Abstract
Objective
Adipose inflammation is crucial to the pathogenesis of metabolic disorders. This study was aimed at identifying the effects of Stearoyl-CoA desaturase-1 (SCD1) on the inflammatory response of a paracrine network involving adipocytes, macrophages and endothelial cells.
Methods and Results
Loss of SCD1 in both genetic (Agouti) and diet-induced obesity (high-fat-diet) mouse models prevented inflammation in white adipose tissue (WAT) and improved its basal insulin signaling. In SCD1 deficient (SCD1-/-) mice, WAT exhibited lower inflammation with a reduced response to lipopolysaccharide (LPS) in isolated adipocytes, but not in peritoneal macrophages. Mimicking the in vivo paracrine regulation of WAT inflammation, SCD1-/- adipocyte-conditioned medium (CM) attenuated the induction of TNFα/IL-1β gene expression in RAW264.7 macrophages and reduced the adhesion response in endothelial cells. We further demonstrated that the adipocyte-derived oleate (18:1n9), but not palmitoleate (16:1n7), mediated the inflammation in macrophages and adhesion responses in endothelial cells.
Conclusions
Loss of SCD1 attenuates adipocyte inflammation and its paracrine regulation of inflammation in macrophages and endothelial cells. The reduced oleate level is linked to the inflammation-modulating effects of SCD1 deficiency.
Keywords: SCD1, adipocyte, macrophage, endothelial cells, inflammation, oleate, palmitoleate
Chronic inflammation plays a causative role in the emergence of various metabolic disorders including type 2 diabetes, insulin resistance and atherosclerosis.1 This inflammatory condition is provoked by diverse factors including reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, hypoxia, lipotoxicity and protein kinase C isoforms (PKCs).2-4 Importantly, lipids have been implicated in the coordinate regulation of metabolism, inflammatory and immune responses.5 The modulation of inflammation by lipids has been further demonstrated by a recent study which showed that fatty acids are ligands for Toll-like receptor-4 (TLR4) in macrophages. 6
In the initiation of chronic inflammation, white adipose tissue (WAT) plays a central role, though other tissues such as liver might also be involved. 7 The cross-talk among adipocytes, macrophages and endothelial cells in WAT orchestrates the inflammatory response in this tissue. The subsequent increased production of proinflammatory cytokines and chemokines, such as TNFα, IL-6, PAI-1, MCP-1 in WAT, leads to insulin resistance and increases the risk of cardiovascular disease associated with obesity.8, 9 Therefore, prevention of WAT inflammation is potentially beneficial for controlling chronic inflammation.
Stearoyl-CoA desaturase (SCD) catalyzes the rate-limiting step in the conversion of saturated to monounsaturated fatty acids (MUFAs) (mainly oleate (18:1n9) and palmitoleate (16:1n7)). This enzyme plays a central role in lipogenesis in rodents and human.10 Several SCD gene isoforms (SCD1-4) have been identified in the mouse, and two SCD isoforms (SCD1 and 5) that are highly homologous to the mouse SCDs are well characterized in human. Despite the high abundance of oleate (18:1n9) as a major MUFA from diet, the expression of SCD is highly regulated by developmental, dietary, hormonal, and environmental factors. Because of the involvement of MUFAs in the regulation of diverse processes including signal transduction, cell differentiation and neuronal development,11-13 SCD is regarded as an important enzyme in the regulation of normal and patho-physiological processes. Altered SCD activity has been implicated in a variety of morbidities such as obesity, diabetes, atherosclerosis, cancer and immune disorders.13
Although SCD1 has been well-established as a key regulator of metabolism, recent studies have also reported its influence on inflammatory processes.14, 15 However, the function of SCD1 expression in regulating adipocyte and WAT inflammation is poorly understood. In the current study, we demonstrate that SCD1 deficiency prevents WAT inflammation and improves insulin signaling under the challenge of obesity. We further demonstrate for the first time the effects of SCD1 deficiency on adipocyte inflammation, and reveal unique functions of oleate as a lipid inflammation mediator in linking the network of adipocytes, macrophages and endothelial cells.
Methods
Animals and Diets
All mice used in the study were C57BL6/J males. The homozygous SCD1 deficient (SCD1-/-) mice were generated, genotyped and maintained as described 16. The breeding of mice was in accordance with the protocols approved by the animal care research committee of the University of Wisconsin-Madison. A standard Purina formula 5008 chow diet was used as regular food. Male chow-fed mice of 12 to 15 weeks old were used for primary cell preparation. For studies in genetically obese mice, the SCD1 deficiency was introduced into agouti (Ay/a) mice by crossing Agouti:SCD1+/- with SCD1-/- mice and generated Agouti:SCD1-/- mice. Wild-type (Wt) control mice, Agouti and Agouti:SCD1-/- mice were fed ad libitum with chow diet until 24 weeks of age. In diet-induced obesity, 8-week-old wild-type and SCD1-/- mice were fed a high-fat diet (Research Diets, RD12492) until 24 weeks old.
