Abstract
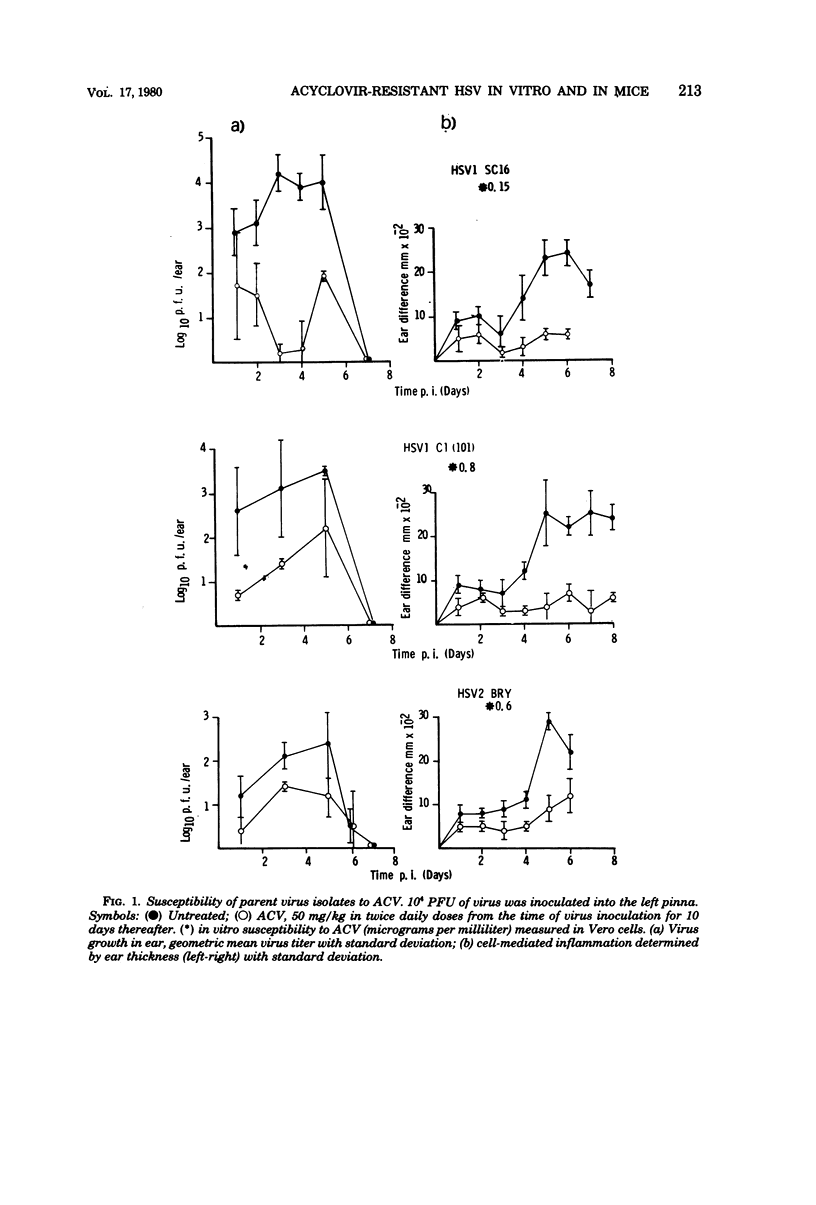
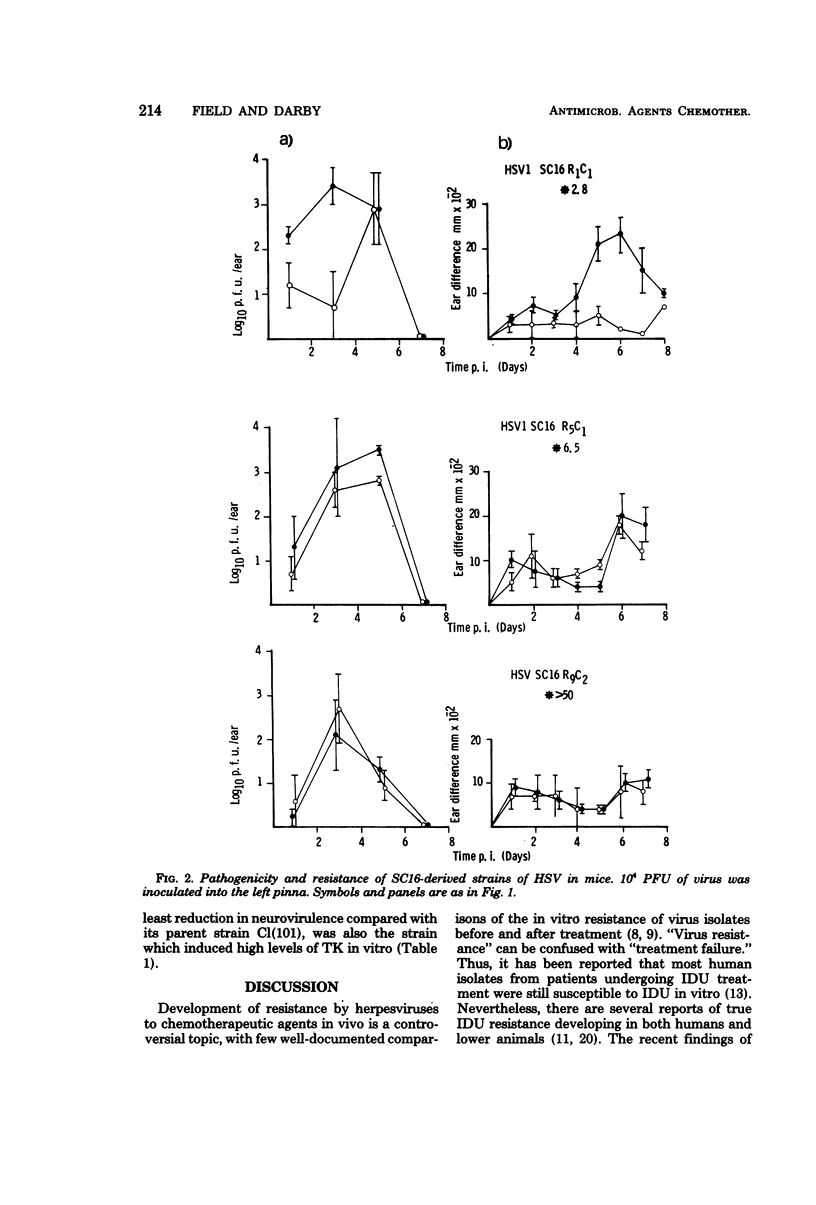
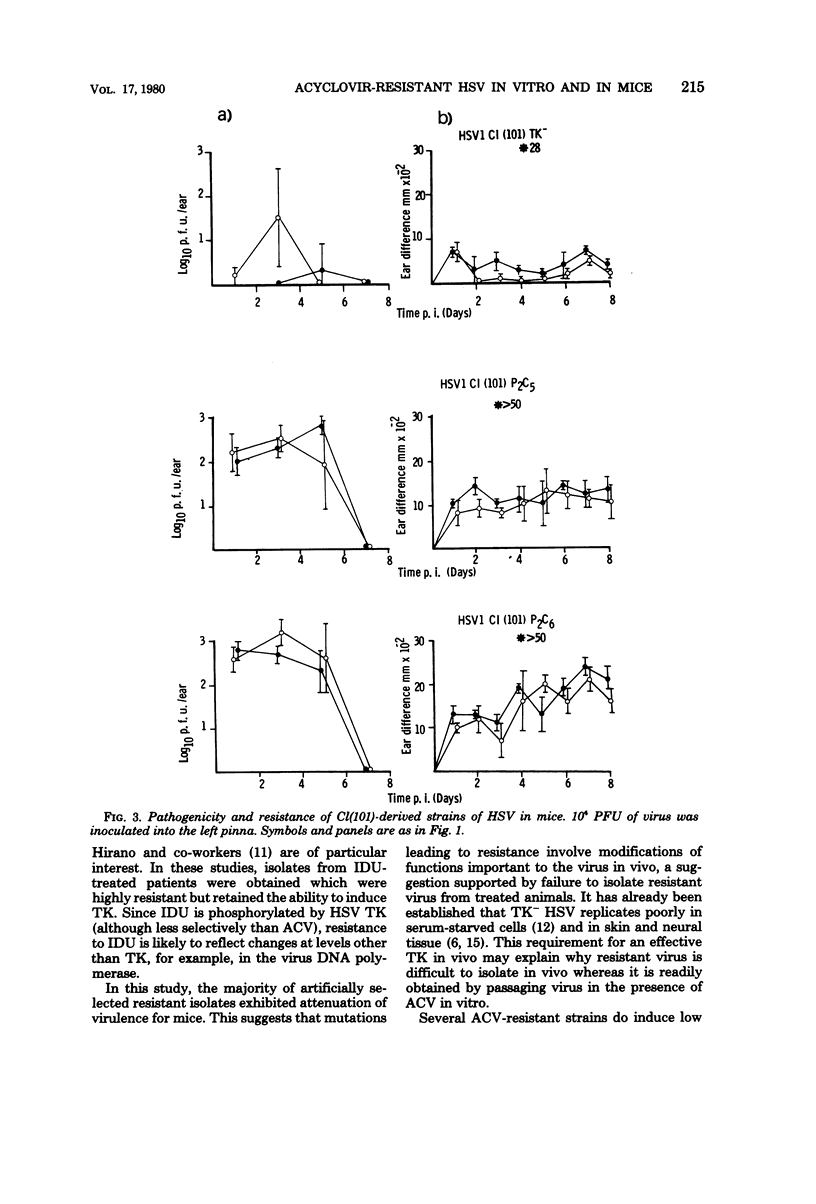
Mice infected with three different isolates of herpes simplex virus (HSV) and treated with acyclovir (acycloguanosine; ACV) showed low levels of virus replication during the acute phase of infection. However, virus isolated from such treated mice did not show increased resistance to ACV. In contrast, resistant virus was readily isolated in vitro by passaging HSV in the presence of the drug. The degree of resistance was determined, in part, by the nature of the cells used to test the virus. The majority of ACV-resistant strains induced low or undetectable levels of HSV-specified thymidine kinase (TK), the enzyme responsible for phosphorylating ACV in infected cells. The TK-resistant strains were attenuated when injected into mice as indicated by reductions in virus replication, inflammation, and establishment of latent infections in sensory ganglia. The reduced virulence of the TK- strains was most marked after intracerebral inoculation, where the lethal dose was increased more than 100-fold compared with the parental isolates. However, one mutant is described which induced high levels of TK but was highly resistant to ACV and retained virulence for mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Dundarov S., Dundarova D., Todorov S., Kavaklova L., Falke D. Induction capacity and influence of dThdMP on thymidine kinase activity of type 1 and 2 strains of herpes simplex virus. Arch Virol. 1978;56(3):243–249. doi: 10.1007/BF01317853. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Bell S. E., Elion G. B., Nash A. A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979 Apr;15(4):554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Gauri K. K. Anti-herpesvirus polychemotherapy. Adv Ophthalmol. 1979;38:151–163. [PubMed] [Google Scholar]
- Herrmann E. C., Jr, Herrmann J. A. A working hypothesis--virus resistance development as an indicator of specific antiviral activity. Ann N Y Acad Sci. 1977 Mar 4;284:632–637. doi: 10.1111/j.1749-6632.1977.tb21997.x. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Hirano A., Yumura K., Kurimura T., Katsumoto T., Moriyama H., Manabe R. Analysis of herpes simplex virus isolated from patients with recurrent herpes keratitis exhibiting "treatment-resistance" to 5-iodo-2'-deoxyuridine. Acta Virol. 1979 May;23(3):226–230. [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- Marcialis M. A., La Colla P., Schivo M. L., Flore O., Firinu A., Loddo B. Low virulence and immunogenicity in mice and in rabbits of variants of Herpes simplex virus resistant to 5-iodo-2-deoxyuridine. Experientia. 1975 Apr 15;31(4):502–503. doi: 10.1007/BF02026404. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Chen M. S., Fischer P. H., Lin T. S., Shiau G. T., Schinazi R. F. Role of nucleosides in virus and cancer chemotherapy. Adv Ophthalmol. 1979;38:3–16. [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E. Serological properties of thymidine kinase produced in cells infected with type 1 or type 2 herpes virus. J Gen Virol. 1972 Dec;17(3):307–315. doi: 10.1099/0022-1317-17-3-307. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Wildy P. Deoxypyrimidine kinases of herpes simplex viruses types 1 and 2: comparison of serological and structural properties. J Gen Virol. 1975 Feb;26(2):159–170. doi: 10.1099/0022-1317-26-2-159. [DOI] [PubMed] [Google Scholar]
- Underwood G. E., Elliott G. A., Buthala D. A. Herpes keratitis in rabbits: pathogenesis and effect of antiviral nucleosides. Ann N Y Acad Sci. 1965 Jul 30;130(1):151–167. doi: 10.1111/j.1749-6632.1965.tb12549.x. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Yamamoto H., Notkins A. L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976 Dec 9;264(5586):554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]



