Abstract

The biomimetic total syntheses of both malbrancheamide and malbrancheamide B are reported. The synthesis of the two mono-chloro species enabled the structure of malbrancheamide B to be unambiguously assigned. The syntheses each feature an intramolecular Diels-Alder reaction of a 5-hydroxypyrazin-2(1H)-one to construct the bicyclo[2.2.2]diazaoctane core, which has also been proposed as the biosynthetic route to these compounds.
Introduction
Our research group has exhibited a longstanding interest in the synthesis and biosynthetic study of a number of unique prenylated indole alkaloids containing a characteristic bicyclo[2.2.2]diazaoctane core.1 This class of natural products includes such highly biologically active fungal metabolites as the paraherquamides,2 brevianamides,3 notamides,4 and stephacidins,5 among others which we have shown all arise biogenetically from tryptophan, mevalonate-derived isoprene units, and proline or derivatives of proline.1 Sammes originally proposed that the bicyclo[2.2.2]diazaoctane core common to all of these natural products arises in Nature via an intramolecular hetero-Diels-Alder reaction of a 5-hydroxypyrazin-2(1H)-one,6 and work from this laboratory has extensively supported such a proposal.1 In fact, we have applied such a [4+2] hetero-Diels–Alder cycloaddition strategy to the total synthesis of several of these prenylated indole alkaloids, including stephacidin A,7 brevianamide B,8 marcfortine C,9 notoamide B,7b and VM55599.10
Malbrancheamide (1)11 and malbrancheamide B (2) were recently isolated from Malbranchea aurantiaca RRC1813, a fungus collected on bat detritus collected in a cave in Mexico by Mata and coworkers. These new substances are the first alkaloids in this class of prenylated indole alkaloids to contain a halogenated indole ring (Figure 1). The lack of a tertiary amide in the bicyclo[2.2.2]diazaoctane core also serves to characterize the malbrancheamides. In addition to these notable structural features, malbrancheamide has been shown to be a calmodulin (CaM) antagonist that inhibits the activity of CaM-dependent phosphodiesterase (PDE1) in a concentration dependent manner.11 The chemotherapeutic potential of PDE1 inhibitors includes applications in the treatment of neurodegenerative diseases, cancers, and vascular diseases, due to the effect on intracellular cAMP and cGMP concentrations.12 New pharmacological properties of malbrancheamide may be discovered through the study of malbrancheamide, malbrancheamide B, and other analogs, as specific PDE1 inhibitors are scarce and the exact function of the enzyme has not been fully characterized.
Figure 1.
Malbrancheamide and Malbrancheamide B
While compelling spectroscopic evidence indicated that the structure of 1 was as shown,11 the precise structure of malbrancheamide B (2) was less certain. Isolation and structural characterization of 2 indicated the presence of a single chlorine on the indole ring, and further, preliminary biosynthetic experiments indicated that malbrancheamide B (2) is a putative biosynthetic precursor to 1,13 which is thought to arise by sequential halogenation events. However, the question as to whether malbrancheamide B (2) was constituted as the 5-chloro- or the 6-chloro-derivative was unclear from the preliminary characterization data due to the scarce supply of the natural material. With these issues at the forefront, we undertook the synthesis of both natural substances to determine the exact identity of malbrancheamide B. To this end, we envisioned that malbrancheamide (1) and malbrancheamide B (2) would arise from the aforementioned hetero-IMDA of the 5-hydroxypyrazin-2(1H)-one 3, which we could access by enolization and tautomerization of the enamide 4 (Scheme 1). Using chemistry previously established in our laboratory,7a we planned on assembling the enamide 4 from a reverse prenylated tryptophan 5, which could be obtained in a few steps from the corresponding chlorinated indole 6.
SCHEME 1.
Retrosynthetic plan.
