Abstract
Background:
Findings from a small clinical study suggested that statins may counteract the therapeutic effects of interferon beta (IFNβ) in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods:
We conducted a post hoc analysis of data from the Safety and Efficacy of Natalizumab in Combination With IFNβ-1a in Patients With Relapsing-Remitting Multiple Sclerosis (SENTINEL) study to determine the effects of statins on efficacy of IFNβ. SENTINEL was a prospective trial of patients with RRMS treated with natalizumab (Tysabri®, Biogen Idec, Inc., Cambridge, MA) plus IM IFNβ-1a (Avonex®, Biogen Idec, Inc.) 30 μg compared with placebo plus IM IFNβ-1a 30 μg. Clinical and MRI outcomes in patients treated with IM IFNβ-1a only (no-statins group, n = 542) were compared with those of patients taking IM IFNβ-1a and statins at doses used to treat hyperlipidemia (statins group, n = 40).
Results:
No significant differences were observed between treatment groups in adjusted annualized relapse rate (p = 0.937), disability progression (p = 0.438), number of gadolinium-enhancing lesions (p = 0.604), or number of new or enlarging T2-hyperintense lesions (p = 0.802) at 2 years. More patients in the statins group reported fatigue, extremity pain, muscle aches, and increases in hepatic transaminases compared with patients in the no-statins group. Statin treatment had no ex vivo or in vitro effect on induction of IFN-stimulated genes.
Conclusions:
Statin therapy does not appear to affect clinical effects of IM interferon beta-1a in patients with relapsing-remitting multiple sclerosis or the primary molecular response to interferon beta treatment.
GLOSSARY
- ANCOVA
= analysis of covariance;
- CI
= confidence interval;
- EDSS
= Expanded Disability Status Scale;
- GAPDH
= glyceraldehyde-3-phosphate dehydrogenase;
- Gd+
= gadolinium-enhancing;
- IFNβ
= interferon beta;
- ISG
= IFN-stimulated gene;
- RRMS
= relapsing-remitting multiple sclerosis;
- SENTINEL
= Safety and Efficacy of Natalizumab in Combination With IFNβ-1a in Patients With Relapsing-Remitting Multiple Sclerosis.
In addition to interfering with cholesterol synthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are pleiotropic compounds with anti-inflammatory, immunomodulatory, and antithrombotic effects.1 Statins are beneficial in experimental autoimmune encephalomyelitis, an animal model of relapsing-remitting multiple sclerosis (RRMS).2,3 We conducted a post hoc analysis of the Safety and Efficacy of Natalizumab in Combination With IFNβ-1a in Patients With Relapsing-Remitting Multiple Sclerosis (SENTINEL) study of natalizumab4 (Tysabri®, Biogen Idec, Inc., Cambridge, MA) to explore the effects of statins on the efficacy of IM IFNβ-1a (Avonex®, Biogen Idec, Inc.) in patients with RRMS. We also conducted studies to assess the impact of statins on interferon (IFN)-induced gene expression.
METHODS
Design.
The methods for the SENTINEL study were described previously.4 Patients from the IM IFNβ-1a monotherapy arm (n = 582) were divided into two groups: a statins group (n = 40) and a no-statins group (n = 542). The statins group patients took statins during the course of the study. Ethics committee approval and informed patient consent were obtained for the original SENTINEL study.4 The present post hoc analysis did not involve interaction with patients, acquisition of new data, or identifying patients. Therefore, additional approval and informed consent was not requested.
Endpoints.
Analyses were conducted on 2-year endpoints, including cumulative probability of sustained disability progression as defined in the SENTINEL study4; rate of clinical relapse; number of new or enlarging T2-hyperintense lesions; and number of gadolinium-enhancing (Gd+) lesions.4 Safety data also were collected.
Biological response study.
The biological effects of concomitant statins and IM IFNβ-1a administration compared with IM IFNβ-1a alone were assessed in patients with RRMS and in an in vitro model of the effects of statins on IFN-stimulated gene (ISG) expression.
