Abstract
Background:
There are limited data on the relationship between patient and site characteristics and clinical outcomes after intracranial stenting.
Methods:
We performed a multivariable analysis that correlated patient and site characteristics with the occurrence of the primary endpoint (any stroke or death within 30 days of stenting or stroke in the territory of the stented artery beyond 30 days) in 160 patients enrolled in this stenting registry. All patients presented with an ischemic stroke, TIA, or other cerebral ischemic event (e.g., vertebrobasilar insufficiency) in the territory of a suspected 50–99% stenosis of a major intracranial artery while on antithrombotic therapy.
Results:
Cerebral angiography confirmed that 99% (158/160) of patients had a 50–99% stenosis. In multivariable analysis, the primary endpoint was associated with posterior circulation stenosis (vs anterior circulation) (hazard ratio [HR] 3.4, 95% confidence interval [CI] 1.2–9.3, p = 0.018), stenting at low enrollment sites (<10 patients each) (vs high enrollment site) (HR 2.8, 95% CI 1.1–7.6, p = 0.038), ≤10 days from qualifying event to stenting (vs ≥10 days) (HR 2.7, 95% CI 1.0–7.8, p = 0.058), and stroke as a qualifying event (vs TIA/other) (HR 3.2, 95% CI 0.9–11.2, p = 0.064). There was no significant difference in the primary endpoint based on age, gender, race, or percent stenosis (50–69% vs 70–99%).
Conclusions:
Major cerebrovascular complications after intracranial stenting may be associated with posterior circulation stenosis, low volume sites, stenting soon after a qualifying event, and stroke as the qualifying event. These factors will need to be monitored in future trials of intracranial stenting.
GLOSSARY
- CI
= confidence interval;
- HR
= hazard ratio.
Large artery intracranial atherosclerosis accounts for approximately 8–10% of ischemic strokes in the United States.1,2 Ethnic and racial differences result in higher frequencies of stroke from this disease in Asian, black, and Hispanic subjects as compared with white subjects.1,3,4
The Warfarin vs Aspirin for Symptomatic Intracranial Disease study revealed that certain subgroups of patients with intracranial arterial stenosis remained at a high risk of stroke while on antithrombotic therapy. Analysis revealed that the severity of stenosis was the most powerful predictor of stroke in the territory and patients with a history of TIA or stroke in the territory of a 70–99% stenosis had a stroke rate of 18% at 1 year while on antithrombotic therapy.5
Intracranial angioplasty and stenting are evolving as possible treatment options for these high-risk patients but results from single-center (≥10 patients) series to date show wide variability in periprocedural complications. Major periprocedural complications including stroke or death within 30 days of stenting have been reported in 0 to 36% of patients.6–16
Patient and site characteristics may contribute to this wide variability in outcomes after intracranial angioplasty and stenting but there are limited data available. The NIH Multicenter Wingspan Intracranial Stent registry provided a unique opportunity to evaluate risk factors associated with major cerebrovascular complications after intracranial stenting.
METHODS
This is a post hoc analysis of a previously published study. Details of the study population, recommended treatment protocol, and stenting procedure have been described previously.17 Each participating site was required to obtain institutional review board approval for the registry data collection, performed in accordance with the Health Insurance Portability and Accountability Privacy Act. Sixteen sites met all these criteria and participated in the registry.
Briefly, all patients undergoing intracranial angioplasty and stenting with Gateway® balloon angioplasty and placement of the Wingspan® intracranial stent (Boston Scientific, Fremont, CA) under the humanitarian device exemption (patients with 50–99% stenosis of a major intracranial artery with a cerebral ischemic event while on antithrombotic therapy) at 16 participating sites were potential candidates for the NIH Multicenter Wingspan Registry. Patients were excluded if they received concurrent treatment with two stents for tandem intracranial stenoses or if stenting was used to treat an acute ischemic stroke.
