Abstract
Five patients with end-stage kidney disease received combined kidney and bone marrow transplants from HLA haploidentical donors following nonmyeloablative conditioning to induce renal allograft tolerance. Immunosuppressive therapy was successfully discontinued in four patients with subsequent follow-up of 3 to more than 6 years. This allograft acceptance was accompanied by specific T-cell unresponsiveness to donor antigens. However, two of these four patients showed evidence of de novo antibodies reactive to donor antigens between 1 and 2 years posttransplant. These humoral responses were characterized by the presence of donor HLA-specific antibodies in the serum with or without the deposition of the complement molecule C4d in the graft. Immunofluorescence staining, ELISA assays and antibody profiling using protein microarrays demonstrated the co-development of auto- and alloantibodies in these two patients. These responses were preceded by elevated serum BAFF levels and coincided with B-cell reconstitution as revealed by a high frequency of transitional B cells in the periphery. To date, these B cell responses have not been associated with evidence of humoral rejection and their clinical significance is still unclear. Overall, our findings showed the development of B-cell allo- and autoimmunity in patients with T-cell tolerance to the donor graft.
Keywords: Antibodies, B cell, tolerance induction
Introduction
We have recently reported on five patients with end-stage renal diseases who received combined kidney–bone marrow transplant from the same haploidentical donor, to induce tolerance to the organ grafts (1). One patient developed acute humoral rejection and lost his graft 10 days posttransplant. For the four remaining patients, immunosuppression was discontinued 9–14 months after transplant and their renal function has remained stable for more than 2.1–5.6 years. Functional T-cell-based assays demonstrated that graft acceptance was accompanied by specific peripheral blood T-cell unresponsiveness to donor antigens, although mixed chimerism was lost within 2–4 weeks posttransplant. These findings provided evidence that all four recipients had achieved T-cell tolerance to the allograft.
Remarkably, three of these four patients developed de novo antibodies reactive to donor antigens and/or C4d deposition in the graft. Two types of responses were distinguished. Transient alloantibody responses, one without donor reactivity, were detected within 2 months posttransplant in two patients (namely Patients 2 and 5) while they were still being treated with immunosuppressive medications. These reactions responded well to treatment. In contrast, one of these two early responders (Patient 5), together with another patient (Patient 4), developed antibody responses late posttransplant, after immunosuppression had been discontinued and at the time of T-cell unresponsiveness to donor antigens. These late responses were characterized by the development of donor-specific antibodies. Class-switched B-cell responses are thought to involve antigen-specific T-cell help. In these patients, however, donor-specific T-cell unresponsiveness did not support the view that antibody responses had necessitated cognate T-cell help. This apparent contradiction suggested that an alternate immune mechanism had been at play. The goal of our study was to characterize these B-cell responses and examine the immune context in which they developed.
Materials and Methods
Patient characteristics
Five patients were enrolled in this study. Their treatments and clinical outcomes have been reported separately (1). A brief summary is provided in Table 1 and supporting Figure S1. Patient 3 lost his graft on Day 10 because of acute humoral rejection. He is not described in this report. The protocol was modified for Patients 4 and 5 to prevent humoral responses. Anti-CD20 antibody (rituximab) and a 10-day course of steroids were added to the conditioning regimen. As described previously, all four subjects included in this report developed mixed chimerism for 1–3 weeks (1). Functional assays revealed T-cell unresponsiveness to donor antigens while responsiveness to third party antigens was progressively restored (1). All patients are currently off immunosuppressive drugs. All samples were collected with IRB approval and after the informed consent was obtained.
Table 1.
Summary of patient clinical outcome
| Day of IS withdrawal | Graft survival (days) | T-cell unresponsiveness | Humoral rejection and DSA | C4d | IS at the time of antibody response (ng/mL) | Treatment of antibody responses | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 240 | >2300 | Yes | None | No | N/A | None |
| Patient 2 | 422 | >2120 | Yes | Early transient humoral rejection (Day 45) no DSA | Yes | CyA alone (192) | PEX × 5, thymoglobulin (1.5 mg/kg) × 4 doses, solumedrol 500 mg × 2, IVIG × 1; maintenance on tacrolimus, MMF and prednisone |
| Patient 3 | 10 | N/A | Early humoral reaction (Day 10) DSA | Yes | CyA alone (219) | PEX × 10, thymoglobulin (1.5 mg/kg) × 7, solumedrol × 2, rituximab (375 mg/kg/m2) × 2, IVIG × 1, HD × 2; maintenance on tacrolimus, MMF and prednisone | |
| Patient 4 | 244 | >1294 | Yes | Late persistent DSA (Day 303) | Yes | None | None |
| Patient 5 | 272 | >1100 | Yes | Early transient humoral rejection (Day 13) DSA | Yes | CyA alone (469) | PEX × 3, thymoglobulin × 3, solumedrol 500 mg × 2, rituximab (375 mg/kg/m2) × 1, IVIG × 1; maintenance on tacrolimus, MMF and prednisone |
| Yes | Late persistent DSA (Day 660) | No | None | None |
DSA = donor-specific antibody; IS = immunosuppression; PEX = plasma exchange; IVIG = intravenous immunoglobulin; MMF = mycophenolate mofetil; HD = hemodialysis.