Isolation of primary adipocytes, stromal vascular cells and resident peritoneal macrophages
Primary adipocytes, WAT stromal vascular cells (SVC) and peritoneal macrophages were isolated as described 17 with minor modifications. Age-matched wild type (Wt) and SCD1-/- mice were used in the isolation. The WAT used in all studies was from epididymal fat pad.
Statistical analysis
Values shown in the study were expressed as mean ± S.E.M. Statistical analysis with three or more groups was done using one-way ANOVA with Bonferroni Post test, and the difference between two groups was tested by two-tail, unpaired Student's t-test, in both cases, significance was considered at p<0.05.
For additional methods and details, please refer to http://atvb.ahajournals.org for supplemental materials.
Results
Loss of SCD1 prevents WAT inflammation in obesity mouse models
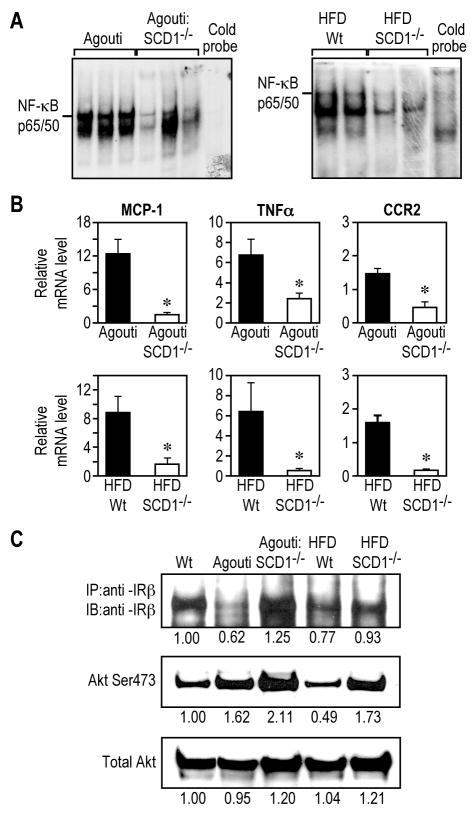
WAT is a critical site in the initiation of the chronic inflammation in obesity. 18 We first examined the effects of SCD1 deficiency on the overall inflammatory status of WAT in both genetic Agouti mutation induced (Agouti) and high-fat-diet induced (HFD) obese mouse models. The activation of nuclear factor κB (NF-κB), a master pro-inflammation transcriptional factor, has been linked to adipose tissue inflammation in obesity. 7 In the Agouti and HFD-fed mice, SCD1 deficiency substantially reduced WAT inflammation, which was shown by a decrease in the DNA binding activity of NF-κB p65/50, the transcriptional active dimer complex of NF-κB (Figure 1A). The expression of MCP-1, TNFα, PAI-1, and VCAM-1 as well as CC-chemokine receptor 2 (CCR2) and colony-stimulating factor-1 receptor (Csf-1R) were also reduced (Figure 1B and Supplemental Figure 1). In addition, cell death in WAT, which is closely correlated with WAT inflammation, was also prevented in SCD1-/- mice as shown by TUNEL staining of WAT sections (Supplemental Figure 1). These results further indicate that loss of SCD1 suppresses obesity-associated WAT inflammation.
Figure 1.
Loss of SCD1 prevents WAT inflammation and improves basal WAT insulin signaling under obese states. A, NF-κB DNA binding activity. B, Expression of proinflammatory genes. C, Levels of IRβ and Akt Ser473 phosphorylation. 4-7 mice per group for gene expression analysis. *, p<0.05.
Inflammation has been linked to insulin resistance, 7 we next analyzed basal insulin signaling in WAT from SCD1-/- mice. The decreased expression of insulin signaling components such as insulin receptor β subunit (IRβ) has been observed in obesity-related chronic insulin resistance.19 In SCD1-/- mice, the decreased protein level of IRβ in WAT from Agouti and HFD-fed Wt mice was improved (Figure 1C). Consistently, the levels of serine 473 phosphorylation (pSer473) of Akt were increased (Figure 1C). Furthermore, the decreased mRNA levels of IR and IRS-1 in WAT from obese mice were also prevented with loss of SCD1 in these mice (Supplemental Figure 1). These results suggest that, consistent with the prevention of WAT inflammation, SCD1 deficiency improves basal WAT insulin signaling under the challenges of obesity.
