Abstract
Background:
This analysis was initiated to define the predictive value of the area under the curve of high-dose methotrexate (AUCHD-MTX) in patients with primary central nervous system lymphoma (PCNSL).
Patients and methods:
We included 55 patients with PCNSL and available pharmacokinetic (PK) data from the International Extranodal Lymphoma Study Group (IELSG) no. 20 trial, randomised to HD-MTX (n=30) or HD-MTX and high-dose cytarabine (HD-AraC) (n=25). Individual AUCHD-MTX from population PK analysis was tested on drug toxicity and clinical outcome using multivariate logistic regression analysis and Cox hazards modelling.
Results:
AUCHD-MTX, the IELSG score and treatment group were significant predictors for treatment response (complete or partial) in the adjusted model. The AUCHD-MTX did not predict toxicity, with the exception of liver toxicity and neutropaenia. A high AUCHD-MTX was associated with better event-free survival (EFS) (P=0.01) and overall survival (OAS) (P=0.02). Both the AUCHD-MTX and the IELSG score were significant predictors of EFS and OAS in the adjusted model, with a hazard ratio of 0.82 and 0.73, respectively, per 100 μmol l−1 h−1 increase in AUCHD-MTX.
Conclusions:
Individualised dosing of HD-MTX might have the potential to improve clinical outcome in patients with PCNSL, even when administered concurrently with HD-AraC. In the future, this could be carried out by using first-cycle PK modelling with determination of potential dose adaptations for later cycles using Bayesian analysis.
Keywords: methotrexate, high-dose chemotherapy, CNS lymphoma, cytarabine, pharmacokinetics
Primary central nervous system lymphomas (PCNSLs) represent 4–6% of extranodal non-Hodgkin's lymphomas, but their incidence in the general population is increasing (Ferreri et al, 2003a). High-dose methotrexate (HD-MTX) is a cornerstone of PCNSL treatment (Reni et al, 1997; Ferreri et al, 2002). Only recently, the International Extranodal Lymphoma Study Group (IELSG) concluded the first randomised study in immunocompetent patients with PCNSL (IELSG no. 20). A significant increase in complete remission rate and event-free survival (EFS) was found when adding high-dose cytarabine (HD-AraC) to HD-MTX (Ferreri et al, 2009). Accordingly, combined HD-MTX/HD-AraC should be seen as the new standard upfront treatment in PCNSL, as it is supported by the best level of evidence available in this disease (Ferreri et al, 2009). Treatment with HD-MTX is hampered by a highly variable pharmacokinetic (PK) behaviour, in part related to renal elimination and the potential for drug interactions (Evans and Christensen, 1985; Thyss et al, 1986; Ferrazzini et al, 1990; Reid et al, 1993; Ronchera et al, 1993; Takeda et al, 2002; Joerger et al, 2006). However, achieving a minimum area under the curve (AUC) of HD-MTX (AUCHD-MTX) might be important for clinical outcome in patients with PCNSL (Ferreri et al, 2004). In this study, we report a PK–pharmacodynamic (PKPD) analysis of HD-MTX in patients enrolled into the IELSG no. 20 trial, to define the predictive value of AUCHD-MTX and to identify clinical and therapeutic variables that could be manipulated to improve MTX efficacy in patients with PCNSL.
Materials and methods
Patient population and treatment
We included 79 immunocompetent patients with PCNSL, randomised to receive either HD-MTX alone (n=40) or HD-MTX with sequential HD-AraC (n=39) from the IELSG no. 20 trial (Ferreri et al, 2009). High-dose MTX was administered at 3.5 g m−2 (0.5 g m−2 in 15 min, followed by 3 g m−2 as a 3 h infusion) on day 1 in both arms, and HD-AraC was administered at 2 g m−2 as a 1 h infusion every 12 h on days 2 and 3 in the combined treatment arm. Radiotherapy was given after chemotherapy in 36 patients, and at progression in 18 patients. Study design and inclusion criteria have been published previously (Ferreri et al, 2009). The determination of MTX serum levels was performed before and immediately after the end of drug infusion, and repeated every 24 h until the MTX serum concentration fell under the threshold concentration of 0.05 μmol l−1. The concentration data of MTX – collected at 0, 24, 48, 72 and 96 h from drug infusion – during the first course of chemotherapy were considered for PK analysis. Leucovorin rescue started 24 h after the start of HD-MTX infusion, administered at a dose of 15 mg m−2 intravenous push every 6 h for 12 times or more until MTX serum levels were undetectable. After 48 h from MTX infusion, leucovorin rescue was modified according to MTX serum levels.
