simply not elevated sufficiently to overcome regulatory mechanisms and induce cachexia. In patients with prostate cancer and cachexia, serum MIC-1 concentrations were significantly higher (mean 12 416±s.d. 10 235 pg ml–1) (Johnen et al, 2007) than those measured in the present cohort of OGC patients. Furthermore, in mice xenografted with prostate tumours overexpressing human MIC-1, only animals with serum concentrations of >8500 pg ml–1 showed clinically significant levels of weight loss (Johnen et al, 2007). Of the OGC patients in this study, only 5 (1.7%) showed plasma concentrations of MIC-1 >8500 pg ml–1. In these individuals, plasma CRP was also elevated (median 58 mg l–1; range 16–92) but there was no significant reduction in BMI (median 30.1 kg m–2; range 21.7–32.0) or increase in weight loss (median 6.5% range 0.0–12.5) compared with the rest of the group. The reasons for low circulating MIC-1 concentrations in OGC patients may include low levels of activation of MIC-1 through p53-dependent mechanisms (Li et al, 2000; Albertoni et al, 2002; Yang et al, 2003), as many oesophago-gastric tumours show p53 deletion, mutation, and loss of heterozygosity (Huang et al, 1992; Renault et al, 1993).
In this study, elevated plasma MIC-1 was associated with worsened survival on univariate but not multivariate analysis. Such findings may vary depending on the definition of normal MIC-1 concentration used. Other studies that have analysed relatively large cohorts of healthy controls have suggested that the upper limit of normal plasma MIC-1 concentration lies between 1070 and 1600 pg ml–1 (Li et al, 2000; Brown et al, 2003; Koopmann et al, 2004, 2006), which is lower than the definition used in this study. However, the present data also suggest that plasma MIC-1 concentration may increase with patient age, thus implying that the normal range is not static. A lack of age-matched healthy controls could be considered a disadvantage in both previous studies and this study.
The variation in plasma MIC-1 concentrations observed between different tumour types, grades, and stages implicates a tumour-specific mechanism in the induction of MIC-1 expression. MIC-1 is often secreted in an unprocessed propeptide form that regulates the balance between extracellular matrix stores and circulating mature MIC-1 (Bauskin et al, 2005). The absence of propeptide in xenograft animal tumour models is associated with a 20-fold increase in serum MIC-1 (Bauskin et al, 2005). In low-grade localised prostate cancer, the level of proMIC-1 stromal stores was the best predictor of future disease relapse when compared with other clinicopathological variables (Bauskin et al, 2005). The mechanisms surrounding the processing and extracellular storage of MIC-1 may represent one explanation for the observed differences in plasma MIC-1 between different tumour variables.
In conclusion, although plasma MIC-1 correlates with tumour grade, disease stage, dietary intake, and systemic inflammation, it does not seem to mediate weight loss or nutritional depletion significantly in OGC.
Acknowledgments
All authors were involved in study conception and design, drafting and revising the manuscript, and final approval of the manuscript. RJES was supported by the Maurice WohI Fellowship from the Royal College of Surgeons of Edinburgh. DAB and SNB were supported by grants from the National Health and Medical Research Council of Australia (NHMRC) and Cancer Council, New South Wales. DAB and SNB declare that St Vincent's Hospital Sydney, Ltd has taken out patents and provisional patents covering some aspects of the area of MIC-1 research. Under this institution's intellectual property policy, should aspects of MIC-1 be commercialised and should financial returns flow, the inventors would receive a share of those returns, in line with the institutional policy.
