Abstract
In common with other Mendelian diseases, the presentation and progression of autosomal dominant polycystic kidney disease (ADPKD) varies widely in the population. The typical course is of adult onset disease with ESRD in the 6th decade. However, a small proportion has adequate renal function into the 9th decade, whereas others present with enlarged kidneys as neonates. ADPKD is genetically heterogeneous and the disease gene is a major determinant of severity; PKD1 on average is associated with ESRD 20 years earlier than PKD2. The majority of PKD1 and PKD2 mutations are likely fully inactivating, although recent studies indicate that some alleles retain partial activity, hypomorphic alleles. Homozygotes for such alleles are viable and in combination with an inactivating allele can result in early onset disease. Hypomorphic alleles and mosaicism may also account for some cases with unusually mild disease. The degree of phenotypic variation detected in families indicates that genetic background influences disease severity. Genome-wide association studies are planned to map common variants associated with severity. Although ADPKD is a simple genetic disease, fully understanding the phenotypic variability requires consideration of influences at the genic, allelic and genetic background level, and so ultimately is complex.
Introduction
Simple monogenic diseases (Mendelian disorders) are particularly amenable to determining the underlying etiology because of the reliable way that they are inherited in families. Hence, employing linkage studies (and other methods), and exploiting the fruits of the Human Genome Project, the primary defect has been identified in more than 2500 such disorders.1 In contrast to complex genetic diseases (such as diabetes) the single gene in a Mendelian disorder, such as autosomal dominant polycystic kidney disease (ADPKD), determines if the individual develops the disease. However, even in this simple highly penetrant disease (almost everyone that inherits an ADPKD mutation gets multiple renal cysts during their lifetime) there are several layers of complexity that contribute to the observed phenotype. This complexity is seen at the genic level, where more than one gene causes ADPKD; several other genes are also associated with overlapping phenotypes. Increasingly it seems that the specific mutation can influence the phenotype (allelic effect), while other genetic variants elsewhere in the genome are also thought to modify how the disease presents and progresses. While we have a good understanding of the genes that cause ADPKD, we are only now getting a glimpse of the other genetic (and environmental) factors that fully influence how the disease manifests in an individual. This genetic information is of diagnostic importance and is starting to be of prognostic significance. The goal of this review is to highlight factors that are known to influence the variability of disease in ADPKD and to show how this helps us to understand pathogenesis.
The ADPKD phenotype is highly variable
ADPKD is typically an adult onset disease characterized by progressive, bilateral cyst development and expansion of the kidneys. This cystic deformation of the kidneys often results in ESRD. Measured by serum creatinine and glomerular filtration rate (GFR), kidney function usually appears normal over many decades, with a decline in GFR, which occurs relatively rapidly (4.4–5.9ml/min/year),2 only evident in the decade or so preceding ESRD. In a total ADPKD population, the mean age at onset of ESRD was calculated as 59 years (quartiles = 49y and 70y)3 with ~15% retaining adequate renal function at 80y; illustrating the phenotypic variability of ADPKD. Factors that have been associated with more severe disease include, early onset of hypertension and gross hematuria.3, 4
The progressive nature and variability of the renal disease in ADPKD was clearly illustrated by the Consortium of Radiological Imaging Studies of PKD (CRISP) study where total renal and cyst volumes were measured by MRI annually over a three year period.5 The mean total kidney volume at baseline was 1060ml (sd±642ml), ranging from ~300ml to ~3000ml, with a mean annual rate of increase of 5.27% (sd±3.9%). Patients showed an exponential rate of kidney and cyst growth over the three years, although the amount of growth varied considerably between patients from close to zero to nearly 2000ml. Analysis of patients with volumes >1500ml showed a decline in GFR (4.33ml/min/yr; sd±8.07), indicating a link between larger kidneys and impaired renal function.