Population PK analysis
Population PK analysis was performed using the nonlinear mixed-effects modelling program (NONMEM) version VI (double precision, level 1.1) (Beal and Sheiner, 1998). First, a basic PK model was developed for MTX concentration–time data. Model selection was based on the minimum value of objective function, the precision of parameter estimates and the fit of the model to the data as approached by graphical plots. Inter-individual variability was estimated using a proportional error model. Second, the following covariates were tested for their relationship with CLMTX: patient gender, age, body-surface area (BSA), creatinine clearance (CLCREA according to the Cockroft–Gault formula, assessed before the start of MTX infusion and capped at 140 ml min−1), as well as co-medication with anticonvulsant drugs and steroids. Forward selection and backward elimination were used for covariate testing, with a significance level of P<0.01.
Toxicity and response assessment
Adverse events were separately assessed for each chemotherapy course and graded according to the NCI-NCIC CTC version 3.0 (Trotti et al, 2003). The worst toxicity per organ and per patient was considered for analysis. Treatment response was assessed on contrast-enhanced brain MRI performed within 7 days before chemotherapy and repeated after the second and fourth treatment cycle and after WBRT. Response definition was based on changes in tumour size of enhanced lesions on T1-weighted MRI, and following the NCI standardised response criteria (Cheson et al, 1999). In cases with concomitant positive CSF, cytology examination was performed after the second and fourth treatment cycle and after treatment completion. A reduction of >50% of cell number was considered PR, whereas a lower reduction was considered SD. The maximum response recorded from treatment start was considered for activity analyses. Follow-up examinations were conducted as reported previously (Ferreri et al, 2003a).
Statistical analysis
Individual AUCHD-MTX was compared between treatment groups, patient gender and treatment response using Student's t-test. Patients were categorised into those having no response to chemotherapy (SD, PD) and responders (CR or PR). Analysis of variance (ANOVA) was used to compare individual AUCHD-MTX with treatment-associated toxicity. To assess any potential relationship between AUCHD-MTX and clinical outcome, the former was categorised into tertiles, with the higher tertile corresponding to an AUC >980 μmol l−1 h and the lower tertile corresponding to an AUC <830 μmol l−1 h. Both EFS and overall survival (OAS) were calculated per AUCHD-MTX category using survival analysis and log-rank test, respectively. The following potential predictors for chemotherapy response were studied using multivariate logistic regression analysis: AUCHD-MTX (tertiles), gender, categorical IELSG prognostic score (Ferreri et al, 2003b) (0–1, 2–3, 4–5 points) and treatment group. The following potential predictors for clinical outcome (EFS, OAS) were studied using multivariate Cox hazards modelling: gender, IELSG score, treatment group and AUCHD-MTX. Both EFS and OAS curves were calculated using the Kaplan–Meier method, and the log-rank test was used to detect potential differences per AUCHD-MTX category. A previously described threshold of AUCHD-MTX ⩾1100 μmol l−1 h (Ferreri et al, 2004) was additionally analysed on chemotherapy response and clinical outcome. All tests of significance were two-sided; P<0.05 was considered significant. All statistical analyses were performed using STATA 10.1 software (STATA Corp, College Station, TX, USA).
Results
Patient population and data set
Patient characteristics have been described previously (Ferreri et al, 2009). Out of the 79 patients, 55 had available PK data of HD-MTX and were included into this analysis with the following characteristics: median age 56 years, 32 female (58%) and 23 male (42%); 30 patients were randomised to receive HD-MTX and 25 patients to combined HD-MTX/HD-AraC, with a median IELSG-score of 2. Patient characteristics of the PKPD subgroup and of the total population were not significantly different. After chemotherapy, 7 HD-MTX and 18 HD-MTX/HD-AraC patients achieved a CR (18 vs 46% P=0.006); 9 MTX and 9 MTX/AraC patients achieved a PR, for an ORR of 40 and 69%, respectively (P=0.009). At a median follow-up of 30 months, 31 MTX and 23 MTX/AraC patients experienced failure (PD, relapse, death), with a 3-year EFS of 21 and 38%, respectively (P=0.01). In all, 12 MTX and 20 MTX/AraC patients are alive, with a 3-year OAS of 32 and 46%, respectively (P=0.07).