References
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC (2005) Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8: 421–432 [DOI] [PubMed] [Google Scholar]
- Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME (2002) Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene 21: 4212–4219 [DOI] [PubMed] [Google Scholar]
- Baek KE, Yoon SR, Kim JT, Kim KS, Kang SH, Yang Y, Lim JS, Choi I, Nam MS, Yoon M, Lee HG (2009) Upregulation and secretion of macrophage inhibitory cytokine-1 (MIC-1) in gastric cancers. Clin Chim Acta 401: 128–133 [DOI] [PubMed] [Google Scholar]
- Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, Liu T, Smith D, Kuffner T, Pankhurst GJ, Johnen H, Russell PJ, Barret W, Stricker PD, Grygiel JJ, Kench JG, Henshall SM, Sutherland RL, Breit SN (2005) The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res 65: 2330–2336 [DOI] [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM (1997) MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 94: 11514–11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle GM, Pedley J, Martyn AC, Banducci KJ, Strutton GM, Brown DA, Breit SN, Parsons PG (2009) Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J Invest Dermatol 129: 383–391 [DOI] [PubMed] [Google Scholar]
- Brown DA, Bauskin AR, Fairlie WD, Smith MD, Liu T, Xu N, Breit SN (2002) Antibody-based approach to high-volume genotyping for MIC-1 polymorphism. Biotechniques 33: 118–120, 122, 124 [DOI] [PubMed] [Google Scholar]
- Brown DA, Lindmark F, Stattin P, Bälter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Grönberg H, Breit SN, Wiklund FE (2009) Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res 15(21): 6658–6664. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN (2003) MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res 9: 2642–2650 [PubMed] [Google Scholar]
- Buchanan JB, Johnson RW (2007) Regulation of food intake by inflammatory cytokines in the brain. Neuroendocrinology 86: 183–190 [DOI] [PubMed] [Google Scholar]
- Corish CA, Kennedy NP (2003) Anthropometric measurements from a cross-sectional survey of Irish free-living elderly subjects with smoothed centile curves. Br J Nutr 89: 137–145 [DOI] [PubMed] [Google Scholar]
- Davis MP, Walsh D, Lagman R, Yavuzsen T (2006) Early satiety in cancer patients: a common and important but underrecognized symptom. Support Care Cancer 14: 693–698 [DOI] [PubMed] [Google Scholar]
- Deans DA, Tan BH, Wigmore SJ, Ross JA, de Beaux AC, Paterson-Brown S, Fearon KC (2009) The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 100: 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans DA, Wigmore SJ, Gilmour H, Paterson-Brown S, Ross JA, Fearon KC (2006) Elevated tumour interleukin-1beta is associated with systemic inflammation: a marker of reduced survival in oesophago-gastric cancer. Br J Cancer 95: 1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong CH, Busquets S, Moses AG, Schrauwen P, Ross JA, Argiles JM, Fearon KC (2005) Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep 14: 257–263 [PubMed] [Google Scholar]
- Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C (2009) Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 150: 1688–1696 [DOI] [PubMed] [Google Scholar]
- Dostálová I, Roubícek T, Bártlová M, Mráz M, Lacinová Z, Haluzíková D, Kaválková P, Matoulek M, Kasalicky M, Haluzík M (2009) Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol 161: 397–404 [DOI] [PubMed] [Google Scholar]
- Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN (1999) MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol 65: 2–5 [DOI] [PubMed] [Google Scholar]
- Fairlie WD, Russell PK, Wu WM, Moore AG, Zhang HP, Brown PK, Bauskin AR, Breit SN (2001) Epitope mapping of the transforming growth factor-beta superfamily protein, macrophage inhibitory cytokine-1 (MIC-1): identification of at least five distinct epitope specificities. Biochemistry 40: 65–73 [DOI] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC (1994) Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg 219: 325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC (1995) Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer 75: 2077–2082 [DOI] [PubMed] [Google Scholar]
- Fearon KC, Voss AC, Hustead DS (2006) Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83: 1345–1350 [DOI] [PubMed] [Google Scholar]
- Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, Zeillinger R, Leipold H, Lass H, Grimm C, Tempfer CB, Reinthaller A (2008) Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 14: 710–714 [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang X, Jiang ZW, Liu BZ, Li N, Li JS (2005) Hypoleptinemia in gastric cancer patients: relation to body fat mass, insulin, and growth hormone. JPEN J Parenter Enteral Nutr 29: 229–235 [DOI] [PubMed] [Google Scholar]
- Huang Y, Boynton RF, Blount PL, Silverstein RJ, Yin J, Tong Y, McDaniel TK, Newkirk C, Resau JH, Sridhara R (1992) Loss of heterozygosity involves multiple tumour suppressor genes in human oesophageal cancers. Cancer Res 52: 6525–6530 [PubMed] [Google Scholar]
- Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, Ohnita K, Mizuta Y, Inoue K, Nakazato M, Kohno S (2005) Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci 50: 833–838 [DOI] [PubMed] [Google Scholar]
- Johnen H, Lin S, Kuffner T, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN (2007) Tumour-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med 13: 1333–1340 [DOI] [PubMed] [Google Scholar]
- Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban RH, Breit SN, Kinzler KW, Vogelstein B, Goggins M (2004) Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res 10: 2386–2392 [DOI] [PubMed] [Google Scholar]
- Koopmann J, Rosenzweig CN, Zhang Z, Canto MI, Brown DA, Hunter M, Yeo C, Chan DW, Breit SN, Goggins M (2006) Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res 12: 442–446 [DOI] [PubMed] [Google Scholar]
- Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Terlecki G, Gamian A (2008) Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin Chem Lab Med 46: 359–364 [DOI] [PubMed] [Google Scholar]
- Li PX, Wong J, Ayed A, Brade AM, Arrowsmith C, Austin RC, Klamut HJ (2000) Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumour cells to DNA damage and p53 overexpression. J Biol Chem 275: 20127–20135 [DOI] [PubMed] [Google Scholar]
- McKernan M, McMillan DC, Anderson JR, Angerson WJ, Stuart RC (2008) The relationship between quality of life (EORTC QLQ-C30) and survival in patients with oesophago-gastric cancer. Br J Cancer 98: 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12: 223–226 [DOI] [PubMed] [Google Scholar]
- Moore AG, Brown DA, Fairlie WD, Bauskin AR, Brown PK, Munier ML, Russell PK, Salamonsen LA, Wallace EM, Breit SN (2000) The transforming growth factor-β superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab 85: 4781–4788 [DOI] [PubMed] [Google Scholar]
- O’Riordain MG, Falconer JS, Maingay J, Fearon KC, Ross JA (1999) Peripheral blood cells from weight-losing cancer patients control the hepatic acute phase response by a primarily interleukin-6 dependent mechanism. Int J Oncol 15: 823–827 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR (2000) Central nervous system mechanisms contributing to the cachexia-anorexia syndrome. Nutrition 16: 1009–1012 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR (2001) Cytokines and feeding. Int J Obes Relat Metab Disord 25(Suppl 5): S48–S52 [DOI] [PubMed] [Google Scholar]
- Renault B, van den Broek M, Fodde R, Wijnen J, Pellegata NS, Amadori D, Khan PM, Ranzani GN (1993) Base transitions are the most frequent genetic changes at P53 in gastric cancer. Cancer Res 53: 2614–2617 [PubMed] [Google Scholar]
- Schober A, Bottner M, Strelau J, Kinscherf R, Bonaterra GA, Barth M, Schilling L, Fairlie WD, Breit SN, Unsicker K (2001) Expression of growth differentiation factor-15/ macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J Comp Neurol 439: 32–45 [DOI] [PubMed] [Google Scholar]
- Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R (2002) The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer 87: 264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander KS, Brown DA, Sequeiros GB, Hunter M, Desmond R, Parpala T, Risteli J, Breit SN, Jukkola-Vuorinen A (2007) Serum macrophage inhibitory cytokine-1 concentrations correlate with the presence of prostate cancer bone metastases. Cancer Epidemiol Biomarkers Prev 16: 532–537 [DOI] [PubMed] [Google Scholar]
- Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, Schluep M, Ostermann S, Hamou MF, Stupp R, Breit SN, de Tribolet N, Hegi ME (2009) Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int J Cancer 125: 2624–2630 [DOI] [PubMed] [Google Scholar]
- Sobin LH, Wittekind C (2002) TNM Classification of Malignant Tumours. 6th edn. John Wiley & Sons: Hoboken, NJ [Google Scholar]
- Turrin NP, Ilyin SE, Gayle DA, Plata-Salamán CR, Ramos EJ, Laviano A, Das UN, Inui A, Meguid MM (2004) Interleukin-1beta system in anorectic catabolic tumour-bearing rats. Curr Opin Clin Nutr Metab Care 7: 419–426 [DOI] [PubMed] [Google Scholar]
- Wakchoure S, Swain TM, Hentunen TA, Bauskin AR, Brown DA, Breit SN, Vuopala KS, Harris KW, Selander KS (2009) Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate 69: 652–661 [DOI] [PubMed] [Google Scholar]
- Weinstein PS, Skinner M, Sipe JD, Lokich JJ, Zamcheck N, Cohen AS (1984) Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol 19: 193–198 [DOI] [PubMed] [Google Scholar]
- Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson Jr HF, Hampton GM (2003) Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA 100: 3410–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT (2003) Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther 2: 1023–1029 [PubMed] [Google Scholar]
- Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, Sutherland RL, Henshall SM, Horvath LG (2009) Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res 69: 7696–7703 [DOI] [PubMed] [Google Scholar]
- Zhao X, Huang K, Zhu Z, Chen S, Hu R (2007) Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol 22: 1317–1321 [DOI] [PubMed] [Google Scholar]