Flow cytometry
CD4+ T-cell and CD 19+ B-cell count was performed on whole blood after red blood cell lysis using mAbs specific for CD3, CD4 and CD19 (Becton Dickinson, Mountain View, CA). Analysis was performed on a LSR II flow cytometer (BD biosciences, San Jose, CA). Cell counts were calculated based on the phenotypic data.
Phenotyping analysis of B cells was performed by labeling PBMC or T-cell depleted PBMC (for Patient 5) with anti-CD24 FITC (BD biosciences), anti-CD38 PE (Beckman Coulter, Fullerton, CA) and anti-CD20 PC5 (Beckman Coulter). Cells were analyzed using a FACScan flow cytometer (BD biosciences).
Detection of alloantibodies
Initial detection of HLA antibodies was done with ELISA panel reactivity antibodies (PRA) (One Lambda, Los Angeles, CA). The presence of antibodies to mismatched donor HLA antigens was confirmed using a Luminex based assay (One Lambda). Anti-HLA-DQ2 and -DR1 7 beads were used to detect DSA in Patient 4 while the assay was carried out with anti-HLA DR53 beads in Patient 5.
ELISA assays
ELISA assays for the detection of antibodies to generic autoantigens were performed as previously described (2).
ELISA assays to confirm the presence of antibodies to candidate proteins identified by protein arrays were performed using human recombinant AIF-1 and PSMA4 (Abnova, Taipei city, Taiwan). These proteins were produced in a cell-free eukaryotic translation system and are endotoxin-free Patient serum samples were diluted 1:25 (AIF-1) or 1:100 (PMSA4) in PBS The concentration of BAFF in the serum was measured using a Quantikine ELISA kit (R&D systems, Minneapolis, MN). Serum samples were diluted 1:8 in PBS.
Immunofluorescence assays (IFAs)
IFAs were conducted using Hep-2 cell coated slides (Bion Enterprises, Ltd, Des Plaines, IL) as previously described (2).
Immunoprofiling using protein microarrays
Antibody profiling of selected serum samples was performed using protein microarrays (V4.0 protoarrays, Invitrogen Corp, Carlsbad, CA), consisting of 8000 recombinant proteins spotted in duplicate on a glass slide Arrays were probed with patient serum samples collected at different time points and revealed using a pan-IgG secondary antibody. The signal intensity obtained for each protein reflected the titer of antibody specific to that protein in the serum sample. Arrays were probed according to the manufacturer’s instructions and scanned on a GenePix 4000B scanner (Molecular Devices, Union City, CA). Fluorescence was measured with the GenePix Pro 3.0 software (Molecular Devices) and quantified with the Prospector 4 software (Invitrogen) using the protein–protein interaction (PPI) algorithm. Signals obtained with serum samples collected at two different points (before and during the alloantibody response) were compared to one another to follow changes in antibody titers. Similar two-dimensional scatterplot analysis using Invitrogen protoarrays has already been reported (3). Variations in protein signals were then evaluated by calculating the ratio between signals obtained pre-DSA and at the time of DSA. Because the ratio can be artificially high with values close to background level, values below 50 arbitrary units were not considered.
Results
Immune reconstitution and serum BAFF levels
The reconstitution of B cells and CD4 T cells is shown in Figure 1A. CD4+ T-cell counts remained low at 2 years posttransplant for Patients 2, 4 and 5 (normal range: 600–1500 cells/μl). In contrast, CD4+ T-cell counts reached normal levels within 1.5 years for Patient 1, whereas B-cell reconstitution appeared delayed. Patient 1 was the only patient who did not show any evidence of antibody response to the graft. The B-cell activation factor BAFF plays a major role in B-cell development and function. We measured the concentration of this factor in serum samples collected at various time points posttransplant (Figure 1B). BAFF levels were low for Patient 1, consistent with slow reconstitution of B cells. Patient 2 showed a sudden increase in serum BAFF levels at Day 22 that preceded the onset of humoral rejection (Table 1). This elevation was transient and the BAFF concentration decreased sharply by Day 60, after the patient had been treated. In comparison, elevations in serum BAFF levels were substantially delayed for both Patients 4 and 5, who received rituximab as part of their pretransplant conditioning regimen. In these subjects, BAFF concentrations gradually increased during the first 100 days, and then progressively returned to normal levels.
Figure 1. Immune reconstitution and serum BAFF levels.