Occasionally, ADPKD manifests as very early onset disease, with clinical indications, including renal enlargement, evident before 18 months of age.6–11 This early onset disease is usually found in a family with otherwise typical ADPKD, but an increased recurrence risk of severe disease has been described in sibs.11 This extreme variant form illustrates the full extent of variation in disease severity that is found in ADPKD, which can even occur within the same family.
ADPKD is also associated with a number of extrarenal manifestations that contribute to morbidity and mortality. Liver cysts are commonly associated with ADPKD (found in 94% of 34–46yr olds by MR analysis12), but much less frequently manifest as massively enlarged polycystic liver disease (PCLD) that requires surgical intervention.13 Severe PCLD is much more common in women and is stimulated by estrogen exposure.14, 15 A separate disease, PCLD without renal involvement, is caused by mutations to the PRKCSH or SEC63 genes.16–18
Asymptomatic intracranial aneurysms (ICA) are found in ~8% of ADPKD cases, five times the level in the normal population19–21, and have an average age at rupture of 41yr, that is a decade earlier than sporadic cases.21, 22 Family clustering of ICA is found, with a five times greater chance of detecting an ICA in a patient with relative with a ruptured ICA,20, 23 suggesting that genetic factors may be associated with development of this disease complication.
ADPKD is genetically heterogeneous
ADPKD is caused by mutations to either of two genes, PKD1 (chromosome region 16p13.3)24–26 or PKD2 (4p21).27 Further genetic heterogeneity has been suggested with the identification of apparently unlinked families28–32. However, careful evaluation of at least one of these questions the existence of another locus.33 PKD1 encodes a large transcript (~14kb), has 46 exons and generates a protein, polycystin-1, of 4303aa. PKD2 generates a ~5kb transcript with 15 exons and encodes polycystin-2, a 968aa protein. Polycystin-2 is a transient receptor potential (TRP) type Ca2+ channel and while polycystin-1 has homology to polycystin-2, it overall has a receptor-like structure, including a large extracellular region.34–36 The role of the polycystin proteins, that are thought to form a functional complex, is still under debate37–40 but probably involves the primary cilium.41 From linkage studies, and more recently from mutation analysis of renal clinic patients, PKD1 accounts for approximately 85% of the population and PKD2 15%.42–44 Analysis of some isolated populations, elderly patients, or population-based figures indicate that PKD2 may be under-recognized and more prevalent in certain groups.45–47
Gene type influences the severity of renal disease
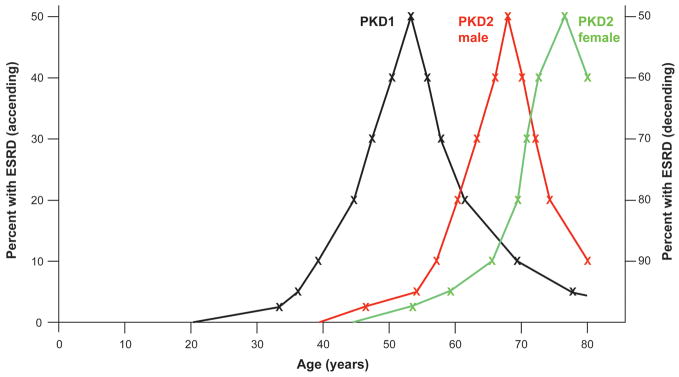
The gene mutated is a major determinant of severity of disease in ADPKD with a range of studies showing the PKD1 is a more severe disease. It has a higher incidence of hypertension and hematuria, with ESRD occurring on average 20 years earlier (54.3y, compared to 74y for PKD2)3, 44, 45, 48 (Figure 1). Consistent with larger renal volumes being associated with more severe disease, age and gender corrected PKD2 total kidney volumes are only two-thirds the size found in PKD1 (623ml vs 1041ml).49 However, although the annual increase in cystic volume is greater in PKD1 (74.9ml vs 32ml), the rate of change (5.68% vs 4.82%) was not significantly different, indicating that PKD2 is not milder because cysts grow more slowly. Instead, PKD1 kidneys were found to have more cysts, especially earlier in the disease process, accounting for their larger size.49, 50
Figure 1.