Patients were treated with aspirin (81–325 mg daily) and clopidogrel (Plavix®, Bristol-Myers Squibb/Sanofi Pharmaceuticals, Bridgewater, NJ) 75 mg at least 3 days before the procedure or loaded with clopidogrel 300 mg and aspirin (81–325 mg daily) within 24 hours of the procedure. Unfractionated heparin was administered during the procedure. Predilation of the lesion was performed with the Gateway balloon diameter estimated at 80% of the normal vessel size and length approximated to the lesion. The stent diameter was sized to be equal to or the next size up from the largest vessel diameter with length estimated to cover the lesion plus 3 mm on either side of the lesion. After the procedure, patients were admitted to an intensive care or stroke unit for 24 hours of monitoring. Aspirin (81–325 mg daily) was recommended throughout follow-up and clopidogrel (75 mg daily) was recommended for at least 4 weeks after stenting.
Evaluation of outcomes.
The primary endpoint for this study was defined as any stroke or death within 30 days of stenting or ischemic stroke in the territory of the stented artery beyond 30 days. Procedural-related complications within 24 hours including arterial dissection, vasospasm, vessel perforation, and acute stent thrombosis were also evaluated. Technical success was defined as balloon angioplasty and stent placement across the target lesion with less than 50% residual stenosis on immediate postprocedure angiography.
Follow-up information was obtained on each patient through review of medical records, personal interview, or telephone contact. Patients were followed to the date of stroke, death, or last contact. Adjudications of all strokes and stenting complications were performed by the local study investigators only.
Statistical analysis.
Baseline features and angiographic results are presented as means (±SD) and as percentages with 95% confidence intervals (CIs) for binary clinical outcomes calculated using the exact binomial method. The cumulative probability of the primary endpoint (any stroke or death within 30 days or stroke in the territory after 30 days) over time was estimated using the Kaplan-Meier (product limit) method with pointwise CIs calculated using the log cumulative hazard transformation.
Univariate analyses to assess patient and site characteristics associated with the primary endpoint were done with the log-rank test. Continuous factors were categorized into two groups; analyses without categorizing yielded similar results. Cox proportional hazards regression was used to calculate the hazard ratio and the 95% confidence interval (CI). A multivariable Cox proportional hazards model incorporated all variables that were associated with the primary endpoint in the univariate analysis at the p < 0.10 level. Since all factors in the model had p values ≤0.064, no further selection procedures were utilized. Cerebrovascular complications within 24 hours between high (enrolled ≥10 patients) and low enrollment sites (enrolled <10 patients) were compared using Fisher exact test.
RESULTS
Baseline and procedural characteristics.
During an 11-month period, 160 patients were treated with the Gateway balloon and Wingspan stent system. Cerebral angiography confirmed that 99% (158/160) of patients had a 50–99% intracranial stenosis and these 158 subjects are included in this analysis. Baseline and procedural characteristics are shown in table 1. The mean age of patients in the registry was 63.9 ± 12.6 years, 83% were white, and 44% were female. Stroke was the indication for stenting in 59%. Median time from qualifying event to stenting was 10 days (range 0–275 days) with 37% of patients undergoing stenting within 5 days of their qualifying event.