ISG expression was determined in ≥30 patients with RRMS after their first dose of IM IFNβ-1a, and included one patient treated with atorvastatin (Lipitor®, Pfizer, Inc., New York, NY) 80 mg per day before initiation and during treatment with IM IFNβ-1a. Gene expression was determined using a customized cDNA macroarray assay.5 RNA was isolated from blood samples 12 hours before and after the first IM IFNβ-1a injection. ISG induction ratio was defined as fold induction 12 hours after the first dose of IM IFNβ-1a relative to preinjection levels, normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) “housekeeping” gene.6 For in vitro studies, HT1080 and Jurkat cells were incubated for 24 hours with 10 μM mevastatin (Sigma-Aldrich, St. Louis, MO) or left untreated, followed by the addition of recombinant IFNβ (1,000 U/mL) for a further 6 hours. Cells were harvested and RNA (10 μg) was analyzed for ISG expression by nuclease protection assay.7,8
To establish mevastatin activity in these cell culture systems, serum-starved HT1080 cells were treated with mevastatin at various doses (1, 5, or 10 μM) for 24 hours or left untreated. Cytoplasmic and membrane protein extracts were prepared9 and probed with anti-Rac-1 antibodies. Incubation with a primary antibody was carried out at 4°C overnight and with a secondary antibody at 37°C for 1 hour. Immunoreactivity was visualized using the ECL Western blotting system (Amersham Biosciences/GE Healthcare, Chalfont St. Giles, UK). The membrane was stripped and reprobed with anti-β-actin antibodies to normalize protein loading.
Statistical analysis.