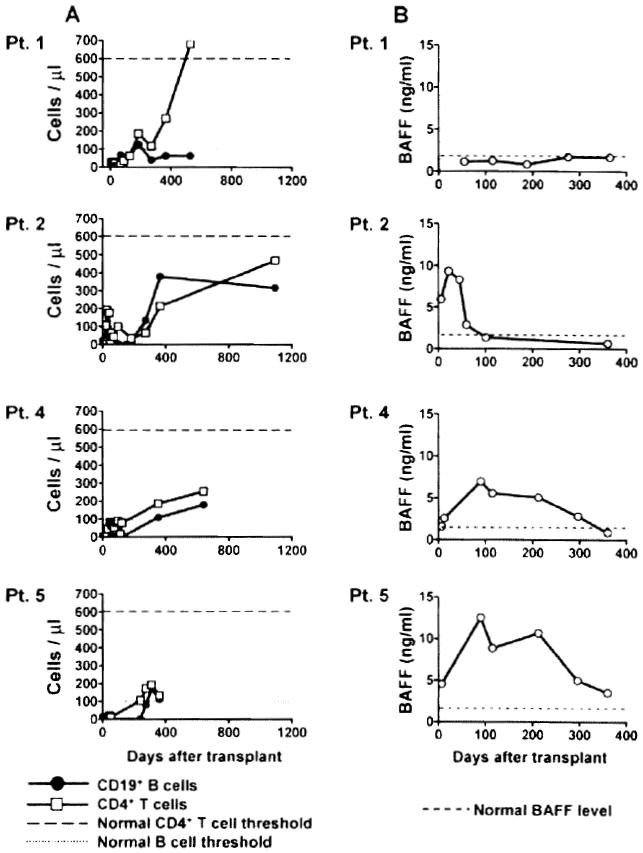
(A) Peripheral CD4+ T-cell counts and CD19+ B-cell counts were determined by flow cytometry on serial samples collected from the four patients. (B) The soluble BAFF concentration was assessed by ELISA in patient serum samples collected at various times after transplantation.
Development of alloantibodies following combined kidney and bone marrow transplantation
Patients 2 and 5 developed early episodes of acute humoral rejection associated with deposition of the complement cleavage product C4d following CKBMT (Table 1 (1)). Additionally, Patients 4 and 5 developed DSA late in association with C4d deposition for Patient 4 but without evidence of rejection. As shown in Figure 2, Class II panel reactive antibodies were detected in the serum of Patients 2, 4 and 5. The presence of antibodies to donor HLA molecules was then assessed by the Luminex assay using beads coated with single HLA molecules. However, the low Class II serum reactivity did not include measurable donor reactivity for Patient 2. DSA were specific for HLA-DQ2 and HLA-DR17 in Patient 4 and for HLA-DR53 in Patient 5 (Figure 2). Despite the presence of DSA, the graft function of these two patients has remained unchanged and their serum creatinine levels have been stable (1).
Figure 2. Development of anti-HLA antibodies after transplant.
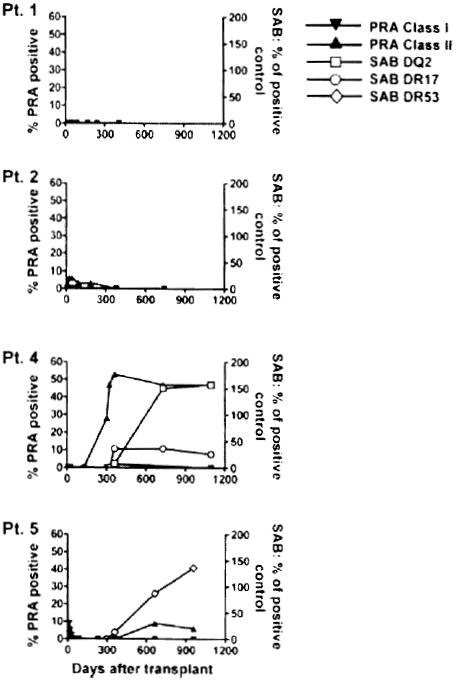
Serial serum samples from the four patients were tested for IgG reactive to a standard commercialized panel of cell lines expressing different HLA Class I and Class II antigens. Results are expressed as percentage of cell lines recognized by serum antibodies (left axis, panel reactive antibodies, PRA). The presence of donor-specific antibodies was assessed by a single HLA antigen bead Luminex assay using anti-HLA-DQ2 and -DR17 beads for Patient 4 and anti-HLA-DR53 beads for Patient 5. Signals are expressed as percentage of positive control (right axis).
Development of autoantibodies following combined kidney and bone marrow transplantation
We investigated whether B-cell responses that developed in these patients also included an autoimmune component as a sign of B-cell deregulation. Patients’ serum samples were tested for the presence of antibodies to double-stranded DNA (dsDNA) and insulin as generic autoantigens by ELISA (Figure 3A). No significant serum reactivity to these antigens was detected for Patients 1 and 2 up to 2 years posttransplant. In contrast, Patients 4 and 5 developed autoantibodies to both antigens 300 and 600 days after transplantation, respectively. These experiments also revealed the pre-existence of a lower level of these autoantibodies prior to transplant in both patients. Patient 4 was originally treated for Alport’s syndrome and did not have any associated autoimmune disease at the time as well as posttransplant. We confirmed the presence of autoantibodies posttransplant for Patient 4 by indirect immunofluorescence staining using the standard carcinoma cell line Hep-2 for the detection of antinuclear antibodies (ANA). Hep-2 cell staining revealed the development of ANA between 10 and 24 months posttransplant (Figure 3B) consistent with serum reactivity to dsDNA (Figure 3A). Hep-2 cell staining also showed a slight increase at Day 360 for Patient 5 (data not shown).
Figure 3. Patient serum reactivity to self antigens.