Results and Discussion
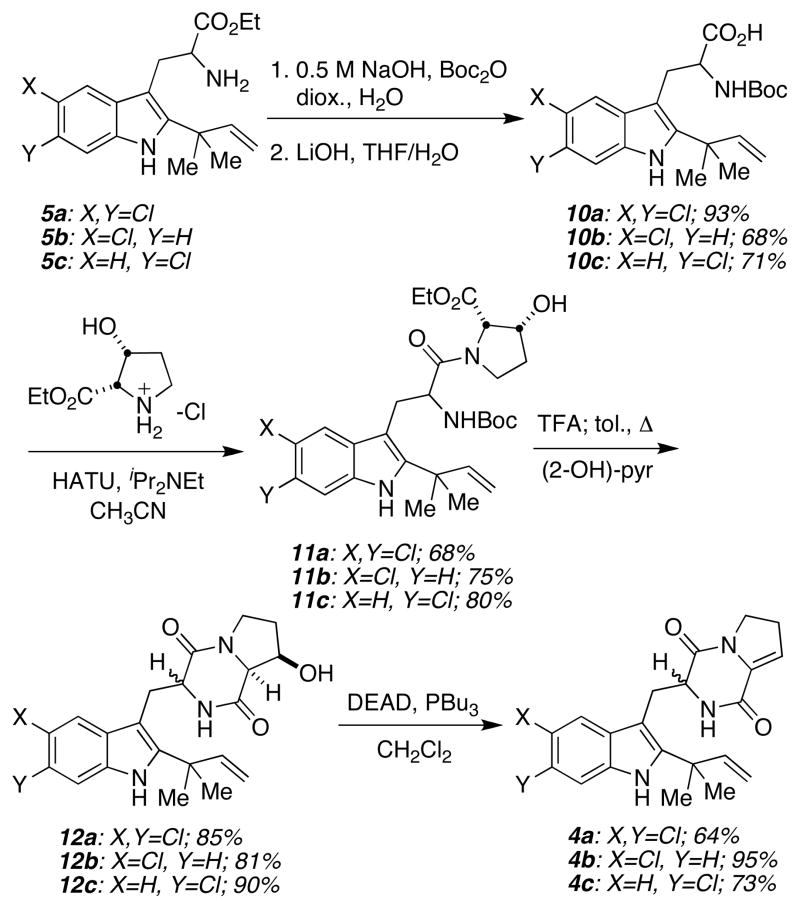
Installation of the reverse prenyl group at the indole 2-position was carried out using a two-step protocol developed by Danishefsky and co-workers.14 Chlorination at the 3-position of indoles 6a–c15 using NCS in DMF gave 7a–c (Scheme 1), which were treated with prenyl-9-BBN in the presence of Et3N to afford the reverse prenylated indoles 8a–c.14 The corresponding gramines 9a–c were prepared by treating 8a–c with formaldehyde and dimethylamine, and subsequent Somei-Kametani coupling16 and imine hydrolysis gave the tryptophan derivatives 5a–c in good yields.
The free amine moieties in 5a–c were protected as the corresponding BOC-carbamates followed by ester hydrolysis under standard conditions to yield acids 10a–c (Scheme 2). Coupling of cis-3-hydroxyproline ethyl ester with the tryptophan derivatives 10a–c in the presence of HATU delivered the amides 11a–c as inseparable mixtures of diastereomers. Treatment of 11a–c with TFA led to carbamate deprotection and the resulting amino esters were immediately cyclized to the corresponding diketopiperazines 12a–c after refluxing with 2-hydroxypyridine. Dehydration of 12a–c under Mitsunobu conditions gave the enamides 4a–c, which would serve as the respective IMDA substrates.17
SCHEME 2.
Reverse Prenylated Tryptophan derivatives.
Treatment of the enamides 4a–c with aqueous KOH in MeOH gave intermediate hydroxy-azadienes by enolization and tautomerizaion, and subsequent IMDA gave mixtures of 13a–c and 14a–c favoring the desired syn-isomers 13a–c in ratios of 2~1.6:1. The observed preference for the IMDA to provide the syn-isomers 13a–c as the major products mirrors the preference we have noted for this cycloaddition in the past.7–9 Considering that previous optimization efforts to improve the syn:anti ratio in related IMDAs were not productive when a variety of solvents and temperatures were studied, we elected to simply separate the major isomers 13a–c and continue the total syntheses without further optimization. 7–9
Completion of the syntheses required selective reduction of the tertiary amide in the presence of the secondary amide, and to that end, 13a–c were treated with excess DIBAL-H18 which cleanly provided malbrancheamide (1) from 12a (80%) and malbrancheamide B (2) from 12c (74%). Synthetic 1 was identical in all respects (1H, 13C, HRMS) to the natural product.11 Comparison of the 1H-NMR spectrum of 15, the 6-chloro derivative, to that of natural malbrancheamide B revealed significant differences in the aromatic region revealing that the correct structure contained a 6-chloroindole ring. Gratifyingly, synthetic 2 was identical in all respects (1H, HRMS) to natural malbrancheamide B.