The cumulative probability of sustained disability progression was estimated using the Kaplan-Meier method, and treatment effect was determined from a Cox proportional hazards model adjusted for baseline Expanded Disability Status Scale (EDSS) score and baseline number of Gd+ lesions. The mean change in EDSS score from baseline to 2 years was analyzed using an analysis of covariance (ANCOVA) model adjusted for baseline score and the number of relapses in the year before study entry. The annualized relapse rate was analyzed using Poisson regression adjusted for the number of relapses in the year before study entry, baseline EDSS score, baseline number of Gd+ lesions, and age. The number of Gd+ lesions was analyzed using a rank-based ANCOVA adjusted for baseline number of Gd+ lesions and age. The number of new or enlarging T2-hyperintense lesions was analyzed using a rank-based ANCOVA adjusted for baseline number of T2 lesions (<9 vs ≥9), baseline number of Gd+ lesions, and age.
RESULTS
Clinical study.
Patients.
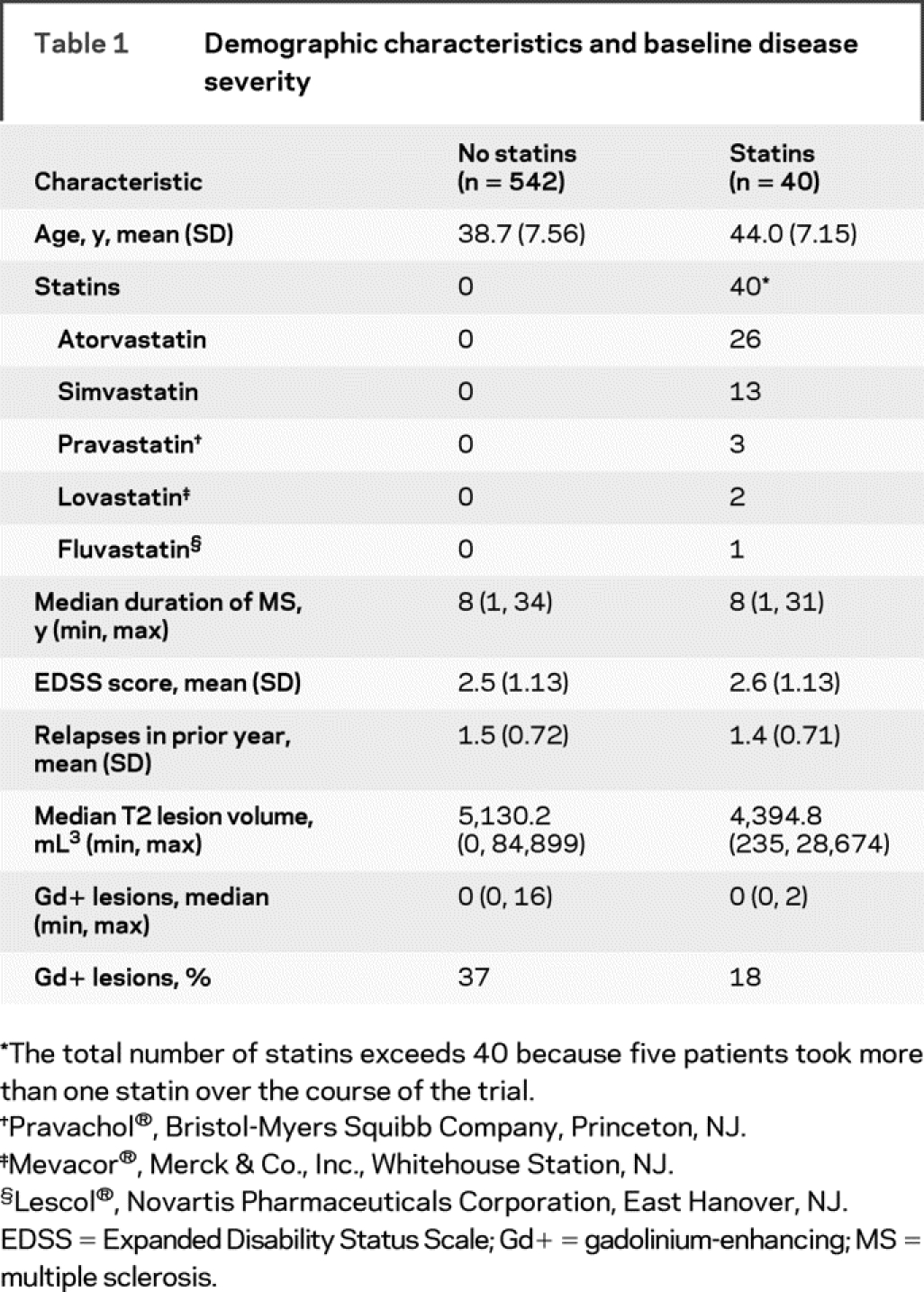
Of the 582 patients randomized to IM IFNβ-1a monotherapy, 40 took at least one dose of statin (statins group) and 542 did not take any statins during the course of the study (no-statins group). Twenty patients were taking statins before randomization; statin therapy was started after the first dose of study medication in 19 patients, and one patient had an unknown statin start date. The median duration of concomitant IM IFNβ-1a and statin administration was 657 days (1.8 years). Most patients took atorvastatin (n = 26) or simvastatin (Zocor®, Merck & Co., Inc., Whitehouse Station, NJ) (n = 13), including four patients who took both throughout the course of the study (table 1). In general, patients were well matched at baseline for disease severity; however, patients in the statins group were older than those in the no-statins group (44.0 vs 38.7 years).