Loss of SCD1 reduces inflammation in WAT and primary adipocytes, but not in peritoneal macrophages
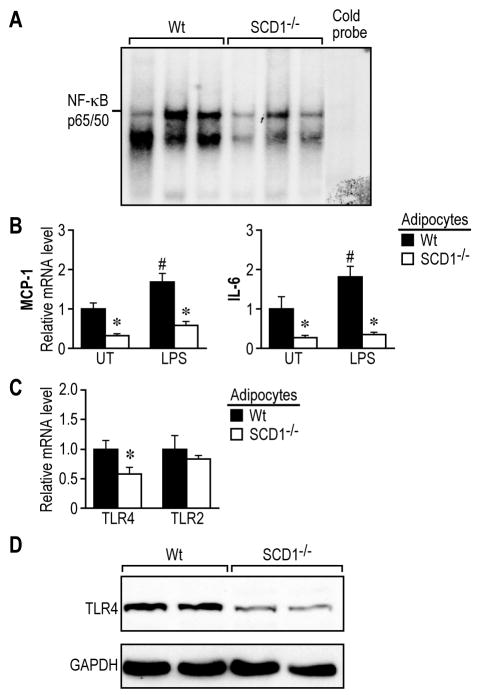
To test the role of SCD1 in WAT inflammation, we next analyzed the inflammatory response in WAT from wild-type (Wt) and SCD1-/- mice which were fed a standard lab chow diet. DNA binding activity of NF-κB p65/50 was reduced in WAT from SCD1-/- mice (Figure 2A). To study the inflammation at cellular level, we further isolated primary adipocytes which were efficiently separated from stromal vascular cells (SVC) in WAT (Supplemental Figure 2). The numbers of isolated adipocytes were comparable between Wt and SCD1-/- mice (Supplemental Figure 3). Consistently, the SCD1-/- adipocytes exhibited lower expression of proinflammatory genes such as monocyte chemoattractant protein 1 (MCP-1) and interleukin-6 (IL-6) (Figure 2B). Upon challenge with lipopolysaccharide (LPS), a Toll-like receptor 4 (TLR4) ligand, the induction of this two genes were also significantly reduced in the SCD1-/- adipocytes (Figure 2B).
Figure 2.
Loss of SCD1 reduces inflammation in WAT and adipocytes. A, NF-κB DNA binding activity in WAT. B, Baseline and LPS (10ng/ml)-induced inflammation in adipocytes. C, mRNA level of TLR4 and TLR2 in adipocytes. D, Protein levels of TLR4 in WAT. p<0.05. *, Wt vs. SCD1-/-; #, LPS vs. un-treated (UT).
We tested the potential contribution of anti-inflammatory factors such as PPARγ and PPARα, and the production of Th2 anti-inflammatory cytokines. The gene expression of PPARγ and its target gene PEPCK, Glut4, ADRP and aP2 were not elevated in SCD1-/- adipocytes (Supplemental Figure 4), and expression of Th2 cytokines IL-4 and IL-13 were undetectable in these adipocytes. The expression of PPARα target genes CPT1 and MCAD were not increased in the adipocytes from SCD1-/- mice (Supplemental Figure 4). These data suggest that the anti-inflammatory pathways were not involved in the inflammation-attenuating effects of SCD1 deficiency. Furthermore, the expression of certain marker genes for mitochondrial function, oxidative stress and ER stress response were not substantially different between Wt and SCD1-/- adipocytes (Supplemental Figure 4, 5), suggesting that these processes may not play dominant role in SCD1-/- adipocytes.
In isolated peritoneal macrophages, the baseline and LPS-stimulated expression of proinflammatory genes MCP-1, TNFα, Cox-2 and IL-6 were not significantly different between Wt and SCD1-/- mice (Supplemental Figure 2). A similar phenomenon was observed upon treatment with lipoteichoic acid (LTA), a TLR2 ligand. Additionally, no genotypic difference was detected in the expression of TLR4 and TLR2, two key receptors mediating inflammation. These data indicate that the modulation of inflammation by SCD1 deficiency is cell-type selective for adipocytes which express high levels of SCD1.
Furthermore, SCD1-/- adipocytes displayed significantly lower TLR4 gene expression, but not TLR2 (Figure 2C). In parallel, TLR4 protein level was also lower in WAT from SCD1-/- mice (Figure 2D). This selective down-regulation of TLR4 expression in SCD1-/- adipocytes may be one of the causative factors in attenuating the inflammatory responses.