Table 1 Baseline and procedural characteristics of patients enrolled in the NIH Multicenter Wingspan Registry (n = 158)
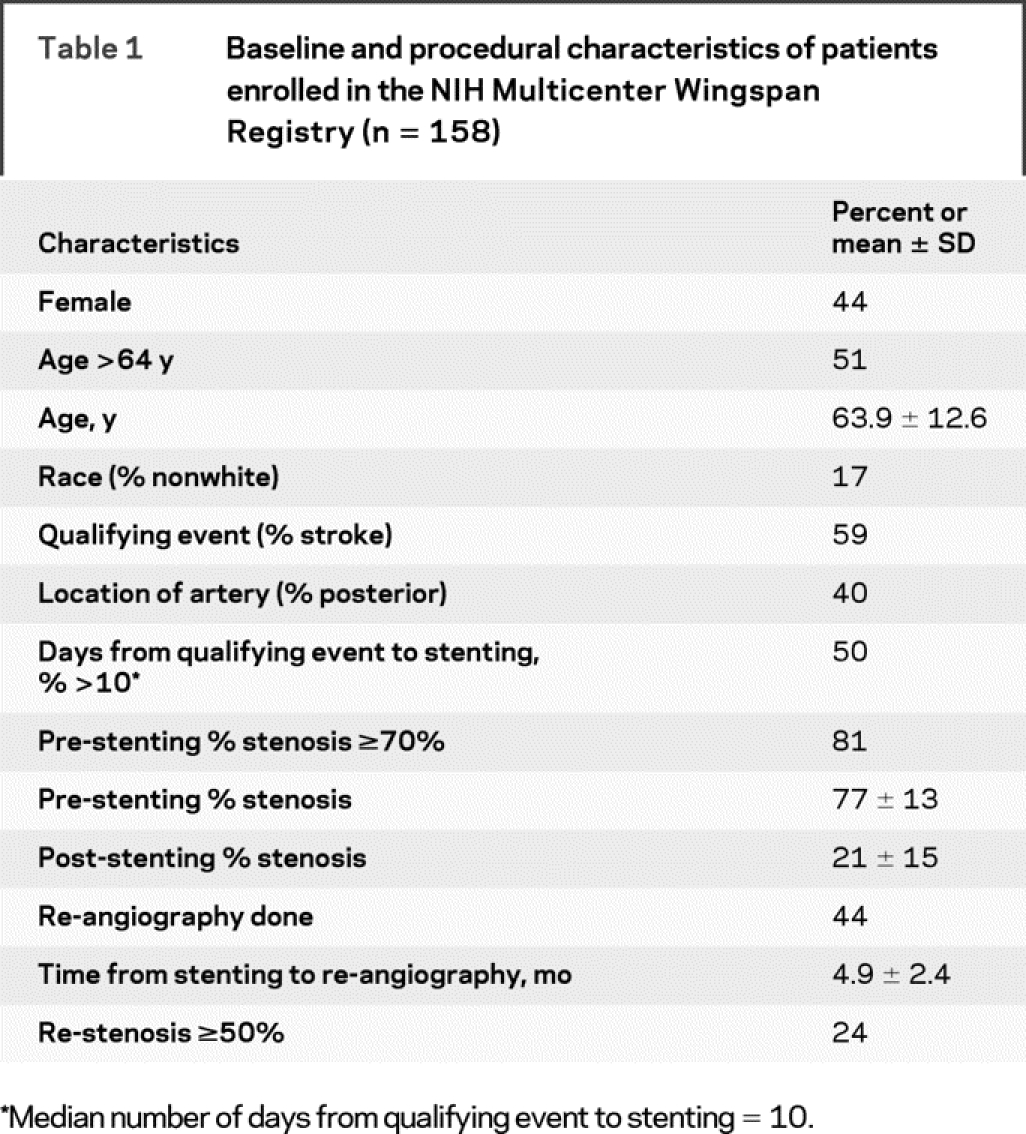
The stented arteries included the middle cerebral artery in 34%, intracranial carotid artery in 26%, intracranial vertebral artery in 23%, and basilar artery in 17%. Mean pre-stenting percent stenosis was 77 ± 13%. Technical success was achieved in 97% with mean post-stenting percent stenosis of 21 ± 15%. Follow-up cerebral angiography was performed in 70 patients (44%) at a mean of 4.9 ± 2.4 months with re-stenosis ≥50% seen in 17 patients (24%).
Primary endpoint during follow-up.
Median follow-up in the study was 5.4 months. Any stroke or death occurred in 5.0% (95% CI = 2.5% to 9.7%) of patients within 24 hours of the stenting procedure and in 9.2% (95% CI = 5.6% to 15.1%) of patients within 30 days. The primary endpoint (any stroke or death within 30 days or stroke in the territory of the stented artery beyond 30 days) at 6 months occurred in 13.9% (95% CI = 5.9% to 21.1%).
Factors associated with the primary endpoint.