It is striking that the initial halogenation of the indole ring during the biosynthesis of malbrancheamide B, occurs at the less-activated 6-position as opposed to the more electron-rich 5-position. Studies to clone and express the putative halogenase from M. aurantiaca are currently under investigation in these laboratories.
In summary, the first total synthesis of malbrancheamide (1) and malbrancheamide B (2) have been completed in twelve steps in 5.3% and 8.2% overall yield respectively. In addition, the structure of malbrancheamide B (2) was confirmed though the synthesis of both the 5-chloro- and the 6-chloro-regioisomers. Experiments to establish the biosynthetic relationship between 1 and 2 and their putative progenitors are in progress and will be reported in due course.
Experimental Section
Representative Procedure for the Hetero-Diels-Alder Reaction of Enamides 4
Preparation of Cycloadducts 13a and 14a
To a solution of 4a (86 mg, 0.21 mmol) in MeOH (16 mL) at 0 °C was added 20% aqueous KOH (4 mL). The reaction was warmed to rt and was stirred for 12 h. The reaction was quenched with sat. NH4Cl (30 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure. The residue was triturated with CHCl3 (30 mL) and the suspension was filtered to provide 53 mg (60%) of 13a as a white amorphous solid. Concentration of the filtrate gave 25 mg (29%) of 14a as a white amorphous solid; data for major isomer 13a: 1H NMR (300 MHz, CD3OD) δ 7.55 (s, 1 H), 7.40 (s, 1 H), 3.57 (d, J = 15.5 Hz, 1 H), 3.47 (m, 1 H), 2.74 (d, J = 15.5 Hz, 1 H), 2.60 (m, 1 H), 2.20–1.96 (comp, 6 H), 1.35 (s, 3 H), 1.11 (s, 3 H); 13C NMR (75 MHz, CD3OD) δ 175.7, 171.3, 144.3, 137.2, 128.1, 125.4, 123.3, 119.8, 113.1, 104.8, 68.3, 61.6, 50.8, 45.2, 36.2, 31.7, 30.1, 28.6, 25.4, 24.9, 22.3; IR (neat) 1663, 1428 cm−1; HRMS (TOF+) calcd for C21H22N3O2Cl2 (M+H) 418.1084, found 418.1084; data for minor isomer 14a: 1H NMR (300 MHz, CDCl3) δ 9.78 (s, 1 H), 7.49 (s, 1 H), 7.33 (s, 1 H), 3.71 (d, J = 17.8 Hz, 1 H), 3.47 (comp, 2 H), 3.25 (s, 1 H), 2.78 (d, J = 17.8 Hz, 1 H), 2.67 (m, 1 H), 2.18–1.78 (comp, 6 H), 1.24 (s, 3H), 1.16 (s, 3 H); 13C NMR (75 MHz, CD3OD/CDCl3 (1:9)) δ 173.7, 169.6, 142.6, 135.7, 127.1, 125.0, 122.9, 119.1, 112.4, 102.9 67.2, 61.5, 45.8, 44.3, 34.8, 32.6, 29.8, 29.1, 28.4, 24.5, 23.2; IR (neat) 1676, 1453 cm−1; HRMS (TOF+) calcd for C21H22N3O2Cl2 (M+H) 418.1084, found 418.1079.