Illustration showing the variability in severity of renal disease in ADPKD. Kaplan-Meier data48, 54 of percent of cases with ESRD (up to 80 years) in total PKD1 (black), PKD2 male (red) and PKD2 female (green) populations. Values from 0–50% ESRD are shown ascending (left scale) and values from 50–100% are shown descending (right scale). PKD1 is significantly more severe than PKD2, and PKD2 males have more severe disease than females. The broad spread of age at ESRD for all three groups indicate that other genetic modifiers and environmental factors influence the phenotype. The milder extremes of the PKD1 (and possibly PKD2) range may partially reflect the presence of hypomorphic alleles and mosaicism. The severe extremes of the ranges may be influenced by in trans inherited PKD1/PKD2 modifying alleles, as is the disease in very early onset cases (not shown).
Extrarenal manifestations may to some extent be influenced by the mutated gene, although ICA are found in PKD2 patients at about the same level as PKD1.51 Severe PCLD is found in PKD2 as well as PKD1, but preliminary data suggest that it may be less common.52, 53
In PKD2, a significant gender difference in the severity of renal disease is found; females on average reach ESRD ~8 years later than males (76.0y vs 68.1y)54 (Figure 1). The data for PKD1 is less clear with some studies finding no difference in time to ESRD48, 55 while two others finding that males have more severe disease.3, 56 In the total CRISP population (PKD1 and PKD2), renal volume was marginally (but significantly) smaller in females and the rate of kidney and cyst volume increase was more rapid in males than females.49
Insights into pathogenesis and genetic mechanisms from rodent models
Mouse models null for Pkd1 or Pkd2 are embryonic lethal, developing renal and pancreatic cysts from E13.5d, depending on the model and genetic background.57, 58 In the heterozygous state, the animals are viable and develop just a small number of hepatic and kidney cysts.59 The Pkd2WS25/− model (WS25 is a hypermutable allele) progressively developed severe cystic kidney and liver disease within 3–6 months.60 Conditional Pkd1 knockouts generate rapidly progressive disease if the gene is inactivated before postnatal day 13 and much slower developing disease if removed after that time, indicating a switch that may correspond to completion of renal development in the mouse.61, 62 The development of cysts following the inactivation of both alleles in animal models, and the identification of somatic mutations in human PKD1 and PKD2 cyst-linings, indicate that cystogenesis can be a two-hit process associated with a germline and somatic mutation.63, 64 However, this may not be the only route to cystogenesis since mice with hypomorphic alleles expressing 15–25% of the wildtype level develop renal and hepatic cysts as viable homozygotes,65, 66 with the severity of disease highly dependant on the genetic background.67 Recently, hypomorphic alleles with characteristics similar to those found in animal models have been described in human ADPKD (see next section).
Mice that are heterozygous for both a Pkd1 and Pkd2 null allele are viable but develop more renal cysts than seen in the Pkd1+/− and Pkd2+/− mutants alone; indicating an additive effect.68 One human family trans-heterozygous for a PKD1 and PKD2 mutation showed more severe disease in the two trans-heterozygous cases than other family members with a single mutation, although the disease in the PKD1-linked cases was milder than typical, indicating that it may be a hypomorphic allele.69, 70
The role of allelic effects in modulating the severity of renal disease
The PKD1 and PKD2 genes display an unusually high level of allelic heterogeneity. Mutation data has now been collected and collated in the ADPKD Mutation Database (PKDB),71 so the full array of disease associated mutations can be analyzed. For PKD1, 436 different mutations have been described accounting for the disease in 544 families. In the case of PKD2, 115 different mutations have been identified in 198 families. A common finding from mutation screening is a novel mutation; only ~30% of mutations in the CRISP cohort were recurrent.43 Even the most common mutations, PKD1: 5014_5015delAG and PKD2: R306X and R872X, each account for less than 2% of total mutant alleles.71
In large mutation screens, likely pathogenic mutations have been identified in 79–91% of families, with ~42–65% of these changes definite, truncating mutations.43, 72–74 The remaining 22–37%, that constitute in-frame changes (many missense) and other non-definite mutations, have been assessed for likely pathogenicity using various bioinformatics tools.43, 73–75 The 9–21% of families where no mutation was detected probably reflect: missed PKD1 and PKD2 mutations, especially outside of the screened exonic and flanking intronic areas; further genetic heterogeneity and phenocopies due to related diseases, such as MODY5, PCLD and ARPKD76; and more complex inheritance (see below for details).