Population PK model
The MTX concentration–time data were best described by a linear two-compartment model with first-order elimination from the central compartment. The MTX clearance was 14.9 l h−1 (relative s.e. 9.95), with an inter-individual variability of 22.3% and a residual variability of 31.8%. Volume distribution was 71.9 l (±51.5), with an inter-individual variability of 30.1%. Inter-compartmental clearance was 11.2 l h−1 (±5.2), with an inter-individual variability of 35%. Two patients had a CLHD-MTX >20 l h−1, six patients <7 l h−1. Median AUCHD-MTX was 931 μmol*l−1 h (range 486–1710 μmol*l−1 h). The AUCHD-MTX was <750 μmol l−1 h in 11 out of 55 cases with available PK data (20%), and >1100 μmol l−1 h in 12 cases (22%). Creatinine clearance was the only significant covariate on CLHD-MTX, resulting in Equation 1 to describe CLHD-MTX as a function of CLCREA (95 ml min−1 is the median CLCREA as found in the study group):
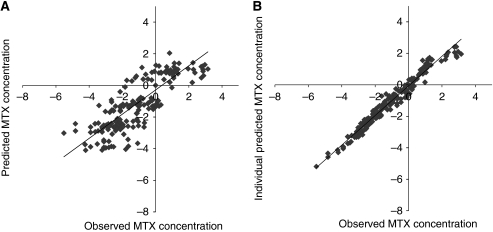
The inclusion of patient age, BSA or concurrent administration of HD-AraC did not improve the model fit. The goodness-of-fit plots supported a good data fit of the final model (Figure 1).
Figure 1.
Goodness-of-fit plots of the final population pharmacokinetic model (all data log-transformed, drug concentration as μmol l−1). Observed MTX concentrations vs model predictions (A) and vs individual Bayesian predictions (B).
Statistical analysis
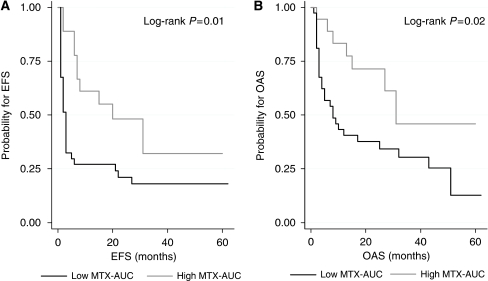
The AUCHD-MTX tertiles are outlined across chemotherapy response and clinical outcome in Table 1. The AUCHD-MTX was not significantly different between treatment groups (902 μmol*l−1 h in the HD-MTX group, 965 μmol*l−1 h in the HD-MTX/HD-AraC group, P=0.16). The AUCHD-MTX was significantly higher in the 29 responding patients compared with the 26 cases without chemotherapy response (1075 vs 867 μmol*l−1 h, P=0.0001). Predictors of a favourable treatment response are outlined in Table 2. Patients with AUCHD-MTX >1100 μmol*l−1 h had an odds ratio of 3.5 for having a favourable treatment response (P=0.03). There was no significant relationship between AUCHD-MTX and toxicity, with the exception of liver dysfunction (AUCHD-MTX 1047 μmol*l−1 h in patients with any treatment-associated liver dysfunction vs 932 μmol*l−1 h in those without liver dysfunction, P=0.02) and neutropaenia (AUCHD-MTX 1036 μmol*l−1 h in patients with grade 3 or 4 neutropaenia vs 914 μmol*l−1 h in those with no or grade 1 or 2 neutropaenia, P=0.007). Patients with the highest AUCHD-MTX exhibited a significantly better EFS and OAS as compared with patients in the lower two tertiles of AUCHD-MTX (Table 2 and Figure 2). The AUCHD-MTX >1100 μmol*l−1 h was associated with a better EFS and OAS by the log-rank test (P=0.023 and P=0.056, respectively). Both the AUCHD-MTX and the IELSG score were significant predictors of EFS and OAS using multivariate Cox regression analysis (Table 3). When AUCHD-MTX >1100 μmol*l−1 h was introduced into Cox regression analysis as a binary covariate, statistical significance was retained (HR=0.51, P=0.033 for EFS, HR=0.50, P=0.044 for OAS). No association was found between the volume of distribution, inter-compartmental clearance and any of the clinical end points.