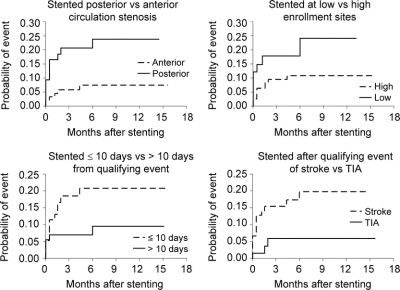
Risk factors that were associated with a significantly higher rate of the primary endpoint after stenting on univariable (table 2) and multivariable analyses were posterior circulation stenosis (vs anterior circulation) (HR 3.4, 95% CI 1.2–9.3, p = 0.018), stenting at low enrollment site (vs high enrollment site) (HR 2.8, 95% CI 1.1–7.6, p = 0.038), ≤10 days from qualifying event to stenting (vs ≥10 days) (HR 2.7, 95% CI 1.0–7.8, p = 0.058), and stroke as a qualifying event (vs TIA/other) (HR 3.2, 95% CI: 0.9–11.2, p = 0.064). Kaplan-Meier curves for each of these risk factors are shown in figure 1. There was no significant difference in the primary endpoint based on age, gender, race, or baseline percent stenosis (<70% vs ≥70%).
Table 2 Baseline characteristics vs primary endpoint for patients enrolled in the NIH Multicenter Wingspan Registry
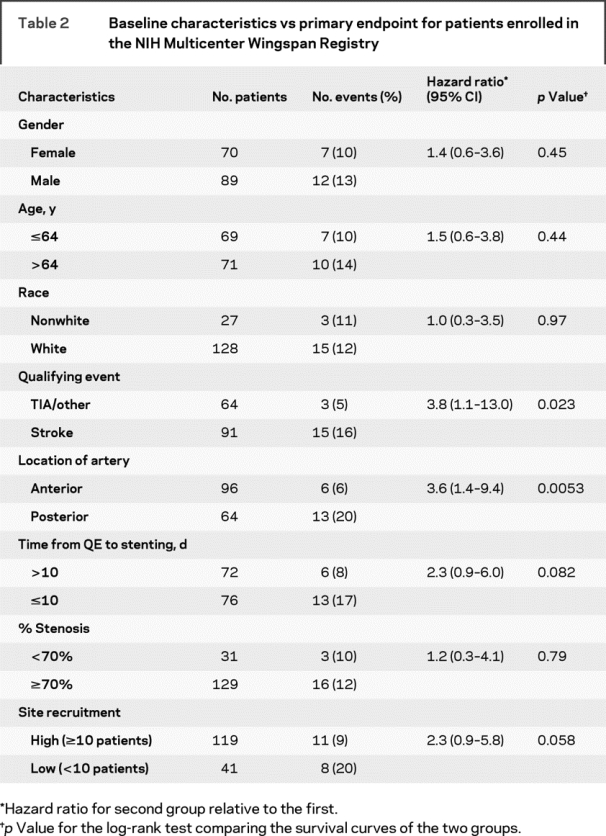
Figure 1 Kaplan-Meier curves for risk factors associated with the primary endpoint (stroke or death within 30 days or stroke in territory after 30 days) over median follow-up of 5.4 months
Complication rates within 24 hours after stenting based on site experience.
Low enrollment sites (enrolled <10 patients each) included 10 centers that collectively enrolled 41 patients. High enrollment sites (enrolled ≥10 patients each) included 6 centers that collectively enrolled 119 patients. Baseline characteristics between the two groups were similar in gender, age, race, stroke as qualifying event, location of symptomatic intracranial stenosis, and the time from qualifying event to stenting. Low enrollment sites had a higher mean pre-stenting percent stenosis than high enrollment sites (low enrollment 81%, high enrollment 76%, p = 0.036), although the percent of patients with high grade stenosis (≥70%) was similar between the two groups (low enrollment 85%, high enrollment 79%, p = 0.37). Technical and angiographic results were also similar in both groups with high rates of technical success (high enrollment 98%, low enrollment 95%, p = 0.28) and low post-stenting percent stenosis (high enrollment 22 ± 22%, low enrollment 20 ± 12%, p = 0.49). Follow-up angiography was performed more frequently at high vs low enrollment sites (high enrollment 52%, low enrollment 20%, p = 0.0003), although time from stenting to follow-up angiography and re-stenosis rates were not significantly different between the two groups (high enrollment 38%, low enrollment 23%, p = 0.39).
