Abstract
Expanded CGG repeats cause chromosomal fragility and hereditary neurological disorders in humans. Replication forks stall at CGG repeats in a length-dependent manner in primate cells and in yeast. Yeast Tof1 and Mrc1 proteins facilitate replication fork progression through CGG repeats. Remarkably, the fork-stabilizing role of Mrc1 does not involve its checkpoint function. Thus, chromosomal fragility might occur when forks stalled at expanded CGG repeats escape the S-phase checkpoint.
Fragile sites are chromosomal loci that look constricted or even broken upon replication inhibition. Rare fragile sites are associated with hereditary neurological disorders in humans, such as fragile X syndrome and FRAXE mental retardation, and are caused by expansions of (CGG)n·(CCG)n repeats2. Both repeat expansions and chromosomal fragility seem to depend on faulty DNA replication caused by unusual structures of the repeats3. CGG repeats adopt hairpin-like and quadruplex-like DNA structures4,5, which arrest DNA replication in vitro6,7 and in unicellular organisms8,9. Is the same true for mammalian cells?
We monitored replication fork progression through CGG repeats in COS-1 fibroblasts using a pSV2neo episome in COS-1 fibroblasts as previously described10. This vector contains a SV40 replication origin functional in cells expressing T-antigen. T-antigen acts as an initiator and a replicative DNA-helicase; the other components of the replisome come from the host cell. (CGG)n repeats of normal (n=18), expansion threshold (n=40) or premutation (n=105) lengths were cloned into the pSV2neo vector either into a non-transcribed area or transcribed area. Each repeat was cloned in two orientations relative to the replication origin, positioning either (CGG)n or (CCG)n runs into the lagging strand template. Their distance from the origin was sufficient to separate stalled intermediates from the un-replicated molecules, avoiding problems in detection of repeat-mediated replication stalls11.
Normal length CGG repeats did not affect replication fork progression in mammalian cells (Fig. 1). CGG repeats of the expansion threshold size caused defined replication stall signals whether they were placed in a transcribed or a non-transcribed area. The replication fork stalling further intensified for 105 CGG repeats. (A diffused descending arm of the Y arc here results from contractions of long repeats during plasmid propagation in E. coli.) Besides being stronger (Fig. 1b), the area of delayed replication spanned a large portion of the Y arc (Fig. 1a). In contrast, replication through a twice longer inverted repeat in mammalian cells produced a defined stall site10. We believe that the extended replication slow zone might be due to the lack of a defined symmetry center in a CGG repeat, allowing it to fold into stable structures at multiple points. Notably, for both 40 and 105 CGG repeats, there was no difference in the severity of the replication blockage between different orientations (Fig. 1b). Thus, CGG repeats stall replication fork progression in mammalian cells in a length-dependent, but orientation-independent manner. These results are qualitatively similar to our previous observations in yeast9, but the minimal length of the repeat required for replication stalling is higher in mammalian cells, closely matching the threshold length for expansions in humans.
Figure 1. Replication fork stalling at CGG repeats in mammalian cells.
(a) Fork progression through CGG repeats in COS-1 fibroblasts. The location of the repeat within the vector is shown above the corresponding gels. The repeat constructs are designated according to the lagging strand template sequence. Bold numbers - the number of repetitive units. Solid arrows - replication stall sites. b. Quantitative analysis of data in (a). Replication stalling was quantified as the ratio between the maximum radioactive count within the bulge normalized to the adjacent arc; error bars show standard deviations.
The lack of orientation dependence in replication stalling can be interpreted in two ways: (i) stalling occurs with equal efficiency on both template strands, or (ii) stalling occurs primarily on the lagging strand template upon the formation of equally stable structures by the repetitive CGG and CCG runs. We favor the second interpretation, since formation of an alternative DNA structure requires a DNA segment to become single-stranded, which is intrinsic to the lagging strand template. In this case, the hairpin-like structures are likely to be responsible for fork stalling by the (CGG)n·(CCG)n repeat, since they can be formed by both strands of the repeat5, in contrast to strand-specific quadruplexes6.