At a population level, no clear correlation between mutation type (truncating or in-frame) and disease severity has been identified in PKD1 or PKD2.54, 55 This indicates that most missense changes fully inactivate the allele. Mutation position has not been correlated with renal disease severity in PKD2, but in PKD1 mutations 5′ to the median position were associated with slightly more severe disease (ESRD at 53y vs 56y).54, 55 A similar correlation was found with a higher frequency of ICA in patients with PKD1, 5′ mutations. This association was especially true for families with more than one vascular case.51 It is unclear if this correlation suggests a role for the N-terminal mutant product, or is associated with the known cleavage of polycystin-1 at the GPS site.77
Although the population data indicates that most missense changes are fully inactivating, recent studies indicate that a few may be hypomorphic, or incompletely penetrant alleles. Viable patients that are homozygous or compound heterozygous for strongly predicted PKD1 mutations, have been described.75 These cases have typical to more severe disease and in each case have evidence of developmental defects of the collecting system, such as dilatation or clubbing of the calyces. These alleles in heterozygosity seem to be associated with only occasional cyst development, but if they are inherited in trans with an inactivating mutation can cause very early onset ADPKD.75 The finding that alleles inherited from the “normal” parent are a cause of infantile ADPKD is consistent with the recurrence risk found in these families.11 A PKD2 variant, F482C, that appears to lower polycystin-2 channel activity, has been suggested as a disease modifier in homozygosity.78 The full extent to which hypomorphic alleles account for mild PKD1 (simple cysts?), modulate the disease variability seen in families, and give the appearance of a negative family history, is yet to be determined.79
Approximately 10% of ADPKD families have a negative family history and in several cases de novo mutations have been demonstrated.24, 80, 81 In two families with apparent de novo mutations, the new changes have been shown to occur as a mosaic and, hence, the patient is a chimera for cells with and without the mutation.72, 82 As mosaicism would not normally be detected from mutation screening, a significant proportion of de novo events may be mosaics. Such cases can explain extreme differences in severity between generations. They are also important to identify as they may dramatically increase the risk of disease in the sib of an apparent de novo case if a parent is mosaic.
Role of genetic background in influencing disease severity
Although genetic and allelic effects can explain some of the phenotypic variability in ADPKD, it is likely that genetic factors elsewhere in the genome also play a significant role. There have been suggestions that intrafamilial variability may be suggestive of anticipation; disease getting successively more severe with each generation83 (usually associated with expanding trinucleotide repeats). However, subsequent studies have highlighted the degree of intrafamilial variability but not found a trend for more severe disease in offspring, or for trinucleotide repeat expansion.9, 56, 84 Comparison of monozygotic twins to siblings showed greater variance in time to ESRD in the siblings, supporting a role for genetic modifiers in influencing disease severity.85 Two studies of large ADPKD populations have analyzed the degree of intrafamilial variability employing variance component analysis.86, 87 Differences due to genetic background were estimated to account for 18–59% of the phenotypic variance prior to ESRD, while the heritability of time to ESRD ranged from ~43–50%.