Cycloadducts 13b and 14b
Prepared from 4b in to give 43% of 13b as a white amorphous solid and 27% of 14b as a white amorphous solid according to the representative procedure described above for 13a and 14a; data for major isomer 13b: 1H NMR (300 MHz, CD3OD) δ 7.54 (s, 1 H), 7.44 (s, 1 H), 7.22 (d, J = 8.6 Hz, 1 H), 7.04 (d, J = 8.6 Hz, 1 H), 3.68 (d, J = 15.4 Hz, 1 H), 3.55–3.40 (comp, 2 H), 2.77 (d, J = 15.4 Hz, 1 H), 2.76 (m, 1 H), 2.61 (m, 1 H), 2.25–1.92 (comp, 5 H), 1.37 (s, 3 H), 1.12 (s, 3 H); 13C NMR (100 MHz, CD3OD) δ 175.8, 171.4, 143.4, 136.8, 129.2, 125.2, 122.1, 118.2, 112.8, 104.5, 68.3, 61.6, 50.8, 45.2, 36.2, 31.7, 30.1, 28.7, 25.5, 25.0, 22.4; IR (neat) 1678, 1441 cm−1; HRMS (TOF+) calcd for C21H23N3O2Cl (M+H) 384.1473, found 384.1460; data for minor isomer 14b: 1H NMR (300 MHz, CD3OD) δ 7.91 (s, 1 H), 7.41 (d, J = 2.0 Hz, 1 H), 7.23 (d, J = 8.6 Hz, 1 H), 7.01 (dd, J = 8.6, 2.0 Hz, 1H), 3.71 (d, J = 17.6 Hz, 1H), 3.53 (comp, 2 H), 2.90 (d, J = 17.6 Hz, 1 H), 2.70 (m, 1 H), 2.23–1.89 (comp, 6 H), 1.34 (s, 3 H), 1.27 (s, 3 H); 13C NMR (100 MHz, CD3OD) δ 175.4, 171.7, 143.4, 136.8, 129.7, 125.3, 122.1, 118.1, 112.8, 103.8, 68.6, 62.7, 47.3, 45.2, 35.9, 33.3, 30.8, 29.9, 28.7, 25.4, 24.8, 23.8; IR (neat) 1675, 1461 cm−1; HRMS (TOF+) calcd for C21H23N3O2Cl (M+H) 384.1473, found 384.1468.
Cycloadducts 13c and 14c
Prepared from 4c in to give 55% of 13c as a white amorphous solid and 31% of 14c as a white amorphous solid according to the representative procedure described above for 13a and 14a; data for major isomer 13c: 1H NMR (400 MHz, DMSO-d6) δ 8.75 (s, 1 H), 7.40–6.98 (comp, 3 H), 3.42 (d, J = 15.3 Hz, 1 H), 3.30 (comp, 2 H), 2.68 (d, J = 15.3 Hz, 1 H), 2.50 (comp, 1 H), 2.10–1.70 (comp, 6 H), 1.27 (s, 3 H), 0.99 (s, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 173.0, 168.4, 142.0, 136.8, 125.3, 125.2, 118.9, 118.5, 110.4, 103.8, 66.0, 59.6, 48.9, 43.6, 34.6, 30.0, 28.7, 27.9, 24.0, 23.7, 21.6; IR (neat) 1671, 1409 cm−1; HRMS (TOF+) calcd for C21H23N3O2Cl (M+H) 384.1473, found 384.1470; data for minor isomer 14c: 1H NMR (400 MHz, CD3OD/CDCl3 (1:9)) δ 9.58 (s, 1 H), 7.33 (d, J = 8.4 Hz, 1 H), 7.23 (d, J = 1.8 Hz, 1 H), 6.97 (dd, J = 8.4, 1.8 Hz, 1 H), 3.75 (d, J = 17.8 Hz, 1 H), 3.47 (comp, 2 H), 3.30 (bs, 1 H), 2.84 (d, J = 17.8 Hz, 1 H), 2.70 (m, 1 H), 2.23–1.77 (comp, 6 H), 1.25 (s, 3 H), 1.18 (s, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 172.43, 169.0, 142.0, 136.8, 125.8, 125.3, 119.0, 118.5, 110.4, 103.1, 79.2, 66.4, 60.5, 45.4, 43.7, 34.2, 31.6, 28.6, 27.7, 24.0, 22.5; IR (neat) 1672, 1410 cm−1; HRMS (TOF+) calcd for C21H23N3O2Cl (M+H) 384.1473, found 384.1468.
Representative Procedure for the Selective Reduction of Tertiary Amides with Excess DIBAL-H
Synthesis of Malbrancheamide (1)
DIBAL-H (0.70 mL, 1 M in toluene, 0.70 mmol) was added to a suspension of 13a (15 mg, 0.036 mmol) in toluene (7 mL) at rt. The reaction was stirred at rt for 12 h, whereupon finely powdered Na2SO4.10H2O was added until bubbling ceased. The mixture was filtered with a medium porosity fritted funnel washing with EtOAc (50 mL) and MeOH (50 mL), and the filtrate was concentrated under reduced pressure. The residue was purified by flash chromatography eluting with MeOH/CH2Cl2 (2:98) to give 12 mg (80%) of 1 as a white amorphous solid; 1H NMR (400 MHz, CD3OD) δ 7.48 (s, 1 H), 7.40 (s, 1 H), 3.43 (d, J = 10.3 Hz, 1 H), 3.06 (m, 1 H), 2.85 (comp, 2 H), 2.54 (m, 1 H), 2.27 (dd, J = 10.2, 1.5 Hz, 1 H), 2.20–1.18 (comp, 6 H), 1.43 (s, 3 H), 1.34 (s, 3 H); 13C NMR (100 MHz, CD3OD) δ 176.6, 145.1, 137.3, 128.2, 125.3, 123.2, 119.6, 113.1, 104.7, 66.1, 59.4, 57.4, 55.4, 48.5, 35.5, 32.4, 30.6, 30.0, 28.1, 24.2, 23.5; IR (neat) 3226, 1658, 1460 cm−1; HRMS (TOF+) calcd for C21H24N3OCl2 (M+H) 404.1291, found 404.1290.