SCD1-/- adipocyte-conditioned medium induces lower inflammation in RAW264.7 macrophages
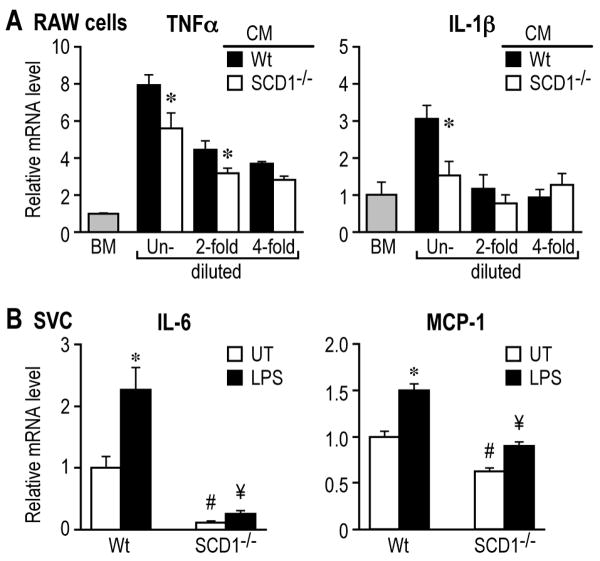
WAT inflammation is attributable to the interaction between adipocytes and resident macrophages in a paracrine manner.20, 21 We collected the Wt and SCD1-/- adipocyte-conditioned media (CM) which contain adipocyte-derived soluble factors, and tested their effects on inflammation. The induction of proinflammatory genes TNFα and IL-1β was significantly lower in RAW264.7 macrophages treated with SCD1-/- CM, compared to the treatment with Wt CM (Figure 3A). As the two adipocyte CMs were further diluted by 2- and 4-fold with basal media (BM), the difference in the induction of TNFα and IL-1β genes was reduced, and comparable induction was observed at 4-fold dilution (Figure 3A). These data indicate that adipocyte-derived soluble factors are the likely mediators of inflammation in macrophages, and that the levels of these factors are lower in SCD1-/- CM.
Figure 3.
SCD1-/- adipocyte-conditioned medium (CM) reduces inflammation in macrophages. A, Induction of TNFα and IL-β in RAW264.7 macrophages. *, p<0.05. B, Expression of IL-6 and MCP-1 in SVC. UT, un-treated; LPS, 10ng/ml. p<0.05, *, LPS vs. UT; #, ¥, SCD-/- vs. Wt.
SVC from WAT contains multiple cell types including tissue macrophages, endothelial cells, preadipocytes and others. With SCD1 deficiency, these cells which are subject to paracrine regulation by adipocytes in WAT in vivo showed reduced expression of proinflammatory genes IL-6 and MCP-1 under both basal and LPS-stimulated conditions (Figure 3B).
SCD1-/- adipocyte-conditioned media reduces the adhesion response of endothelial cells
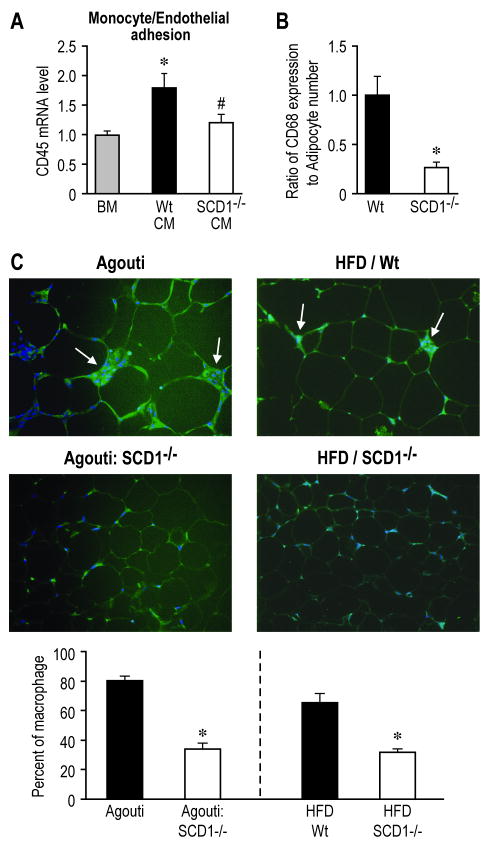
The paracrine regulation of WAT inflammation involving adipocytes also influences adhesion pathways in endothelial cells, which recruit circulating monocytes into WAT.21 We next investigated the effects of SCD1-/- CM on adhesion of mouse monocytes to mouse aortic endothelial cells. Compared to the endothelial cells incubated with Wt CM, SCD1-/- CM significantly decreased the adhesion of monocytes to endothelial cells, as assessed by the expression of leukocyte specific gene CD45 after the adhesion assay (Figure 4A). Consistently, the mRNA levels of adhesion molecules ICAM-1 and P-Selectin were also significantly lower in endothelial cells treated with SCD1-/- CM, as shown in Figure 6C. Furthermore, the SVC from WAT of SCD1-/- mice displayed lower ratio of CD68 (macrophage marker gene) expression to adipocyte number and reduced expression of Mac1 (another macrophage marker gene) (Figure 4B, Supplemental Figure 7), suggesting less abundance of macrophages in WAT.
Figure 4.