Analysis for potential modifiers employing association studies with candidate genes has been relatively disappointing with inconsistent results obtained for both the ACE insertion/deletion polymorphism and the ENOS gene.88–91 As with other candidate association studies,92 the relatively small population sizes, problematic endpoints, and limited genetic characterization of the population have contributed to the inconsistency. Better understanding of the linkage disequilibrium block structure of the genome,93, 94 the development of high resolution single nucleotide polymorphism (SNP) arrays, and the use of large populations has now made genome-wide association studies (GWAS) possible.93, 94 GWAS have been successfully employed to identify quantitative trait loci in a wide range of complex genetic diseases.95, 96 Plans are underway for GWAS analysis of a large population of ADPKD patients in an attempt to identify associations with disease severity.
Another potential source of genotypic variability that may influence the severity of ADPKD are copy number variants (CNV) due to duplication or deletion of larger DNA segments, some of which are common in the human genome.97 These can also be assayed employing specifically designed SNP arrays. As well as the common variants assayed employing a GWAS, rarer variants that individually have a larger phenotypic effect (similar to those identified as PKD1 variants on the “normal” allele) may significantly modulate the ADPKD phenotype. The development of next-generation and third-generation methods, along exon enrichment, may in the near future allow coding regions of all human genes to be screened for such variants.98–100
One clear example of where mutation to a second gene significantly modulates the phenotype is when the tuberous sclerosis 2 (TSC2), that lies immediately adjacent to the 3′ end of PKD1, is disrupted by contiguous PKD1/TSC2 deletions. These patients have various tuberous sclerosis phenotypes but also PKD that is significantly more severe than normally associated with mutation to either gene alone.72, 101, 102 This enhanced phenotypic effect may be due to direct interaction of the TSC2 protein, tuberin, and polycystin-1 or to synergy in their signaling through the mTOR pathway; suggesting mTOR inhibitors as a treatment option in ADPKD.103, 104 Another potential ADPKD modifier is the cystic fibrosis gene (CFTR), with anecdotal reports of cases with mild disease when co-inherited with cystic fibrosis;105, 106 suggesting that CFTR inhibition may be beneficial in ADPKD.107
Understanding the influence that environmental factors have on disease progression is in its infancy. Caffeine exposure has been considered a risk factor in PKD, and evidence that caffeine can increase cAMP levels in cystic epithelium provides some justification for avoiding caffeinated drinks.108, 109 Smoking may be associated with more rapid progression in renal disease, including ADPKD, due to its known vascular effects and higher blood pressure;110 although was not found significant in one screen for modifying factors.87
Conclusions
Whether a patient develops ADPKD is simply due to if they have a pathogenic mutation in the PKD1 or PKD2 gene, however, the severity of disease behaves more like a complex trait. The gene involved is a major determinant of disease severity but increasingly allelic effects appear important, especially for atypical disease presentations. The hunt for other genetic modifiers is only now beginning but identification of factors may help understand pathogenesis, be of prognostic significance, and guide future therapies.