Table 1 Demographic characteristics and baseline disease severity
Outcomes.
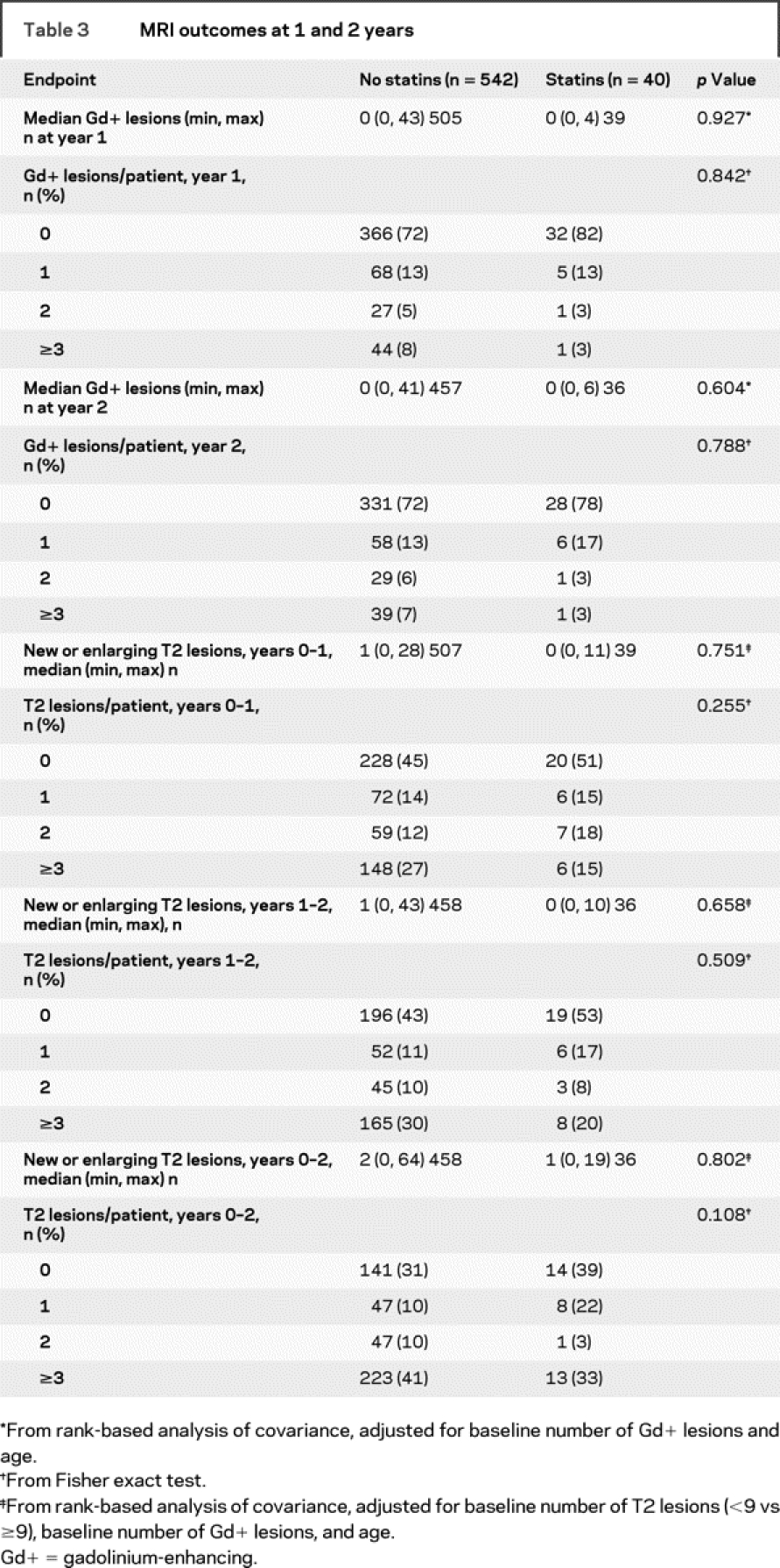
No significant differences were observed between the statins group and the no-statins group on measures of clinical and MRI efficacy (tables 2 and 3). The adjusted annualized relapse rate at 2 years was similar in both groups (p = 0.937), as was the proportion of relapse-free patients and patients with ≥1 relapse (p = 0.466) and the cumulative probability of sustained disability progression (hazard ratio = 1.27 [95% confidence interval (CI) = 0.70, 2.29]; p = 0.438). The median number of Gd+ lesions was 0 for both groups after 1 year (p = 0.927) and 2 years (p = 0.604). Similar proportions of patients in both groups were free of Gd+ lesions at 2 years (p = 0.788). Over 2 years, the median number of new or enlarging T2-hyperintense lesions was one for the statins group compared with two in the no-statins group (p = 0.802).
Table 2 Clinical efficacy outcomes at 2 years
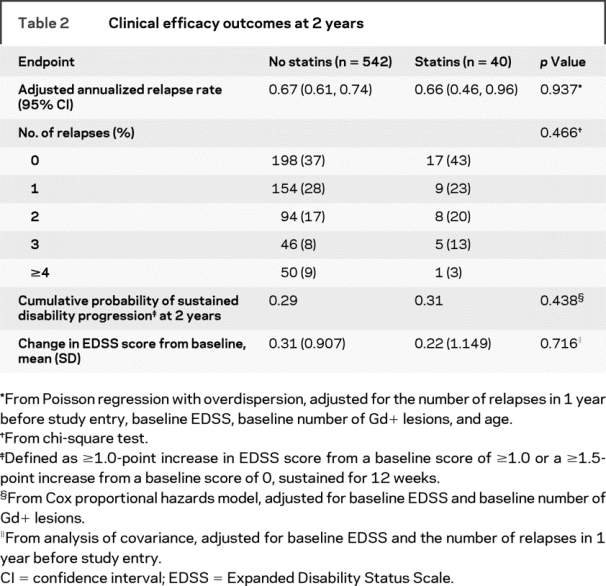
Table 3 MRI outcomes at 1 and 2 years
A sensitivity analysis to determine the effect of uninterrupted therapy on measures of disease progression revealed that there were no significant differences between the 17 patients treated for 2 full years with concomitant statins and IM IFNβ-1a and the 438 patients treated for 2 full years with IM IFNβ-1a monotherapy in adjusted annualized relapse rate (0.59 no statins vs 0.67 statins; p = 0.650); disability progression (0.26 vs 0.18; p = 0.689); or median number of Gd+ lesions (0, both groups; p = 0.997) or T2-hyperintense lesions (2 vs 0; p = 0.612).