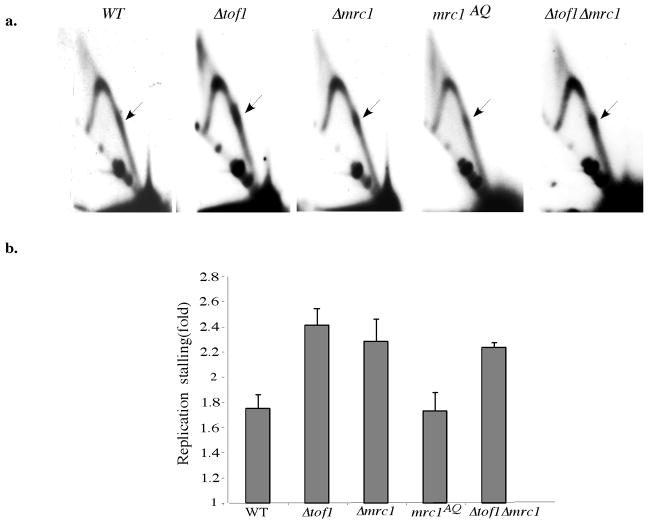
Given the similarity between repeat-mediated replication blockage in mammalian and yeast cells, we studied the mechanisms regulating CGG-mediated replication blockage in yeast. We looked at the effect of fork stabilizing proteins on the replication of CGG repeats in a yeast 2-micron plasmid via 2-dimensional gel-electrophoresis9. Tof1 is a fork stabilizing protein supporting normal replication12 and stabilizing replisomes stalled by hydroxyurea treatment13. In addition, it promotes fork pausing at protein-mediated replication barriers, such as Fob1-DNA complexes, centromeres, or tRNA genes, by counteracting the Rrm3 helicase15. Consequently, replication stalling at these sites is absent in Tof1 knockouts14. In contrast, we found that the strength of the fork stalling at CGG repeats was increased in Δtof1, compared to the wild-type strain (Fig. 2). This increase was not due to a general replication slow down in the Tof1 mutant, since the strength of repeat-mediated stalling was normalized to the strength of the Y arc (Supplementary methods and Figure 1). Tof1 mutation similarly affected fork stalling at inverted repeats10. Thus, Tof1 sustains forks stalled by DNA structures, while its fork pausing, counter-helicase function is limited to protein-mediated barriers.
Fig. 2. Genetic control of replication fork pausing at CGG repeats.
a. Replication fork progression through (CGG)40 in the wild type and mutant S. cerevisiae strains. Arrows show replication stall sites. b. Quantitative analysis of data in (a).
Yeast Mrc1 forms a complex with Tof1 and Csm3 proteins, which travels with the replication fork13. Besides maintaining the integrity of stalled replisomes, Mrc1 mediates replication checkpoint responses16. We found that the severity of replication stalling at the CGG repeat was increased in the Δmrc1 compared to the wild type strain (Fig. 2a,b). To distinguish whether this effect is due to the checkpoint or fork-stabilizing function of the protein, we analyzed its separation of function mutant mrc1AQ, which cannot be phosphorylated by the checkpoint kinases16. When we expressed the mrc1AQ allele in the Δmrc1 strain, the severity of repeat-mediated fork stalling was restored to the wild-type level (Fig. 2a,b). Furthermore, repeat-mediated replication stalling in the Δtof1Δmrc1 double mutant was similar to that in individual mutants. Thus, fork stabilizing, rather than the checkpoint function of the Mrc1 protein helps replisome progress through CGG repeats. By the same token, replisomes stalled at CGG repeats do not seem to trigger an intra-S checkpoint response.