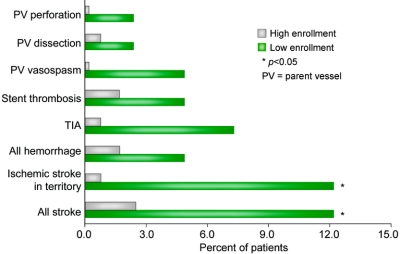
Patients at low enrollment sites had significantly higher rates of cerebrovascular complications (stroke, TIA, intracerebral hemorrhage, vasospasm, parent vessel dissection, parent vessel perforation, or acute stent thrombosis) at 24 hours compared with patients at high enrollment sites (low volume 29.3%, high volume 5.0%, p = 0.0001). Specific cerebrovascular complication rates of both groups are shown in figure 2. Low enrollment sites had significantly higher rates of all stroke and ischemic stroke in the territory than high enrollment sites. Other complications, including hemorrhage, TIA, acute stent thrombosis, parent vessel vasospasm, dissection, and perforation, were numerically higher at low enrollment sites compared with high enrollment sites but were not significantly different. An analysis of patients treated early in the sequence (up to the initial five patients) at low and high enrollment sites revealed that cerebrovascular complications remained significantly higher at low enrollment sites (low enrollment 32.4%, high enrollment 6.7%, p = 0.013).
Figure 2 Cerebrovascular complication rates within 24 hours at low enrollment vs high enrollment sites
DISCUSSION
We found that patients who underwent intracranial angioplasty and stenting for symptomatic intracranial stenosis of 50–99% had a significantly higher risk of the primary endpoint (any stroke or death within 30 days of stenting or ischemic stroke in the territory of the stented artery beyond 30 days) if they had a posterior circulation stenosis, were treated at low volume sites, were stented soon after a qualifying event, or had a stroke as the qualifying event rather than a TIA. As seen on the Kaplan-Meier curves, patients with any of these risk factors appeared to have an elevated risk of the primary endpoint beginning within the first week after intracranial stenting.
Several case series have previously reported clinical outcomes in patients who underwent intracranial angioplasty and stenting of the posterior circulation.6,13,15,18,19 In one early case series, any stroke or death within 30 days was reported in 4 of 11 patients treated with a coronary stent.6 A more recent and larger case series from China included 79 patients treated with a coronary or intracranial stent and reported a 30-day stroke or death rate of 4.6%.15 The Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries study, a multicenter nonrandomized prospective study using the NEUROLINK system, reported a 30-day risk of stroke in 6.6% (4/61) of patients, including three ischemic strokes in the posterior circulation and one subarachnoid hemorrhage.20 Some authors have suggested that there may be an increased risk of perforator strokes associated with stenting of the basilar artery due to compression of plaque into perforator ostia.21 In our patient registry, we found that patients who underwent stenting of their basilar arteries had a trend toward higher rates of the primary endpoint than patients who underwent vertebral artery stenting, although this did not reach significance (basilar artery 30%, vertebral artery 14%; logrank p = 0.12).
Patients treated at low enrollment sites also had significantly higher rates of the primary endpoint than patients treated at high enrollment sites, with the majority (51%) of events at low enrollment sites occurring within 24 hours of the procedure. Mean pre-stenting percent stenosis was higher at low volume sites compared with high volume sites, suggesting that the patients at low volume sites may have been at a higher risk. It is important to note, however, that the number of patients with pre-stenting stenosis of ≥70% was similar between the two groups. Other potential differences in baseline features of patients at low vs high enrolling sites that were not measured in this study could have contributed to the differences in outcomes of these two groups.
Differences in periprocedural adverse events between low and high enrollment sites in our study may represent differences in the procedural experience of interventionalists at the various sites before enrollment in the registry. Interventionalists at high enrollment sites likely performed more intracranial angioplasty and stenting procedures before enrollment in the registry than interventionalists at low enrollment sites. A similar procedural learning curve has previously been demonstrated to influence clinical outcomes in carotid endarterectomy22–24 and carotid stenting25,26 with a decrease in periprocedural complications associated with more procedural experience. If this learning curve is not achieved with stenting for intracranial arterial stenosis, the high rate of periprocedural complications may outweigh any potential benefit of stenting.