The most commonly reported adverse events in patients in the statins group were fatigue, headache, back pain, extremity pain, arthralgia, depression, and asthenia. For patients in the no-statins group, the most common adverse events were headache, nasopharyngitis, fatigue, back pain, arthralgia, and hypoesthesia (table e-1 on the Neurology® Web site at www.neurology.org). The incidence of muscle-related pain was higher for patients in the statins group than for patients in the no-statins group (back pain, 38% statins vs 27% no statins; extremity pain, 35% statins vs 20% no statins; myalgia, 18% statins vs 9% no statins). Patients in the statins group were more likely than patients in the no-statins group to experience a shift from baseline to high alanine transaminase, alkaline phosphatase, and aspartate transaminase levels (table e-1).
Biological response study.
ISG expression analysis.
Macroarray assays documented strong ISG expression (mean number of ISG induction ratios ≥2 = 57) in ≥30 patients with RRMS. One patient concurrently using atorvastatin 80 mg/day expressed 47 ISGs with induction ratio ≥2 (figure e-1).
In vitro effect of mevastatin on ISG expression.
Pretreatment of HT1080 (a fibrosarcoma cell line) or Jurkat (a T-cell line) with mevastatin did not alter IFNβ-1a–induced expression of four well-characterized ISGs (TRAIL, β-R1, 9-27, and 6-16) (figure e-2). Mevastatin reduced the association of Rac-1 with cell membranes indicating biological activity despite lack of effect on ISG induction (figure e-3).
DISCUSSION
In the current analysis of data from SENTINEL, there was no evidence that standard doses of statins used to treat hyperlipidemia reduced the efficacy of IM IFNβ-1a with respect to annualized relapse rates, sustained disability progression, or lesion burden. The adverse events experienced by patients in both groups were similar, although more patients in the statins group reported musculoskeletal pain and hepatic enzyme abnormalities. Additional studies demonstrated robust upregulation of ISGs ex vivo in a patient taking atorvastatin 80 mg at the time of the initial IM IFNβ-1a injection, equivalent to that observed in patients treated with IM IFNβ-1a alone. Mevastatin did not alter ISG induction in vitro, while Rac-1 translocation assays showed that the statin was biologically active in vitro.
These findings contrast with another study that found significantly greater risk of new and enhancing lesions or relapses in patients taking atorvastatin in combination with subcutaneous IFNβ-1a.10 However, there were several methodologic differences between studies. Our study was a retrospective analysis of 2 years of treatment with IM IFNβ1-a, whereas the other was a prospective study of 6 months of treatment with SC IFNβ1-a.10 Patients in our study took five different statins at a variety of doses, most of which were ≤20 mg/day; patients assigned to statin treatment in the other study received atorvastatin 40 or 80 mg/day only.10 Also, the number who received no statins in our study (n = 542) was larger than that in the other study (n = 9).10
Based on the results of the current study, we could discern no significant effect of statins at usual therapeutic doses on treatment with IM IFNβ-1a in patients with RRMS, and biological studies failed to demonstrate an effect of statins on the primary molecular response to IFNβ.
AUTHOR CONTRIBUTIONS
Statistical analysis for this manuscript was conducted by Amy Pace, ScD, Biogen Idec, Inc., Cambridge, MA.
ACKNOWLEDGMENT
The authors thank Matthew Hasson, Scientific Connections, Newtown, PA, for providing copyediting and word processing assistance in preparing this manuscript for submission.