Isomalbrancheamide B (15)
Prepared from 13b in 68% yield as a white amorphous solid according to the representative procedure described above for 1; 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1 H), 7.33 (s, 1 H), 7.26 (d, J = 8.5 Hz, 1 H), 7.01 (d, J = 8.5 Hz, 1 H), 3.33 (d, J = 7.2 Hz, 1 H), 3.26 (d, J = 9.9 Hz, 1 H), 2.76 (s, 2 H), 2.42 (m, 1 H), 2.15–1.73 (comp, 7 H), 1.33 (s, 3 H), 1.27 (s, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 173.1. 143.4, 134.9, 127.7, 122.8, 120.4, 116.7, 112.1, 103.4, 64.1, 58.6, 55.3, 53.9, 47.0, 34.0, 31.1, 30.0, 28.7, 26.6, 23.7, 22.5; IR (neat) 3311, 1637, 1458 cm−1; HRMS (TOF+) calcd for C21H25N3OCl (M+H) 370.1680, found 370.1675.
Malbrancheamide B (2)
Prepared from 13c in 74% yield as a white amorphous solid according to the representative procedure described above for 1; 1H NMR (400 MHz, DMSO-d6) δ 8.41 (s, 1 H), 7.32 (d, J = 8.4 Hz, 1 H), 7.27 (d, J = 1.7 Hz, 1 H), 6.95 (dd, J = 8.4, 1.7 Hz, 1 H), 3.36 (s, 1 H), 3.27 (d, J = 10.0 Hz, 1 H), 2.95 (m, 1 H), 2.76 (s, 2 H), 2.43 (m, 1 H), 2.13 (d, J = 9.9 Hz, 1 H), 2.10–1.70 (comp, 6 H), 1.32 (s, 3 H), 1.26 (s, 3 H); 13C NMR (100 MHz, DMSO-d6) δ 173.1, 142.6, 136.8, 125.3, 125.2, 118.7, 118.5, 110.3, 103.7, 64.1, 58.5, 55.3, 53.9, 47.0, 34.0, 31.1, 30.0, 28.7, 26.6, 23.7, 22.5; IR (neat) 3297, 1652, 1457 cm−1; HRMS (TOF+) calcd for C21H25N3OCl (M+H) 370.1680, found 370.1670.
Supplementary Material
SCHEME 3.
Enamide Diels-Alder precursors.
SCHEME 4.
Hetero-IMDA reactions.
SCHEME 5.
Amide reductions.
Acknowledgments
Financial support from the National Institutes of Health (CA70375 to R.M.W) and the Hans W. Vahlteich Professorship (to D.H.S.) is gratefully acknowledged. We are grateful to Prof. Rachel Mata and Dr. María del Carmen González of the Departamento de Farmacia, Facultad de Química, Universidad Nacional Autónoma de México for providing 1H NMR spectra of natural malbrancheamide and malbrancheamide B as well as an authentic specimen of malbrancheamide. We also acknowledge Prof. Mata for sharing a pre-print of their manuscript describing the structural elucidation of malbrancheamide B (ref. 11b). We thank Dr. Anthony E. Glenn of the USDA for providing M. aurantiaca RRC1813 strain (originally obtained from Dr. María del Carmen González). Mass spectra were obtained on instruments supported by the NIH Shared Instrumentation Grant GM49631.