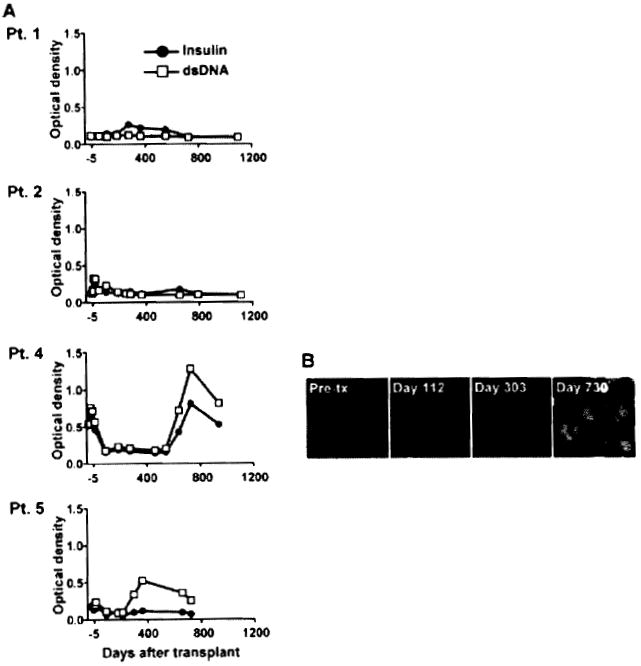
(A) Serial serum samples collected from the four patients were tested for IgG reactivity to double stranded DNA and insulin by ELISA. (B) Hep-2 reactivity of Patient 4 serial serum samples collected before transplant, before the detection of DSA (Day 112), when DSA were detected for the first time (Day 303) as well as later posttransplant (Day 730), when DSA were still present. Staining was revealed using an anti-IgG secondary antibody conjugated to FITC.
Immunoprofiling using protein microarrays
We next used a protein array approach to identify additional targets of autoimmune B-cell responses that developed in Patients 4 and 5. To examine fluctuations of autoantibody titers with time, we compared signals obtained from two arrays probed with serum samples collected at two different time points, i.e. before and at the time of antibody responses. We first examined changes in autoantibody titers for Patient 2, for whom no reactivity was detected in Hep-2 or ELISA assays (Figure 3A). Protein arrays were probed with a sample collected prior to (Day 6) and at the time of suspected humoral rejection (Day 45), when intragraft C4d deposition was documented. Figure 4A shows the scatter-plot of signals obtained for all 8000 proteins in duplicate (16 000 dots) using the two serum samples. As shown in this figure, signals from almost all proteins fall within the range of the control diagonal line, indicating that there was no substantial variation in IgG titer to these proteins between Days 6 and 45. The signal ratio between Day 6 and Day 45 was used to rank variations in antibody binding to all proteins (supporting Table S1). This signal ratio was notably higher for a single target: allograft inflammatory factor-1 (AIF-1) (supporting Table S1). We confirmed the increase in the serum antibody concentration to this single antigen by ELISA using recombinant AIF-1 protein obtained from a different source from the one used in the array (Figure 4B). As shown in this figure, the serum reactivity to AIF-1 decreased between Day 45 and Day 249, indicating that the antibody response to this protein was transient.
Figure 4. Protein array profiling of Patient 2 antibody response.
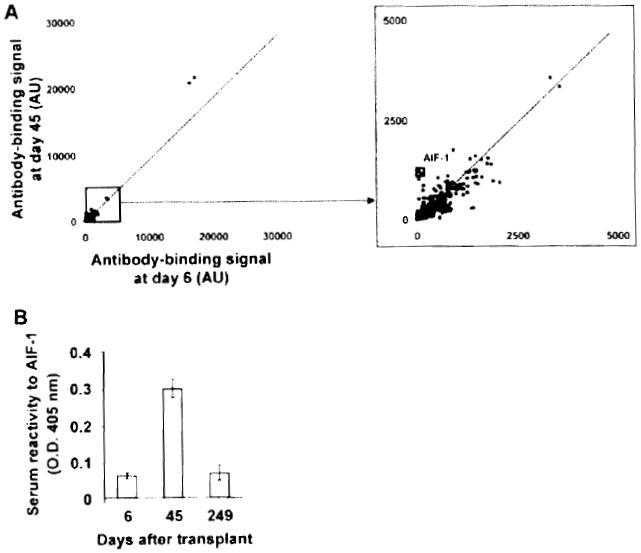
(A) Two-dimensional scatterplot of IgG binding values obtained for all 8000 proteins on the array using serum samples collected before antibody response to the graft (Day 6; x-axis) and at the time of response (Day 45; y-axis). All proteins are spotted in duplicate. Signals are expressed as arbitrary units (AU). Framed dots correspond to allograft inflammatory factor 1 (AIF-1), for which signal was most increased between the two time points. The depicted line was established using values obtained for array internal control proteins. Signal values falling within range of this trend line correspond to proteins for which antibody titers did not change between the two samples. (B) Protein array data confirmation for AIF-1 protein. The reactivity of Patient 2 serum samples collected at Day 6 and Day 45 to recombinant AIF-1 was assessed by ELISA using an anti-IgG secondary antibody.