DISCLOSURE
R.A. Rudick has received consulting fees from Biogen Idec, Inc., Genzyme, Millennium Pharmaceuticals, Novartis, Teva, and Wyeth. A. Pace, R. Hyde, and M. Panzara are employees of and have equity interest in Biogen Idec, Inc. S.L. Maurer is an employee of Scientific Connexions, which provided editorial support in preparing this manuscript for submission; this support was funded by Biogen Idec. P.A. Calabresi has received grants from Biogen Idec, EMD-Merck-Serono, Genentech, and Teva, and has served as a consultant for Amplimmune, Biogen Idec, Centocor, Eisai, EMD-Merck-Serono, Genentech, Teva, and Vertex. C. Confavreux has received grants for research support and honoraria from Biogen Idec and has given expert testimony for Biogen Idec. S. Galetta has received research support and honoraria from Biogen Idec. F. Lublin has received grants for research from Acorda Therapeutics, Biogen Idec, Genentech, Genzyme, Novartis, and Teva Neuroscience; honoraria from Actelion, AmGen, Avigen, Bayer, BioMS, GenMab, Medicinova, Pfizer, Serono, and Teva Neuroscience; and has an ownership interest in Cognition Pharma. R.M. Ransohoff conducts research supported by the NIH and the National MS Society. Dr. Ransohoff has received consultant fees from Bayer, Biogen Idec, Merck, Millennium Pharmaceuticals, and Schering-Plough. Dr. Ransohoff serves or has served on the Chemocentryx Scientific Advisory Board, the Boehringer Ingelheim MS Advisory Board, a safety monitoring panel for Merck, and the Immunology/Inflammation Subgroup of the Vertex Pharmaceuticals Scientific Advisory Board. The SENTINEL study was supported by Biogen Idec, Inc., and Elan Pharmaceuticals, Inc. Copyediting was supported by Biogen Idec, Inc.
MEDICATIONS AND MEDICAL DEVICES
Atorvastatin (Lipitor®, Pfizer, Inc., New York, NY); ECL Western blotting system (Amersham Biosciences/GE Healthcare, Chalfont St. Giles, UK); fluvastatin (Lescol®, Novartis Pharmaceuticals Corporation, East Hanover, NJ); IFNβ-1a (Avonex®) and natalizumab (Tysabri®, Biogen Idec, Inc., Cambridge, MA); lovastatin (Mevacor®) and simvastatin (Zocor®, Merck & Co., Inc., Whitehouse Station, NJ); pravastatin (Pravachol®, Bristol-Myers Squibb Company, Princeton, NJ).
Supplementary Material
Address correspondence and reprint requests to Dr. Richard A. Rudick, Mellen Center for Treatment and Research in Multiple Sclerosis, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195 rudickr@ccf.org
Supplemental data at www.neurology.org
The interferon biological response studies were supported by the US National Institutes of Health (NINDS PO1 NS38667 and NCRR MO1 RR-018390) and the National Multiple Sclerosis Society (RG 3604A6/1).
Disclosure: Author disclosures are provided at the end of the article.
Medications and Medical Devices: A list of medications and medical devices used in this study is provided at the end of the article.
Presented in part at the 23rd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Prague, Czech Republic, October 11–14, 2007.
Received September 30, 2008. Accepted in final form March 10, 2009.
REFERENCES
- 1.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol 2005;46:1855–1862. [DOI] [PubMed] [Google Scholar]
- 2.Wekerle H. Tackling multiple sclerosis. Nature 2002;420:39–40. [DOI] [PubMed] [Google Scholar]
- 3.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420:78–84. [DOI] [PubMed] [Google Scholar]
- 4.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 5.Schlaak JF, Hilkens CM, Costa-Pereira AP, et al. Cell-type and donor-specific transcriptional responses to interferon-alpha. Use of customized gene arrays J Biol Chem 2002;277:49428–49437. [DOI] [PubMed] [Google Scholar]
- 6.Lindbom J, Ljungman AG, Lindahl M, Tagesson C. Increased gene expression of novel cytosolic and secretory phospholipase A (2) types in human airway epithelial cells induced by tumor necrosis factor-alpha and IFN-gamma. J Interferon Cytokine Res 2002;22:947–955. [DOI] [PubMed] [Google Scholar]
- 7.Rani MRS, Foster GR, Leung S, et al. Characterization of β-R1, a gene that is selectively induced by interferon-β (IFN-β) compared with IFN-α. J Biol Chem 1996;271:22878–22884. [DOI] [PubMed] [Google Scholar]
- 8.Rani MRS, Pandalai S, Shrock J, et al. Requirement of catalytically active TYK2 and accessory signals for the induction of TRAIL mRNA by IFN-β. J Interferon Cytokine Res 2007;27:767–779. [DOI] [PubMed] [Google Scholar]
- 9.del Pozo MA, Price LS, Alderson NB, et al. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J 2000;19:2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology 2008;71:1390–1395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