SCD1-/- CM attenuates endothelial adhesion response and SCD1 deficiency prevents macrophage infiltration into WAT in obesity. A, The adhesion response of endothelial cells treated with CMs. p<0.05, *, Wt vs. BM; # SCD1-/- vs. Wt. B, The ratio of CD68 expression in SVC to adipocyte number. * p<0.05. C, Macrophage infiltration in WAT. * p<0.05.
Figure 6.
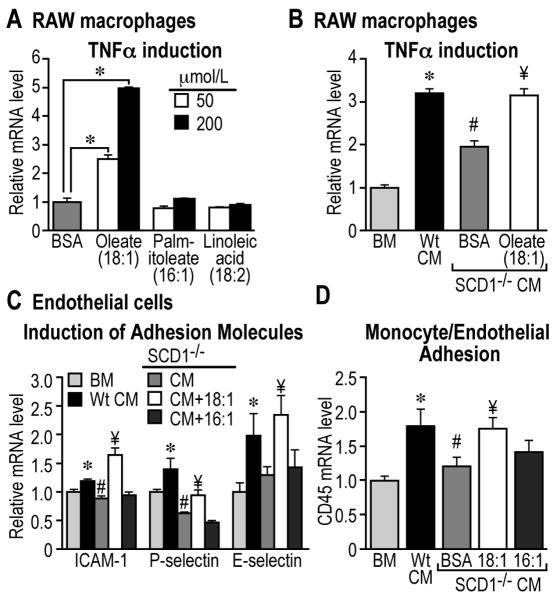
Oleate (18:1n9) promotes macrophage inflammation and endothelial adhesion. A, Induction of TNFα in RAW264.7 macrophages. * p<0.05. Supplementation of oleate to SCD1-/- CM enhances TNFα expression (B), elevates adhesion molecules expression (C), and promotes monocyte/endothelial adhesion (D). p<0.05, *, Wt vs. BM; # SCD1-/- vs. Wt; ¥, 18:1 vs. BSA.
SCD1-/- mice are resistant to obesity-associated macrophage infiltration into WAT
Given the reduced adhesion response in endothelial cells upon treatment with SCD1-/- CM, we further tested the effects of SCD1 deficiency on macrophage infiltration into WAT in obesity. SCD1-/- mice under two different obesity challenges (the genetic model Agouti 22 and HFD model) exhibited significantly decreased macrophage abundance as shown by Emr1 (F4/80) staining in WAT sections (Figure 4C) and lower expression of Emr1 (F4/80) and CD68 (Supplemental Figure 1). In addition, analysis of the cell morphology in WAT from SCD1-/- mice also revealed diminished multi-nucleated cell structures which are typical of the infiltrated macrophages in WAT of obese mice (Supplemental Figure 1).
Loss of SCD1 leads to reduced content of unsaturated fatty acids in adipocytes and the adipocyte conditioned media
Next, we set out to identify the adipocyte-derived inflammatory mediators in the CM that were affected by loss of SCD1. Adiponectin and leptin are two adipocyte-specific adipokines which are known to exert endocrine and paracrine regulation on inflammation.9 The levels of adiponectin in CMs were not significantly different between Wt and SCD1-/- adipocytes (Supplemental Figure 6). Levels of leptin in Wt CM tended to be slightly higher, but were not statistically different from that in SCD1-/- CM.
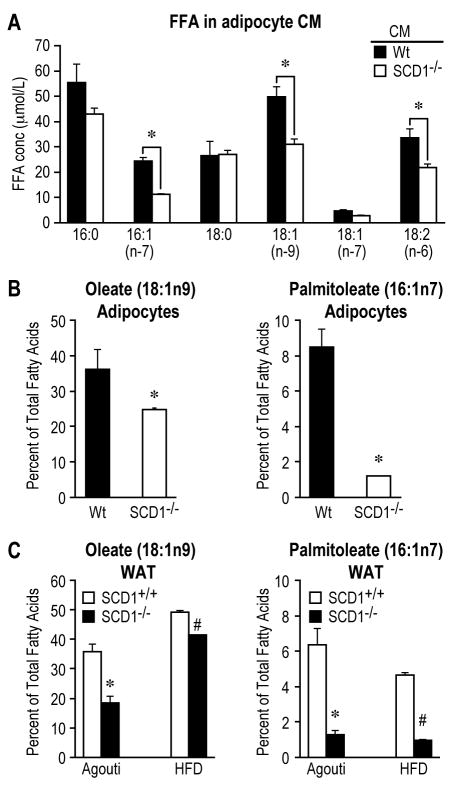
Free fatty acids (FFA), which are released by adipocytes, have been well-established as active regulators for inflammation.6, 20, 23 SCD1 is a key lipogenic enzyme responsible for the de novo synthesis of monounsaturated fatty acids (MUFA), mainly oleate (18:1n9) and palmitoleate (16:1n7). Thus, loss of SCD1 might cause an alteration in the profile of adipocyte-derived FFAs which in turn modulates inflammation. Indeed, compared to Wt CM, SCD1-/- CM exhibited significantly lower levels of palmitoleic (16:1n7) and oleic (18:1n9), but comparable levels of the corresponding saturated FFAs, palmitic (16:0) and stearic acid (18:0), respectively (Figure 5A). These FFAs were mainly derived from adipocytes, as their levels in plain 10%FBS/DMEM media were substantially lower (Supplemental Figure 7). Consistently, the contents of oleate (18:1n9) and palmitoleate (16:1n7) were also significantly reduced in SCD1-/- adipocytes (Figure 5B). Furthermore, WAT from Agouti and HFD-fed mice with SCD1 deficiency exhibited decreased oleate (18:1n9) and palmitoleate (16:1n7) levels (Figure 5C). The level of linoleic acid (18:2n6) was also reduced in the CM of SCD1-/- adipocytes (Figure 4A), but the content of this FA in WAT was comparable in Wt and SCD1-/- mice (data not shown). In parallel with the reduced concentrations of FFAs in adipocyte CM, WAT and adipocytes from SCD1-/- mice exhibited decreased levels of LPL, the enzyme responsible for fatty acid uptake in various tissues including adipose tissue (Supplemental Figure 7).