Table 1. Comparison between AUCHD-MTX tertiles, chemotherapy response and clinical outcome.
|
AUClow (<830 μmol l−1 h)
|
AUCmid (830–980 μmol l−1 h)
|
AUChigh (>980 μmol l−1 h)
|
|||||
|---|---|---|---|---|---|---|---|
| Covariate | Pts (%) | OR (95% CI) | Pts (%) | OR (95% CI) | Pts (%) | OR (95% CI) | P-value |
| ORR | |||||||
| SD/PD | 14 (54) | Ref | 10 (39) | Ref | 2 (7) | Ref | |
| CR/PR | 5 (17) | 0.18 (0.05–0.61) | 8 (28) | 0.61 (0.19–1.89) | 16 (55) | 14.7 (2.93–74.4) | <0.001a |
| Pts (%) | % | Pts (%) | % | Pts (%) | % | ||
| Outcome | |||||||
| 3-Year EFS | 19 (34) | 21.1 | 18 (33) | 20.8 | 18 (33) | 32.1 | 0.04b |
| 3-Year OAS | 19 (34) | 30.7 | 18 (33) | 30.5 | 18 (33) | 46.0 | 0.06b |
Abbreviations: AUCHD-MTX=area under the curve of high-dose methotrexate; Pt=patient; OR=odds ratio; CI=confidence interval; ORR=objective response rate; SD=stable disease; PD=progressive disease; CR=complete response; PR=partial response; Ref=reference; EFS=event-free survival; OAS=overall survival.
Analysis of variance.
log-rank test.
Table 2. Predictors for chemotherapy response (complete and partial remission) using multivariate regression modeling.
| Covariate | OR | 95% CI | P-value |
|---|---|---|---|
| Patient gender | |||
| Male | Ref | ||
| Female | 0.48 | 0.08–2.92 | 0.42 |
| IELSG risk score | |||
| 0–1 | Ref | ||
| 2–3 | 0.05 | 0.005–0.44 | 0.007 |
| 4–5 | 0.03 | 0.002–0.48 | 0.01 |
| Treatment group | |||
| HD-MTX | Ref | ||
| HD-MTX/HD-AraC | 9.33 | 1.33–65.53 | 0.02 |
| AUCHD-MTX (tertiles, μmol l−1 h−1) | |||
| <830 | Ref | ||
| 830–980 | 5.21 | 0.73–37.3 | 0.10 |
| >980 | 121.9 | 7.80–190.1 | 0.001 |
Abbreviations: OR=odds ratio; CI=confidence interval; IELSG=International Extranodal Lymphoma Study Group; HD-MTX=high-dose methotrexate; HD-AraC=high-dose cytarabine; AUC=area under the curve; Ref=reference.
Figure 2.
Kaplan–Meier plots for event-free survival (A) and overall survival (B) grouped according to the highest AUCHD-MTX tertile (>980 μmol*l−1 h) and the lower two tertiles of AUCHD-MTX (<980 μmol*l−1 h).
Table 3. Predictors for event-free and overall survival using multivariate Cox regression analysis.
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Event-free survival | |||
| Patient gender | |||
| Female | Ref | ||
| Male | 1.12 | 0.52–2.40 | 0.77 |
| IELSG score | |||
| 0–1 vs 2–3 vs 4–5 points | 1.71 | 1.04–2.81 | 0.03 |
| Treatment group | |||
| HD-MTX | Ref | ||
| HD-MTX/AraC | 0.65 | 0.34–1.25 | 0.19 |
| AUCHD-MTX | |||
| Per 100 μmol l−1 h increase | 0.82 | 0.69–0.98 | 0.03 |
| Overall survival | |||
| Patient gender | |||
| Female | Ref | ||
| Male | 1.77 | 0.76–4.10 | 0.19 |
| IELSG score | |||
| 0–1 vs 2–3 vs 4–5 points | 1.82 | 1.00–3.31 | 0.05 |
| Treatment group | |||
| HD-MTX | Ref | ||
| HD-MTX/AraC | 0.80 | 0.39–1.65 | 0.54 |
| AUCHD-MTX | |||
| Per 100 μmol l−1 h increase | 0.73 | 0.59–0.89 | 0.002 |
Abbreviations: HR=hazard ratio; CI=confidence interval; IELSG=International Extranodal Lymphoma Study Group; HD-MTX=high-dose methotrexate; HD-AraC=high-dose cytarabine; AUC=area under the curve; Ref=reference.