The same approach was used to study Patients 4 and 5, who developed both antidonor HLA and self-antibodies late posttransplant. For Patient 4, arrays were probed with samples collected before any evidence of humoral response (Day 112) and at the time DSA were first detected in the serum (Day 303). This patient experienced a sharp increase in antibody titers to a variety of target antigens (signals above control diagonal) between these two time points (Figure 5A). The highest ratios were observed for the proteasome subunit alpha type 4 (PSMA4). Comparatively, there were no significant decreases in the antibody-binding signal below control diagonal. As the immunosuppressive therapy was discontinued in Patient 4 only 60 days prior to antiself antibody detection at Day 303, we next examined the long-term effect of immunosuppression on antibody production by Day 730 posttransplantation. Antibody concentrations continued to increase between Days 303 and 730 for a significant number of antigens but also decreased for others (Figure 5B). Remarkably, reactivity to PSMA4 decreased abruptly to a level comparable to that seen at Day 112. The transient elevation of antibody titers to PSMA4 around Day 303 was further confirmed by ELISA using a recombinant PSMA4 protein produced in a cell-free expression system (Figure 5C). Table 2 reports the top 10 ranked target autoantigens identified between Day 112 and Day 303, and between Day 303 and Day 730 based on signal ratios.
Figure 5. Protein array profiling of Patient 4 antibody response.
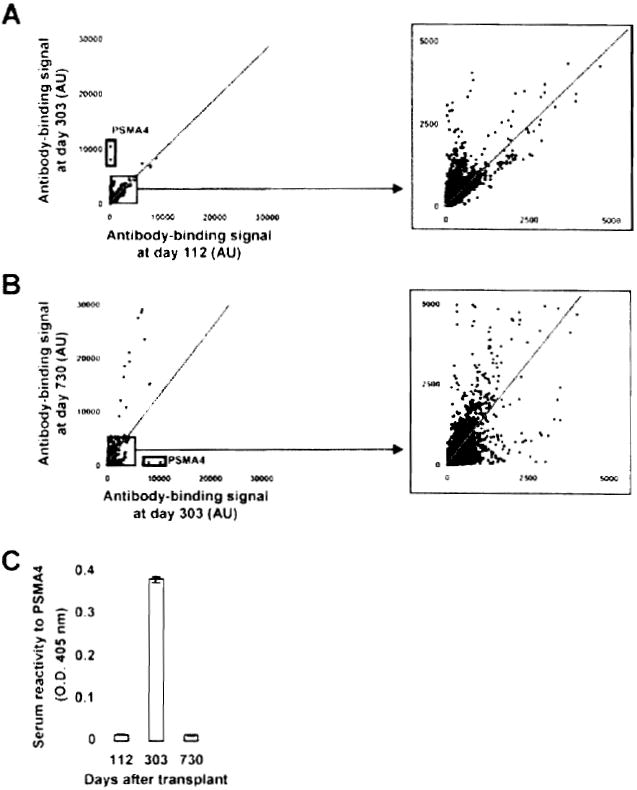
(A) Two dimensional scatterplot of IgG binding values obtained for all 8000 proteins on the array using serum samples collected at Day 112 (before detection of DSA and C4d; x-axis) and at Day 303 (time of detection of DSA and C4d; y-axis). All proteins are spotted in duplicate. Signals are expressed as arbitrary units (AU). Framed dots correspond to PSMA4, for which signal was most increased between the two time points. The depicted line was established using values obtained for array internal control proteins. Signal values falling within the range of this trend line correspond to proteins for which antibody titers did not change between the two samples. (B) Two-dimensional scatterplot comparison of IgG binding values obtained samples collected at Day 303 (time of detection of DSA and C4d; x-axis) and Day 730 (persistence of DSA and C4d; y-axis). Framed dots correspond to PSMA4, for which signal was the most decreased between the two time points. (C) Protein array data confirmation for PSMA4. The reactivity of Patient 4 serum samples collected at Day 112, Day 303 and Day 730 to recombinant PSMA4 was assessed by ELISA using an anti-IgG secondary antibody.
Table 2.