Footnotes
Supporting Information Available Spectroscopic data and experimental details for the preparation of all new compounds as well as copies of 1H NMR and 13C NMR spectra. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.(a) Williams RM, Cox RJ. Acc Chem Res. 2003;36:127. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; (b) Williams RM. Chem Pharm Bull. 2002;50:711. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]; (c) Williams RM, Stocking EM, Sanz-Cevera JF. Topics Curr Chem. 2000;209:98. [Google Scholar]
- 2.(a) Yamazaki M, Okuyama E. Tetrahedron Lett. 1981;22:135. [Google Scholar]; (b) Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J Antibiot. 1990;43:1375. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; (c) Liesch JM, Wichmann CF. J Antibiot. 1990;43:1380. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; (d) Banks RM, Blanchflower SE, Everett JR, Manfer BR, Reading CJ. J Antibiot. 1997;50:840. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 3.(a) Birch AJ, Wright JJ. J Chem Soc Chem Commun. 1969:644. [Google Scholar]; (b) Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; (c) Birch AJ, Russell RA. Tetrahedron. 1972;28:2999. [Google Scholar]
- 4.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem, Int Ed. 2007;46:2254. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 5.(a) Qian-Cutrone J, Huang S, Shu YZ, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J Am Chem Soc. 2002;124:14556. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]; (b) Qian-Cutrone J, Krampitz K, Shu Y-Z, Chang LP. 6, 291, 461. US Patent. 2001
- 6.(a) Porter AEA, Sammes PG. J Chem Soc Chem Commun. 1970:1103. [Google Scholar]; (b) Baldas J, Birch AJ, Russell RA. J Chem Soc Perkin Trans I. 1974:50. [Google Scholar]
- 7.(a) Greshock TJ, Williams RW. Org Lett. 2007;9:4255. doi: 10.1021/ol701845t. [DOI] [PubMed] [Google Scholar]; (b) Greshock TJ, Grubbs AW, Tsukamoto S, Williams RW. Angew Chem Int Ed. 2007;46:2262. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]
- 8.(a) Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. J Am Chem Soc. 1998;120:1090. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; (b) Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. Bioorg Med Chem. 1998;6:1233. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; (c) Sanz-Cervera JF, Williams RM, Marco JA, López-Sánchez JM, González F, Martínez ME, Sancenón F. Tetrahedron. 2000;56:6345. [Google Scholar]; (d) Adams LA, Valente MWN, Williams RM. Tetrahedron. 2006;62:5195. [Google Scholar]
- 9.Greshock TJ, Grubbs AW, Williams RM. Tetrahedron. 2007;63:6124. doi: 10.1016/j.tet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Stocking EM, Sanz-Cervera JF, Williams RM. J Am Chem Soc. 2000;122:1675. doi: 10.1021/ja005655e. [DOI] [PubMed] [Google Scholar]; (b) Sanz-Cervera JF, Williams RM. J Am Chem Soc. 2002;124:2556. doi: 10.1021/ja017425l. [DOI] [PubMed] [Google Scholar]
- 11.(a) Martinez-Luis S, Rodriguez R, Acevedo L, Gonzalez MC, Lira-Rocha A, Mata R. Tetrahedron. 2006;62:1817. [Google Scholar]; (b) Figueroa M, del Carmen González M, Mata R. unpublished results. [Google Scholar]
- 12.(a) Zhu HJ, Wang JS, Guo QL, Jiang Y, Liu GQ. Biol Pharm Bull. 2005;28:1974. doi: 10.1248/bpb.28.1974. [DOI] [PubMed] [Google Scholar]; (b) Leisner TM, Liu MJ, Jaffer ZM, Chernoff J, Parise LV. J Cell Biol. 2005;170:465. doi: 10.1083/jcb.200502090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Y, Sherman DH, Williams RM. unpublished results. [Google Scholar]
- 14.Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am Chem Soc. 1999;121:11964. [Google Scholar]
- 15.For the preparation of 5,6-dichloroindole see: Bromidge SM, et al. J Med Chem. 1998;41:1598. doi: 10.1021/jm970741j.
- 16.(a) Somei M, Karasawa Y, Kaneko C. Heterocycles. 1981;16:941. [Google Scholar]; (b) Kametani T, Kanaya N, Ihara M. J Chem Soc Perkin Trans. 1981;1:959. [Google Scholar]
- 17.Curiously, attempts to effect the one-step dehydration/IMDA reaction sequence from 12a–c directly to 13a–c + 14a–c failed under the same conditions used successfully for stephacidin A (ref. 7a) and marcfortine C (ref. 9).
- 18.Fukuyama T, Liu G. J Am Chem Soc. 1996;118:7426–7427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.