We propose that chromosomal fragility arises when a replication fork stalled at the expanded repeat escapes the S-phase checkpoint (Fig. 3). This could result in the continuation of the cell cycle before the completion of replication around the repeat17,18. As mitosis proceeds, the under-replicated areas would convert into constrictions and/or double-stranded breaks.
Fig. 3.

Model of chromosomal fragility at expanded CGG repeats.
Recent data indicate that breakage and instability of trinucleotide repeats is increased in yeast Tof1 and Mrc1 knockouts19,20. Combined with our current data, these results point to the role of fork stalling in repeat instability. Similarly to our observations, Mrc1 prevented repeat contractions independently of its checkpoint function. The checkpoint function, however, specifically prevented repeat expansions. Thus, replication fork stalling may trigger repeat contractions, while expansions may additionally involve a DNA damage checkpoint-inducing event.
Supplementary Material
Acknowledgments
We thank Kate Mirkin for her help with plasmid construction, Catherine Freudenreich for many useful suggestions, Steve Elledge for the plasmid with the mrc1AQ allele, and John and Penny White for their generosity. Supported by the NIH grant GM60987 to S.M.M.
Footnotes
AUTHOR CONTRIBUTIONS
I.V. designed and performed experiments in yeast and mammalian cells, and wrote a paper, C.F.S. performed replication studies in mammalian cells, A.A.S. performed cassettes for yeast knockouts, M.M.K. contributed to plasmid construction, S.M.M. designed experiments, supervised the whole project and wrote the paper.
References
- 1.Lukusa T, Fryns JP. Biochim Biophys Acta. 2008;1779:3–16. doi: 10.1016/j.bbagrm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Fu YH, et al. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 3.Mirkin SM. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 4.Fry M, Loeb LA. Proc Natl Acad Sci USA. 1994;91:4950–4954. doi: 10.1073/pnas.91.11.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gacy AM, Goellner G, Juranic N, Macura S, McMurray CT. Cell. 1995;81:533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 6.Usdin K, Woodford KJ. Nucleic Acids Res. 1995;23:4202–4229. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, Ohshima K, Shimizu M, Amirhaeri S, Wells RD. J Biol Chem. 1995;270:27014–27021. doi: 10.1074/jbc.270.45.27014. [DOI] [PubMed] [Google Scholar]
- 8.Samadashwily GM, Raca G, Mirkin SM. Nat Genet. 1997;17:298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier R, Krasilnikova MM, Samadashwily GM, Lahue RS, Mirkin SM. Mol Cell Biol. 2003;23:1349–1357. doi: 10.1128/MCB.23.4.1349-1357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voineagu I, Narayanan V, Lobachev KS, Mirkin SM. Proc Natl Acad Sci USA. 2008;105:9936–9941. doi: 10.1073/pnas.0804510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichol Edamura K, Leonard MR, Pearson CE. Am J Hum Genet. 2005;76:302–311. doi: 10.1086/427928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson B, Calzada A, Labib K. Mol Biol Cell. 2007;18:3894–902. doi: 10.1091/mbc.E07-05-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katou Y, et al. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- 14.Calzada A, Hodgson B, Kanemaki M, Bueno A, Labib K. Genes Dev. 2005;19:1905–1919. doi: 10.1101/gad.337205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohanty BK, Bairwa NK, Bastia D. Proc Natl Acad Sci USA. 2006;103:897–902. doi: 10.1073/pnas.0506540103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborn AJ, Elledge SJ. Genes Dev. 2003;17:1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Rosell J, et al. Science. 2007;315:1411–1415. doi: 10.1126/science.1134025. [DOI] [PubMed] [Google Scholar]
- 18.Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- 19.Freudenreich CH, Lahiri M. Cell Cycle. 2004;3:1370–1374. doi: 10.4161/cc.3.11.1246. [DOI] [PubMed] [Google Scholar]
- 20.Razidlo DF, Lahue RS. DNA Repair. 2008;7:633–640. doi: 10.1016/j.dnarep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.