Patient 4 antibody titer increase
| Protein | Accession # | Fold increase |
|---|---|---|
| Between Day 112 and Day 303: 10 top ‘hits’ | ||
| Proteasome subunit, alpha type, 4 (PSMA4) | NM_002789.2 | 51.5 |
| Transglutaminase 2 | BC003551.1 | 21.4 |
| BAI 1-associated protein 2 | BC014020.1 | 14.4 |
| Family with sequence similarity 120B (FAM120B) | NM_032448.1 | 14.3 |
| Adenylate kinase 2 (AK2) | NM_001625.1 | 12.7 |
| Zeta-chain (TCR) associated protein kinase 70 kDa | BC053878.1 | 10.9 |
| SH3 domain binding glutamic acid-rich protein like 3 (SH3BGRL3) | NM_031286.1 | 10.8 |
| WW domain containing adaptor with coiled coil | BC010356.1 | 10.8 |
| Splicing factor, arginine/serine-rich 9 (SFRS9) | NM_003769.2 | 8.8 |
| Protein kinase C, zeta | BC008058.1 | 8.1 |
| Between Day 303 and Day 730: 10 top ‘hits’ | ||
| Glycolipid transfer protein domain containing 1 (GLTPD1) | NM_030575.1 | 19.4 |
| Small nuclear ribonucleoprotein polypeptide C (SNRPC) | NM_003093.1 | 15 |
| Phosphatidic acid phosphatase type 2C (PPAP2C) | NM_003712.1 | 13.7 |
| Src-like-adaptor2 (SLA2) | NM_032214.1 | 13.5 |
| Chromosome 10 open reading frame 116 (C10 or f116) | NM_006829.1 | 9.9 |
| Embryonal Fyn-associated substrate (EFS) | NM_032459.1 | 9.1 |
| Major Histocompatibility Complex, Class II, DR beta 1 | BC024269.1 | 8.6 |
| Disabled homolog 1 (Drosophila) | BC067446.1 | 7.2 |
| Eukaryotic translation initiation factor 5 | BC032866.2 | 6.5 |
| Dual specificity phosphatase 22 (DUSP22) | NM_020185.2 | 5.7 |
Immunoprofiling of serum from Patient 5 also demonstrated the development of autoantibodies between Day 180, before the detection of anti-DNA antibodies, and Day 660, when DSA were detected (Figure 6). Signal ratios for the top 10 antibody targets are reported in Table 3.
Figure 6. Protein array profiling of Patient 5 antibody response.
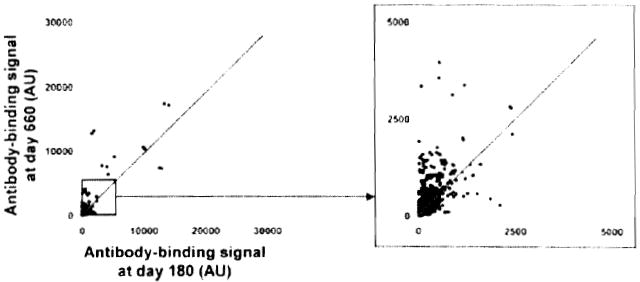
Two-dimensional scatterplot of IgG binding values obtained for all 8000 proteins on the array using serum samples collected at Day 180 (before detection of anti-DNA antibodies; x-axis) and at Day 660 (time of detection of DSA; y-axis). All proteins are spotted in duplicate. Signals are expressed as arbitrary units (AU). The depicted line was established using values obtained for array internal control proteins. Signal values falling within the range of this trend line correspond to proteins for which antibody titers did not change between the two samples.
Table 3.
Patient 5 antibody titer increase between Day 180 and Day 660: 10 top ‘hits’
| Protein | Accession # | Fold increase |
|---|---|---|
| Family with sequence similarity 50, member A (FAM50A) | NM_004699.1 | 37.3 |
| Glutaminyl-tRNA synthetase | BC001772.1 | 19.3 |
| MAX-like protein X (MLX), transcript variant 1 | NM_198205.1 | 11.3 |
| MAX-like protein X (MLX), transcript variant 2 | NM_198204.1 | 10.9 |
| Serine/arginine repetitive matrix 2 | BC032416.1 | 10.4 |
| Protein (peptidylprolyl cis/trans isomerase) NIMA-interacting, 4 (parvulin) (PIN4) | NM_006223.1 | 8.8 |
| Annexin A5 (ANXA5) | NM_001154.2 | 8.3 |
| Myosin, light chain 1, alkali; skeletal, fast (MYL1) | NM_079420.1 | 7.5 |
| Myosin, light chain 6B, alkali, smooth muscle and nonmuscle (MYL6B) | NM_002475.2 | 7.1 |
| Spermatogenesis associated 1 | BC064144.1 | 6.8 |
Overall, the number of target proteins for which the signal increased more than 3-fold between the two time points was 3 for Patient 2, 2108 and 125 for Patient 4 (between 4–10 months and 10–24 months, respectively) and 399 for Patient 5. A similar immunoprofiling procedure was carried out in control experiments using serial samples collected from three kidney transplant recipients with stable renal function and without any detectable anti-HLA antibodies. Microarray analyses showed a very stable level of autoantibodies for these three patients. A representative immunoprofile is shown in supporting Figure S2.
Allo- and autoantibody responses coincided with B-cell reconstitution and high frequency of peripheral transitional B cells
The distribution of B-cell subsets was monitored in the blood of Patients 4 and 5 at the time of antibody responses. Virtually, no circulating CD20+ B cells were detectable in Patient 4 on Day 112 posttransplantation (Figure 7, panel B). CD20+ B cells had started to reconstitute in the periphery when auto- and alloantibodies were detected by Day 303 (Figure 7, panel B). Further staining revealed that nearly 25% of these cells had a CD24high CD38high transitional phenotype as compared to about 3% in healthy donors (Figure 7, panel A and (4)). Transitional B cells were also predominant in the blood of Patient 5 on Day 213 (Figure 7, panel C). The size of this subset decreased thereafter but remained larger than normal on Days 360 and 660 posttransplantation and at the time of auto- and alloantibodies detection. By Day 724, the frequency of circulating transitional B cells had reached normal levels for this patient (Figure 7, panel C).