Figure 5.
SCD1 deficiency reduces levels of unsaturated fatty acids. A, Free fatty acid profile in CMs. B, Contents of oleate (18:1n9) and palmitoleate (16:1n7) in triglyceride (TG) fraction in primary adipocytes. C, Contents of oleate and palmitoleate from TG fraction in WAT from obese mice. *, # p<0.05, Wt vs. SCD1-/-.
Oleate (18:1n9), but not palmitoleate (16:1n7), contributes to the induction of TNFα in RAW264.7 macrophages treated with adipocyte CM
Here we asked whether the decreased levels of unsaturated FFAs in SCD1-/- adipocyte CM were linked to the reduced inflammation in macrophages. Treatment of RAW264.7 macrophages with increasing doses (50 and 200μmol/L) of oleate (18:1n9) induced significantly higher expression of TNFα, whereas the same doses of palmitoleic (16:1n7) or linoleic acid (18:2n6) were ineffective (Figure 6A). Given this selective effect of oleate (18:1n9), we subsequently supplemented SCD1-/- CM with 50μmol/L of oleate, and found that it significantly enhanced the expression of TNFα in RAW macrophages to a level comparable to the treatment with Wt CM (Figure 6B). The observed reduction in LPL activity in SCD1-/- CM did not alter the induction of TNFα in RAW cells (Supplemental Figure 7). These data indicate a unique role of oleate in modulating macrophage inflammation, which is not shared by palmitoleate, another enzymatic product of SCD1.
Supplementation of oleate (18:1n9), but not palmitoleate (16:1n7), in SCD1-/- adipocyte CM enhances the adhesion response of endothelial cells
Next, we examined the effects of decreased oleate and palmitoleate levels on the adhesion response of endothelial cells. Endothelial cells incubated with Wt CM exhibited significantly higher expression levels of adhesion molecules such as ICAM-1, P-Selectin and E-Selectin compared to treatment with basal media, whereas SCD1-/- CM led to significantly lower expression levels of ICAM-1, P-Selectin, and lower tendency of E-Selectin in these cells (Figure 6C). Supplementation of oleate (50μmol/L), but not palmitoleate (50μmol/L), to SCD1-/- CM significantly enhanced the expression of these adhesion molecules in endothelial cells. Consistently, similar pattern was observed on monocytes/endothelial cell adhesion (Figure 6D). In these experiments, the reduced LPL activity in SCD1-/- CM did not alter the adhesion responses in endothelial cells (data not shown). These data indicate that differential regulation on endothelial cell adhesion response exists between oleate and palmitoleate.
To further test the in vivo effects of SCD1 deficiency on endothelial inflammation, we analyzed the expression of adhesion molecules in WAT-derived SVC which contains multiple cell types including endothelial cells. The expression of ICAM-1, VCAM-1 and P-selectin were significantly lower in SVC from WAT of SCD1-/- mice (Supplemental Figure 8). Endothelial inflammation and dysfunction are closely associated with insulin resistance. 24 SVC with SCD1 deficiency exhibited significantly lower expression of endothelial dysfunction markers, Nox4 (NADPH oxidase 4), Nos3 (NO synthase), and a lower tendency of Edn1 (endothelin 1) expression (Supplemental Figure 8). These data suggest that endothelial cells in WAT from SCD-/- mice may have reduced inflammation with enhanced insulin sensitivity.