Intracranial angioplasty and stenting within 10 days of the qualifying event and stroke as the qualifying event were both found to be risk factors for the primary endpoint with trends toward significance. While angioplasty and stenting soon after a stroke may be associated with poststroke cerebrovascular hemodynamic changes, the trend toward a higher risk of major cerebrovascular complications in stenting soon after the qualifying event persisted even after adjusting for stroke as the qualifying event. It is possible that recent events (stroke or TIA) may be associated with unstable atherosclerotic plaque that increases the risk of stenting. Notably, patients with recent ischemic symptoms and stroke as the qualifying event are also at a high risk of recurrent ischemic events on medical therapy.5 Therefore, optimizing the time of stenting to minimize the risk of stroke should be a focus of research in this area.
Limitations of our study include the lack of central adjudication of events and cerebral angiograms and the lack of prospective follow-up of study patients. Because in-person follow-up was not required and relied on patient self-report, the risk of stroke may have been underestimated. In addition, since the main focus of the registry was to collect preliminary data on the periprocedural complications of intracranial stenting, detailed information on medical comorbidities, the number of intracranial stenting procedures performed by interventionalists at the various sites before inclusion into the registry, and violations of the recommended protocol were not collected. This limits our ability to correlate medical comorbidities, previous stenting experience, and protocol violations with outcome following stenting. Finally, because we did not obtain data on patients treated with stents other than Wingspan, it is possible that low enrollment sites may have performed an equivalent number of stenting procedures utilizing coronary stents and only utilized Wingspan stents for more technically challenging cases, resulting in higher rates of periprocedural complications than high enrollment sites.
Nevertheless, our results suggest that adverse events after intracranial angioplasty and stenting may be associated with posterior circulation stenosis, low volume sites, stenting soon after a qualifying event, and stroke as the qualifying event. These factors will need to be monitored in future trials of intracranial stenting.
AUTHOR CONTRIBUTIONS
Statistical analyses completed by Michael Lynn, MS, Department of Biostatistics, Rollins School of Public Health, Emory University, Atlanta, GA.
Supplementary Material
Address correspondence and reprint requests to Dr. Fadi Nahab, 1365 Clifton Road, NE, Building A, 3rd Floor, Atlanta, GA 30322 fnahab@emory.edu
Editorial, page 1974
e-Pub ahead of print on March 18, 2009, at www.neurology.org.
Supported by NIH/NINDS grant R01 NS051688-01 to Dr. Marc Chimowitz.
Disclosure: Drs. Zaidat, Alexander, Barnwell, Lutsep, Chaloupka, and Mawad consult for or have received an honorarium from Boston Scientific Inc., the manufacturers of the Wingspan stent.
Medications and Medical Devices: clopidogrel (Plavix®, Bristol-Myers Squibb/Sanofi Pharmaceuticals, Bridgewater, NJ); Gateway® balloon angioplasty (Boston Scientific, Fremont, CA); Wingspan® intracranial stent (Boston Scientific, Fremont, CA).
Presented in part at the annual meeting of the American Academy of Neurology, Chicago, IL, April 17, 2008; and the International Stroke Conference, New Orleans, LA, February 21, 2008.
Received September 4, 2008. Accepted in final form January 13, 2009.