Footnotes
The authors have no conflict of interest or financial disclosures significant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.OMIM(TM) Online Mendelian Inheritance in Man. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University; Baltimore, MN: National Center for Biotechnology Information; Bethesda, MD: 2009. [Google Scholar]
- 2.Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol. 1995;5:2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 3.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AM, Gabow PA. Identification of pateints with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol. 1997:1560–1567. doi: 10.1681/ASN.V8101560. [DOI] [PubMed] [Google Scholar]
- 5.Grantham JJ, Torres VE, Chapman AB, et al. Volume Progression in Polycystic Kidney Disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 6.Fick GM, Johnson AM, Strain JD, et al. Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1863–1870. doi: 10.1681/ASN.V3121863. [DOI] [PubMed] [Google Scholar]
- 7.Kaariainen H. Polycystic kidney disease in children: a genetic and epidemiological study of 82 Finnish patients. J Med Genet. 1987;24:474–481. [PMC free article] [PubMed] [Google Scholar]
- 8.Michaud J, Russo P, Grignon A, et al. Autosomal dominant polycystic kidney disease in the fetus. Am J Med Genet. 1994;51:240–246. doi: 10.1002/ajmg.1320510314. [DOI] [PubMed] [Google Scholar]
- 9.Peral B, Ong ACM, San Millán JL, Gamble V, Rees L, Harris PC. A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1) Hum Mol Genet. 1996;5:539–542. doi: 10.1093/hmg/5.4.539. [DOI] [PubMed] [Google Scholar]
- 10.Shamshirsaz A, Reza Bekheirnia M, Kamgar M, et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 2005;68:2218–2224. doi: 10.1111/j.1523-1755.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 11.Zerres K, Rudnik-Schöneborn S, Deget F. German working group on paediatric nephrology: Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. J Med Genet. 1993;30:583–588. doi: 10.1136/jmg.30.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 13.Schnelldorfer T, Torres VE, Zakaria S, Rosen CB, Nagorney DM. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250:112–118. doi: 10.1097/SLA.0b013e3181ad83dc. [DOI] [PubMed] [Google Scholar]
- 14.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11:1033–1037. doi: 10.1002/hep.1840110619. [DOI] [PubMed] [Google Scholar]
- 15.Sherstha R, McKinley C, Russ P, et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology. 1997;26:1282–1286. doi: 10.1002/hep.510260528. [DOI] [PubMed] [Google Scholar]
- 16.Davila S, Furu L, Gharavi AG, et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet. 2004;36:575–577. doi: 10.1038/ng1357. [DOI] [PubMed] [Google Scholar]
- 17.Drenth JP, Te Morsche RH, Smink R, Bonifacino JS, Jansen JB. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat Genet. 2003;33:345–347. doi: 10.1038/ng1104. [DOI] [PubMed] [Google Scholar]
- 18.Li A, Davila S, Furu L, et al. Mutations in PRKCSH Cause Isolated Autosomal Dominant Polycystic Liver Disease. Am J Hum Genet. 2003;72:691–703. doi: 10.1086/368295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Eng J Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 20.Huston J, Torres VE, Sullivan PP, Offord KP, Wiebers DO. Value of magnetic resonance angiography for detection of intracranial aneurysm in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 21.Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:269–276. doi: 10.1681/ASN.V131269. [DOI] [PubMed] [Google Scholar]
- 22.Chauveau D, Pirson Y, Verellen-Dumoulin C, Macnicol A, Gonzalo A, Grünfeld J-P. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 1994;45:1140–1146. doi: 10.1038/ki.1994.151. [DOI] [PubMed] [Google Scholar]
- 23.Belz MM, Hughes RL, Kaehny WD, et al. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2001;38:770–776. doi: 10.1053/ajkd.2001.27694. [DOI] [PubMed] [Google Scholar]
- 24.European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 25.Hughes J, Ward CJ, Peral B, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 26.International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 28.Bogdanova N, Dworniczak B, Dragova D. Genetic heterogeneity of polycystic kidney disease in Bulgaria. Hum Genet. 1995;95:645–650. doi: 10.1007/BF00209481. [DOI] [PubMed] [Google Scholar]