Discussion
Ever since the first finding that TNFα is over-produced by adipocytes in obesity, 25 WAT has been regarded as a critical site for promoting chronic and systemic inflammation17, 18 and thereby contributing to the development of atherosclerosis. The present investigation has demonstrated that the inflammation in WAT can be attenuated by SCD1 deficiency under the challenge of obesity. Using isolated primary adipocytes, we showed a reduced response to inflammation with SCD1 deficiency directly in the adipocytes and a decreased paracrine regulation on macrophages and endothelial cells. We further showed that the decreased production of oleate (18:1n9), but not palmitoleate (16:1n7) by SCD1-deficient adipocytes, contributed to these effects. Palmitoleate has been recently demonstrated to be an adipocyte-derived lipokine which improves muscle insulin sensitivity and suppresses hepatosteatosis.26 However, the effects of oleate were not measured in the study. Given that both oleate and palmitoleate are the enzymatic products of SCD1, the current study using SCD1-deficient mice affords a unique model to demonstrate the functional difference of these two MUFAs in regulating inflammation.
It is becoming increasingly clear that in WAT inflammation, adipocytes interact with macrophages and endothelial cells in a paracrine manner.20, 21 An array of protein factors, which include cytokines (TNFα, IL-6), chemokines (MCP-1), adipokines (adiponectin, leptin) and others, are released by WAT and are involved in regulating inflammation in obesity.9 However, it was reported that the adipocyte-secreted TNFα and IL-6 were unlikely to mediate the proinflammatory effects and therefore, other unknown adipocyte-derived soluble factors may exert these effects.27 Under our experimental settings, we did not detect significant difference in the levels of adiponectin and leptin in Wt and SCD1-/- CMs. However, since these experiments were done in an in vitro context and unable to replicate the hormonal or other physiological regulations in vivo, we can not rule out the possibilities that the above protein factors released by adipocytes may contribute in vivo to the regulation of inflammation.
In addition to secreting adipokines and cytokines, adipocytes also actively release FFAs through lipolysis. Recent studies have demonstrated the potent regulation by FFAs on the immune response of macrophages through members of Toll-like receptor family.6, 23 We were therefore prompted to explore the function of this class of soluble mediators in regulating the inflammation in SCD1 deficient system. Although FFAs, most notably saturated FAs such as palmitate (16:0), are important adipocyte-derived mediators in promoting macrophage inflammation,20 the precise contribution of MUFAs, mainly oleate (18:1n9) and palmitoleate (16:1n7), to the cross-talk among adipocytes, macrophages and endothelial cells has remained largely understudied. Given that SCD1 is highly expressed in adipocytes and has direct impact on FA profile, we hypothesized that the alteration in the profile of secreted FAs from adipocytes lacking SCD1 might contribute to the reduced paracrine inflammatory regulation on the network of adipocytes, macrophages and endothelial cells.
Our results demonstrated that oleate, but not palmitoleate, promotes macrophage inflammation and endothelial adhesion response in a paracrine-fashion. SCD1-/- adipocytes released similar levels of saturated FFAs as Wt adipocytes. What was detected to be lower though, are the levels of unsaturated FFAs including palmitoleic (16:1n7), oleic (18:1n9) and linoleic (18:2n6) acids. In this regard, the SCD1-/- CM model provides us with a unique opportunity to selectively examine the roles of these unsaturated FFAs in regulating inflammation independent of the effects from saturated FFAs. Oleic, but not palmitoleic or linoleic acid, contributes to the inflammation in both RAW macrophages and endothelial cells with the doses and timeframe of our treatments. Consistently, oleate has also been reported to significantly promote inflammation in RAW264.7 macrophages in another study.23 Furthermore, of particular interest is the fact that both oleate and palmitoleate are enzymatic products of SCD1, and the only chemical structural difference is the two additional carbons present in the fatty acyl chain of the former. Examination on how this subtle structural difference in the FAs leads to differential outcomes of cell signaling may yield important findings.
In parallel with the reduced paracrine inflammation by SCD1-/- adipocytes, these adipocytes exhibit reduced inflammatory response to LPS. We showed that the gene expression level of TLR4 was lower in SCD1-/- than Wt adipocytes. The role of TLR4 in mediating FFA-induced inflammation has been well-established.6 In adipocytes, another recent study further demonstrated that FAs enhance the expression of TLR4 and induce inflammation through TLR4/NF-κB cascade.28 In addition to the reduced TLR level, we further observed a substantially lower level of lipids (triglycerides) in SCD1-/- than Wt adipocytes (Supplemental Figure 3) and decreased NF-κB DNA binding activity in WAT. Taken together, these data suggest that reduced lipid levels in SCD1-/- adipocytes might lead to reduced expression of TLR4, and subsequently, decreased TLR4/NF-κB pathway, resulting in lower inflammation. In line with this reduced TLR4/NF-κB signaling, the basal, as well as LPS-stimulated, expression of pro-inflammatory factors (MCP-1 and IL-6) are lower in SCD1-/- adipocytes than that in Wt adipocytes.