REFERENCES
- 1.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. Stroke 1995;26:14–20. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke 1998;29:415–421. [DOI] [PubMed] [Google Scholar]
- 3.Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996;27:1974–1980. [DOI] [PubMed] [Google Scholar]
- 4.Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology 1998;50:812–813. [DOI] [PubMed] [Google Scholar]
- 5.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–563. [DOI] [PubMed] [Google Scholar]
- 6.Gomez CR, Misra VK, Liu MW, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke 2000;31:95–99. [DOI] [PubMed] [Google Scholar]
- 7.Zhang QZ, Miao ZR, Li SM, et al. Complications of stent-assisted angioplasty of symptomatic intracranial artery stenosis [in Chinese]. Zhonghua Yi Xue Za Zhi 2003;83:1402–1405. [PubMed] [Google Scholar]
- 8.Jiang WJ, Du B, Wang YJ, et al. Symptomatic intracranial artery stenosis: angiographic classifications and stent-assisted angioplasty [in Chinese]. Zhonghua Nei Ke Za Zhi 2003;42:545–549. [PubMed] [Google Scholar]
- 9.de Rochemont M, Turowski B, Buchkremer M, et al. Recurrent symptomatic high-grade intracranial stenoses: safety and efficacy of undersized stents: initial experience. Radiology 2004;231:45–49. [DOI] [PubMed] [Google Scholar]
- 10.Liu JM, Hong B, Huang QH, et al. Safety and short-term results of stent-assisted angioplasty for the treatment of intracranial arterial stenosis [in Chinese]. Zhonghua Yi Xue Za Zhi 2004;42:169–172. [PubMed] [Google Scholar]
- 11.Jiang WJ, Wang YJ, Du B, et al. Stenting of symptomatic M1 stenosis of middle cerebral artery: an initial experience of 40 patients. Stroke 2004;35:1375–1380. [DOI] [PubMed] [Google Scholar]
- 12.Lylyk P, Vila JF, Miranda C, et al. Endovascular reconstruction by means of stent placement in symptomatic intracranial atherosclerotic stenosis. Neurol Res 2005;27 (suppl 1):S84–S88. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Lee BH, Kim DI, et al. Stent-assisted angioplasty of symptomatic intracranial vertebrobasilar artery stenosis: feasibility and follow-up results. AJNR Am J Neuroradiol 2005;26:1381–1388. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CY, Yim MB. Primary stent therapy for symptomatic intracranial atherosclerotic stenosis: 1-year follow-up angiographic and midterm clinical outcomes. J Neurosurg 2006;105:235–241. [DOI] [PubMed] [Google Scholar]
- 15.Jiang WJ, Xu XT, Du B, et al. Long-term outcome of elective stenting for symptomatic intracranial vertebrobasilar stenosis. Neurology 2007;68:856–858. [DOI] [PubMed] [Google Scholar]
- 16.Freitas JM, Zenteno M, Aburto-Murrieta Y, et al. Intracranial arterial stenting for symptomatic stenoses: a Latin American experience. Surg Neurol 2007;68:378–386. [DOI] [PubMed] [Google Scholar]
- 17.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology 2008;70:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy EI, Horowitz MB, Koebbe CJ, et al. Transluminal stent-assisted angioplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery 2001;48:1215–1223. [DOI] [PubMed] [Google Scholar]
- 19.Levy EI, Hanel RA, Bendok BR, et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg 2002;97:1294–1301. [DOI] [PubMed] [Google Scholar]
- 20.SSYL VIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): study results. Stroke 2004;35:1388–1392. [DOI] [PubMed] [Google Scholar]
- 21.Jiang WJ, Srivastava T, Gao F, et al. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology 2006;66:1868–1872. [DOI] [PubMed] [Google Scholar]
- 22.Wennberg DE, Lucas FL, Birkmeyer JD, et al. Variation in carotid endarterectomy mortality in the Medicare population. JAMA 1998;279:1278–1281. [DOI] [PubMed] [Google Scholar]
- 23.Cebul RD, Snow RJ, Pine R, et al. Indications, outcomes and provider volumes for carotid endarterectomy. JAMA 1998;279:1282–1287. [DOI] [PubMed] [Google Scholar]
- 24.Karp HR, Flanders WD, Shipp CC, et al. Carotid endarterectomy among Medicare beneficiaries. Stroke 1998;29:46–52. [DOI] [PubMed] [Google Scholar]
- 25.Lin PH, Bush RL, Peden EK, et al. Carotid artery stenting with neuroprotection: assessing the learning curve and treatment outcome. Am J Surg 2005;190:850–857. [DOI] [PubMed] [Google Scholar]
- 26.Verzini F, Cao P, De Rango P, et al. Appropriateness of learning curve for carotid artery stenting: an analysis of periprocedural complications. J Vasc Surg 2006;44:1205–1212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.