Discussion
This PKPD analysis of HD-MTX in patients from the IELSG no. 20 trial is of special value, as this is the first prospective, randomised trial in PCNSL with completed accrual (Ferreri et al, 2003a). This study shows that AUCHD-MTX is the most important and independent predictor of clinical outcome in this group of patients. This is an important issue considering the fact that the HD-MTX/HD-AraC combination is the new standard therapeutic approach to patients with PCNSL (Ferreri et al, 2009). Interestingly, this study showed that nearly 75% of patients did not achieve an AUCHD-MTX >1100 μmol*l−1 h, which has been previously reported as an independent predictor for improved clinical outcome (Ferreri et al, 2004). The present results cannot be interpreted as a lack of benefit from combined HD-MTX/HD-AraC treatment, as not all patients had available PK data for HD-MTX, and combined HD-MTX/HD-AraC treatment was still a significant predictor for clinical outcome when AUCHD-MTX was dropped from the Cox model. It still indicates that inter-individual disparities in HD-MTX pharmacology have an important role in clinical outcome, and that optimising individual AUCHD-MTX is an important strategy for improving clinical outcome in PCNSL. Thus, the encouraging results of the IELSG no. 20 trial (Ferreri et al, 2003a) might be further improved by individualised MTX administration aimed to achieve a target AUCHD-MTX of 1000 μmol*l−1 h. This statement is also endorsed by the fact that there was no relevant impact of AUCHD-MTX on drug toxicity. The strengths of this study include a homogeneous patient population, the availability of detailed response, outcome and toxicity data in all patients, first-course PK data of MTX in most patients, as well as population PKPD analysis of HD-MTX time–concentration data. The main limitations of this study are that drug interactions between HD-MTX and HD-AraC could only indirectly be studied because no PK data of HD-AraC were available, and PK data of HD-MTX from later courses were not available. However, the fact that tumour response was usually seen within the first two courses of chemotherapy (Ferreri et al, 2009) does suggest a strong correlation between first-course PK data and clinical outcome.
In a retrospective study (Ferreri et al, 2004), PCNSL patients treated with MTX-based chemotherapy and an AUCHD-MTX >1100 μmol*l−1 h showed significantly better response and survival rates. In the IELSG no. 20 trial, only 22% of patients achieved this AUCHD-MTX, suggesting room for improving HD-MTX administration. Importantly, a suboptimal AUCHD-MTX was obtained equally in both treatment arms in the IELSG no. 20 trial. Therefore, the introduction of a personalised dose of HD-MTX, according to patient age, gender and CLCREA, has the potential to significantly improve treatment activity in these patients, and should be investigated in future trials. According to the present observation, personalisation of the MTX administration schedule should not consider the concomitant use of HD-araC, as the addition of this drug did not change MTX PK. However, this has to be taken with caution, considering the potential for increased toxicity with the combined use of HD-MTX/HD-AraC.
In conclusion, individualised dosing of HD-MTX might have the potential to improve clinical outcome in patients with PCNSL, even when administered concurrently with HD-AraC. In the future, this could be carried out by using first-cycle PK modelling with determination of potential dose adaptations for later cycles using Bayesian analysis.