Despite the observed reduced inflammation in WAT and adipocytes of SCD1-/- mice in our study, a recent study using a SCD1-/-:LDLR-/- mouse model showed that SCD1 deficiency resulted in increased atherosclerosis.29 The mechanism was attributed to the skin inflammation associated with global SCD1 deficiency. Although increased levels of circulating inflammatory factors were detected in the SCD1-/-:LDLR-/- mice in that study, the levels of MCP-1 that is highly produced by WAT were actually lower in these mice. Thus, this result is consistent with the decreased inflammation of WAT and adipocytes observed in our study in SCD1-/- mice. Interestingly, there is another study suggesting that SCD1 knock-down by targeted anti-sense oligos (ASOs) resulted in reduced atherosclerosis in a mouse model of chronic intermittent hypoxia.30 The apparent discrepancy suggests that there are likely other functions of SCD1 in regulating atherosclerosis independent of skin inflammation. In this regard, the pronounced skin inflammation might override the other beneficial effects of SCD1 deficiency on atherosclerosis including the reduced WAT inflammation observed in our study. Therefore, better models of SCD1 deficiency, for instance, the tissue-specific deletion of SCD1, might provide more insights on the exact role of SCD1 in atherosclerosis.
Although adipocytes are sensitive to SCD1 deficiency in inflammation, the inflammatory responses of peritoneal macrophages, one of the major immune cells, are not altered by SCD1 deficiency in our study, which is consistent with the observation made in the SCD1-/-:LDLR-/- mouse model.29 Since we detected decreased macrophage infiltration in WAT from SCD1-/- mice challenged by obesity, it thus suggests that the adipocytes are likely to be the primary cell type regulating WAT inflammation in SCD1-/- mice. In contrast, a recent study showed increased inflammation response in peritoneal macrophages after treating mice with SCD1-targeted ASOs, which may contributed to the increased atherosclerosis in those mice.14 The discrepancy may be partially due to different specificities in deleting SCD1 expression (genetic knock-out verse ASO knock-down), or the varying genetic backgrounds of mouse models used in these studies.
In obesity, increased lipolysis in WAT results in elevated plasma circulating levels of FFAs that cause inflammation and insulin resistance.31 Given that the content of oleate was reduced in WAT of obese mouse models with SCD1 deficiency, it is tempting to speculate that this would contribute to the prevention of WAT inflammation in these mice. However, since SCD1 deficiency protects mice from HFD-10 or Agouti-induced obesity,32 our current study was not intended to address the precise contributions of oleate to inflammation independent of the obesity-preventing effects.
In summary, this study is the first demonstration on the molecular mechanism of SCD1 deficiency in modulating the levels of adipocyte-derived FAs on inflammation. Understanding of the differential functions of different types of MUFAs (oleate and palmitoleate) on the inflammatory crosstalk among adipocytes, macrophages, and endothelial cells will provide a more comprehensive understanding of the relationship between obesity, lipid metabolism, and atherosclerosis.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grants RO1-DK62388 to James Ntambi and by the American Heart Association Fellowship to Xueqing Liu.
Footnotes
Disclosure: None.
Liu, SCD1 deficiency reduces adipocyte inflammation
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 3.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113–121. doi: 10.1016/s0952-3278(02)00261-2. [DOI] [PubMed] [Google Scholar]
- 11.DeWille JW, Farmer SJ. Postnatal dietary fat influences mRNAS involved in myelination. Dev Neurosci. 1992;14:61–68. doi: 10.1159/000111648. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim Y, Lee SD, Lopez I, Arnold RS, Lambeth JD, Suh PG, Ryu SH. Selective activation of phospholipase D2 by unsaturated fatty acid. FEBS Lett. 1999;454:42–46. doi: 10.1016/s0014-5793(99)00745-0. [DOI] [PubMed] [Google Scholar]
- 13.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- 14.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, Shah R, Davis MA, Kelley K, Wilson MD, Kent C, Parks JS, Rudel LL. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idle JR, Gonzalez FJ. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance--is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26:897–904. doi: 10.1038/sj.ijo.0802028. [DOI] [PubMed] [Google Scholar]
- 20.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 21.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 22.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. Faseb J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 24.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 26.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab. 2004;287:E1178–1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 28.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald ML, van Eck M, Hildebrand RB, Wong BW, Bissada N, Ruddle P, Kontush A, Hussein H, Pouladi MA, Chapman MJ, Fievet C, van Berkel TJ, Staels B, McManus BM, Hayden MR. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:341–347. doi: 10.1161/ATVBAHA.108.181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki M, Sampath H, Liu X, Flowers MT, Chu K, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem Biophys Res Commun. 2009;380:818–822. doi: 10.1016/j.bbrc.2009.01.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.