Figure 7. Immunophenotyping of Patient 4 and Patient 5 peripheral B-cell subsets during immune reconstitution.
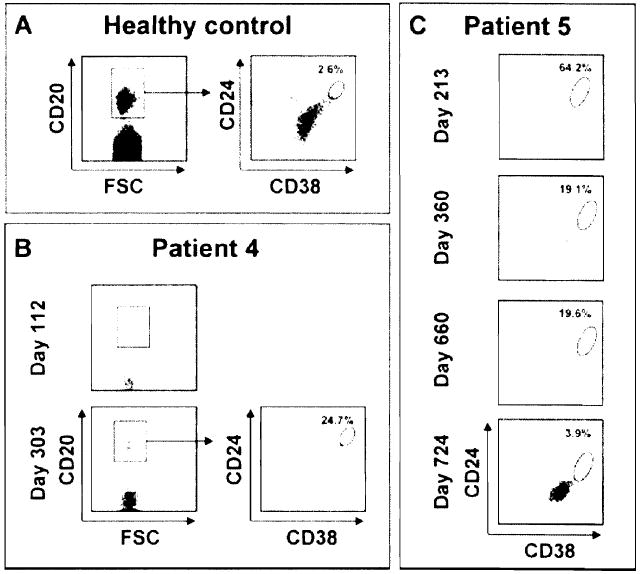
Percentage of CD24high CD38high transitional B cells in the peripheral blood of a healthy subject (panel A), Patient 4 (panel B) and Patient 5 (panel C) during B-cell reconstitution. The frequency of CD24high CD38high transitional B cells was determined after gating on CD20 + cells.
Discussion
Combined kidney and bone marrow transplantation from the same haploidentical donor successfully promoted sustained tolerance of the allograft in four of five patients in a pilot trial at our institution (1). These patients have remained free of maintenance immunosuppressive treatments for 3 to more than 6 years posttransplant while maintaining stable graft function. Tolerance was accompanied by specific direct T-cell unresponsiveness to donor antigens as revealed by cell-based in vitro assays (1). The mechanism whereby graft acceptance was achieved is currently under study. Remarkably, two of these four patients developed late humoral responses to the donor and two developed early humoral rejection episodes, one without evidence of DSA. Our goal was to characterize these antibody responses and elucidate the immune mechanisms that led to their development.
Autoantibodies have been described in solid organ graft recipients independent of anti-HLA antibodies (5-10). Our assays detected the appearance of autoantibodies reactive to a set of generic self-antigens such as DNA and insulin during late responses in both Patients 4 and 5. We also used a protein array technology to further characterize these autoimmune responses. Patient 2 showed a particularly steady antibody profile between 6 and 45 days. A single antigen AIF-1 was identified toward which antibody levels strongly increased. AIF-1 is an IFNγ-induced protein expressed by macrophages during inflammation. AIF-1 appears to play a role in graft rejection (11, 12), and enhanced expression has been associated with rejection in heart transplant recipients. In contrast, Patient 4 revealed a different immunoprofile. Antibody levels to a wide range of proteins were increased between 112 and 303 days posttransplant. Levels continued to increase for several targets between Day 303 and Day 730, indicating that the response was still ongoing. Among all self-proteins identified in our assays, the proteasome subunit alpha type 4 (PSMA4) was the target toward which antibody titers increased the most during B-cell responses. Other subunits of the proteasome complex have previously been identified as targets of autoantibodies in autoimmune diseases and in the context of antitumor immunity (13-16). No functional role has been attributed to these antibodies thus far. Tribbles-1 was also identified as one of the 20 target proteins toward which the antibody concentration increased the most between Day 112 and Day 303. Expression of Tribbles-1 has recently been proposed as a biomarker for chronic antibody-mediated rejection of kidney allografts (17). We also observed that Patient 5 developed autoantibodies to a variety of targets at the time DSA were detected. Collectively, our findings indicate that both Patients 4 and 5 developed antibodies to a set of self-antigens targeted in other clinical situations of autoimmunity.
To date, neither Patient 2 (who only had an early apparent humoral rejection episode in the absence of DSA) nor Patients 4 and 5 (who developed late allo- and autoantibodies) have experienced graft rejection since being removed from immunosuppression therapy. As reported by the group of Terasaki, DSA can be detected years before any sign of humoral rejection (18). However, these studies were conducted in recipients of conventional kidney transplant recipients. While it is possible that the observed antibody responses in Patients 4 and 5 could be early signs of chronic humoral rejection, it is also possible that they have had no deleterious effect on allograft survival in the context of T-cell tolerance. These antibodies might even play a role in promoting graft acceptance since mechanisms of accommodation are still poorly defined but appear to involve graft-specific antibodies (19).
BAFF levels appeared to tightly correlate with antibody responses in CKBMT patients. Patients 2, 4 and 5 had significant elevations in the BAFF concentration to a level comparable with that of hematopoietic stem cell transplant recipients who develop chronic graft versus host disease (20,21). It is likely that BAFF levels correlated with B-cell lymphopenia and reconstitution. Patients 4 and 5 received a revised conditioning regimen including anti-CD20 mab (rituximab) prior to transplantation and demonstrated prolonged elevations of BAFF.