Acknowledgments
We thank all co-investigators who were actively involved in patient accrual, central radiology review and central pathology review: Marco Foppoli, Maurizio Martelli, Marina Siakantaris, Gerasimos Pangalis, Maurizio Frezzato, Maria Giuseppina Cabras, Alberto Fabbri, Gaetano Corazzelli, Fiorella Ilariucci, Giuseppe Rossi, Riccardo Soffietti, Caterina Stelitano, Daniele Vallisa, Francesco Zaja, Marta Gelemur, Gian Marco Aondio, Giuseppe Avvisati, Monica Balzarotti, Alba Brandes, Maria Joao Costa, Henry Gomez, Attilio Guarini, Graziella Pinotti, Luigi Rigacci, Catrina Uhlmann, Maria AngelopoulouAlberto Franzin, Nicoletta Anzalone, Maurilio Ponzoni, Federico Caligaris-Cappio. We also acknowledge Mrs Cristina Morinini, Mrs Oxanna López, Mrs Monica Bertini, and Mrs Elena Porro (The International Extranodal Lymphoma Study Group, Bellinzona, Switzerland).
References
- Beal SL, Sheiner LB (1998) NONMEM User's Guide. NONMEM Project Group, University of San Francisco: San Francisco, CA [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17(4): 1244. [DOI] [PubMed] [Google Scholar]
- Evans WE, Christensen ML (1985) Drug interactions with methotrexate. J Rheumatol 12(Suppl 12): 15–20 [PubMed] [Google Scholar]
- Ferrazzini G, Klein J, Sulh H, Chung D, Griesbrecht E, Koren G (1990) Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. J Pediatr 117(5): 823–826 [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Abrey LE, Blay JY, Borisch B, Hochman J, Neuwelt EA, Yahalom J, Zucca E, Cavalli F, Armitage J, Batchelor T (2003a) Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol 21(12): 2407–2414, doi:10.1200/JCO.2003.01.135 [doi]JCO.2003.01.135 [pii] [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F (2003b) Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 21(2): 266–272 [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Guerra E, Regazzi M, Pasini F, Ambrosetti A, Pivnik A, Gubkin A, Calderoni A, Spina M, Brandes A, Ferrarese F, Rognone A, Govi S, Dell’Oro S, Locatelli M, Villa E, Reni M (2004) Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer 90(2): 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, Aondio GM, Ferrarese F, Gomez H, Ponzoni M, Borisch B, Berger F, Chassagne C, Iuzzolino P, Carbone A, Weis J, Pedrinis E, Motta T, Jouvet A, Barbui T, Cavalli F, Blay JY (2002) A multicenter study of treatment of primary CNS lymphoma. Neurology 58(10): 1513–1520 [DOI] [PubMed] [Google Scholar]
- Ferreri AJM, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabra MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Gelemur M, Aondio GM, Avvisati G, Balzarotti M, Brandes A, Costa MJ, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Angelopoulou M, Tirindelli M, Naso V, Franzin A, Anzalone N, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F (2009) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomized phase 2 trial. Lancet 374(9700): 1512–1520, doi:10.1016/S0140-6736(09)61416-1 [DOI] [PubMed] [Google Scholar]
- Joerger M, Huitema AD, van den Bongard HJ, Baas P, Schornagel JH, Schellens JH, Beijnen JH (2006) Determinants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patients. Br J Clin Pharmacol 62(1): 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T, Yuen A, Catolico M, Carlson RW (1993) Impact of omeprazole on the plasma clearance of methotrexate. Cancer Chemother Pharmacol 33(1): 82–84 [DOI] [PubMed] [Google Scholar]
- Reni M, Ferreri AJ, Garancini MP, Villa E (1997) Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol 8(3): 227–234 [DOI] [PubMed] [Google Scholar]
- Ronchera CL, Hernandez T, Peris JE, Torres F, Granero L, Jimenez NV, Pla JM (1993) Pharmacokinetic interaction between high-dose methotrexate and amoxycillin. Ther Drug Monit 15(5): 375–379 [DOI] [PubMed] [Google Scholar]
- Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H (2002) Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther 302(2): 666–671 [DOI] [PubMed] [Google Scholar]
- Thyss A, Milano G, Kubar J, Namer M, Schneider M (1986) Clinical and pharmacokinetic evidence of a life-threatening interaction between methotrexate and ketoprofen. Lancet 1(8475): 256–258 [DOI] [PubMed] [Google Scholar]
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3): 176–181, doi:S1053-4296(03)00031-6 [pii]10.1016/S1053-4296(03)00031-6 [doi] [DOI] [PubMed] [Google Scholar]