Patients 4 and 5 developed allo- and autoantibody responses in the context of T-cell unresponsiveness to donor antigens. The possibility of undetected, low level indirect allo-specific T-cell help to B cells cannot be ruled out and could explain these humoral responses. On the other hand, it is possible that the combination of CD4+ T-cell lymphopenia and high BAFF levels may have promoted a broad, unregulated B-cell response in Patients 4 and 5, eliciting the observed antibody responses. In this case, antibody responses might be independent of cognate antigenspecific T-cell help. Pan-B-cell activation may also have resulted from nonspecific stimuli such as bystander T cells or signaling through TLR (22).
Overall, our studies demonstrate that donor-specific isotype-switched B-cell immunity can occur in the context of T-cell tolerance to donor antigens following CKBMT. These findings indicate that T-cell unresponsiveness to donor antigens does not necessarily denote tolerance of both arms of the adaptive immune system. Further studies are now required to clarify the precise mechanism whereby these B-cell responses were elicited and to develop strategies to promote B-cell tolerance in addition to T-cell tolerance.
Supplementary Material
Acknowledgments
The four patients under study were initially enrolled in a clinical trial supported by a contract from the Immune Tolerance Network (N01-AI-15416) sponsored by the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Juvenile Diabetes Research Foundation. We are indebted to Drs Christian Leguern and Christene Huang for their thoughtful review of our manuscript
Footnotes
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 3.Marina O, Biernacki MA, Brusic V, Wu CJ. A concentration-dependent analysis method for high density protein microarrays. J Proteome Res. 2008;7:2059–2068. doi: 10.1021/pr700892h. [DOI] [PubMed] [Google Scholar]
- 4.Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 5.Fang JC, Kinlay S, Behrendt D, et al. Circulating autoantibodies to oxidized LDL correlate with impaired coronary endothelial function after cardiac transplantation. Arterioscler Thromb Vase Biol. 2002;22:2044–2048. doi: 10.1161/01.atv.0000040854.47020.44. [DOI] [PubMed] [Google Scholar]
- 6.Wang SX, Ahola H, Palmen T, Solin ML, Luimula P, Holthofer H. Recurrence of nephrotic syndrome after transplantation in CNF is due to autoantibodies to nephrin. Exp Nephrol. 2001;9:327–331. doi: 10.1159/000052628. [DOI] [PubMed] [Google Scholar]
- 7.Szewczyk M, Wielkoszynski T, Zakliczynski M, Zembala M, Szumska-Kostrzewska M. Anti-ox-LDL and anticardiolipin autoantibodies in patients after cardiac transplantation. Transplant Proc. 2007;39:2870–2872. doi: 10.1016/j.transproceed.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 8.Ducloux D, Bourrinet E, Motte G, Chalopin JM. Antiphospholipid antibodies as a risk factor for atherosclerotic events in renal transplant recipients. Kidney Int. 2003;64:1065–1070. doi: 10.1046/j.1523-1755.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 9.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 10.Shin YS, Yang CW, Ahn HJ, et al. Clinical significance of anti-endothelial cell antibody in renal transplant recipients. Korean J Intern Med. 2001;16:24–29. doi: 10.3904/kjim.2001.16.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm PC, McKenna R, Nickerson P, et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–1589. doi: 10.1681/ASN.V1071582. [DOI] [PubMed] [Google Scholar]
- 12.Nagakawa Y, Nomoto S, Kato Y, et al. Over-expression of AIF-1 in liver allografts and peripheral blood correlates with acute rejection after transplantation in rats. Am J Transplant. 2004;4:1949–1957. doi: 10.1111/j.1600-6143.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayo I, Arribas J, Villoslada P, et al. The proteasome is a major autoantigen in multiple sclerosis. Brain. 2002;125(Pt 12):2658–2667. doi: 10.1093/brain/awf274. [DOI] [PubMed] [Google Scholar]
- 14.Feist E, Brychcy M, Hausdorf G, et al. Anti-proteasome autoantibodies contribute to anti-nuclear antibody patterns on human larynx carcinoma cells. Ann Rheum Dis. 2007;66:5–11. doi: 10.1136/ard.2006.055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer NH, Milthers J, Bonde Lauridsen AM, Houen G, Lautrup Frederiksen J. Autoantibodies to the proteasome in monosymptomatic optic neuritis may predict progression to multiple sclerosis. Scand J Clin Lab Invest. 2007;67:696–706. doi: 10.1080/00365510701342062. [DOI] [PubMed] [Google Scholar]
- 16.Stinton LM, Eystathioy T, Selak S, Chan EK, Fritzler MJ. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes, and GW bodies. Clin Immunol. 2004;110:30–44. doi: 10.1016/j.clim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Ashton-Chess J, Giral M, Mengel M, et al. Tribbles-1 as a novel biomarker of chronic antibody-mediated rejection. J Am Soc Nephrol. 2008;19:1116–1127. doi: 10.1681/ASN.2007101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PC, Terasaki PI, Takemoto SK, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 19.Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Hum Immunol. 2007;68:645–651. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B cell homeostasis and excess BAFF in human chronic graft versus host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