- 29.Coto E, de Castro SS, Aguado S, et al. DNA microsatellite analysis of families with autosomal dominant polycystic kidney disease types 1 and 2: evaluation of clinical heterogeneity between both forms of the disease. J Med Genet. 1995;32:442–445. doi: 10.1136/jmg.32.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daoust MC, Reynolds DM, Bichet DG, Somlo S. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics. 1995;25:733–736. doi: 10.1016/0888-7543(95)80020-m. [DOI] [PubMed] [Google Scholar]
- 31.de Almeida S, de Almeida E, Peters D, et al. Autosomal dominant polycystic kidney disease: evidence for the existence of a third locus in a Portuguese family. Hum Genet. 1995;96:83–88. doi: 10.1007/BF00214191. [DOI] [PubMed] [Google Scholar]
- 32.Turco AE, Clementi M, Rossetti S, Tenconi R, Pignatti PF. An Italian family with autosomal dominant polycystic kidney disease unlinked to either the PKD1 or PKD2 gene. Am J Kidney Dis. 1996;28:759–761. doi: 10.1016/s0272-6386(96)90261-9. [DOI] [PubMed] [Google Scholar]
- 33.Consugar M, Rossetti S, Anderson S, et al. PKD3 revisted with improved PKD1 and PKD2 haplotyping and mutation screening. J Am Soc Nephrol. 2005;16:358A. [Google Scholar]
- 34.Gonzalez-Perrett S, Kim K, Ibarra C, et al. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable non-selective cation channel. Proc Natl Acad Sci USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koulen P, Cai Y, Geng L, et al. Polycystin-2 is an intracellular calcium release channel. Nature cell biology. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 36.Vassilev PM, Guo L, Chen XZ, et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun. 2001;282:341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 37.Battini L, Macip S, Fedorova E, et al. Loss of Polycystin-1 Causes Centrosome Amplification and Genomic Instability. Hum Mol Genet. 2008;17:2819–2833. doi: 10.1093/hmg/ddn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Happe H, Leonhard WN, van der Wal A, et al. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet. 2009;18:2532–2542. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- 39.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 40.Sharif-Naeini R, Folgering JH, Bichet D, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 41.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nature reviews. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 42.Peters DJM, Sandkuijl LA. Genetic heterogeneity of polycystic kidney disease in Europe. Contributions to Nephrology: Polycystic Kidney Disease. 1992;97:128–139. doi: 10.1159/000421651. [DOI] [PubMed] [Google Scholar]
- 43.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 44.Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7:2142–2151. doi: 10.1681/ASN.V7102142. [DOI] [PubMed] [Google Scholar]
- 45.Dicks E, Ravani P, Langman D, Davidson WS, Pei Y, Parfey PS. Incident Renal Events and Risk Factors in Autosomal Dominant Polycystic Kidney Disease: A Population and Family-Based Cohort Followed for 22 Years. CJASN. 2006;1:710–717. doi: 10.2215/CJN.01581105. [DOI] [PubMed] [Google Scholar]
- 46.Rossetti S, Adeva M, Kubly V, Consugar MB, Torres VE, Harris PC. An Olmsted County population-based study indicates that PKD2 is more common than previously described. J Am Soc Nephrol. 2007;18:365A. [Google Scholar]
- 47.Torra R, Badenas C, Perez-Oller L, et al. Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am J Kidney Dis. 2000;36:728–734. doi: 10.1053/ajkd.2000.17619. [DOI] [PubMed] [Google Scholar]
- 48.Hateboer N, van Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 49.Harris PC, Bae K, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in ADPKD. J Am Soc Nephrol. 2006;17:3013–3019. doi: 10.1681/ASN.2006080835. [DOI] [PubMed] [Google Scholar]
- 50.Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–116. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–2201. doi: 10.1016/S0140-6736(03)13773-7. [DOI] [PubMed] [Google Scholar]
- 52.Bozza A, Aguiari G, Scapoli C, et al. Autosomal dominant polycystic kidney disease linked to PKD2 locus in a family with severe extrarenal manifestations. Am J Nephrol. 1997;17:458–461. doi: 10.1159/000169141. [DOI] [PubMed] [Google Scholar]
- 53.Rossetti S, Chauveau D, Consugar M, et al. Mutation screening of ADPKD patients with unusally severe or mild cystic disease of the liver. J Am Soc Nephrol. 2005;16:357A. [Google Scholar]
- 54.Magistroni R, He N, Wang K, et al. Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:1164–1174. doi: 10.1097/01.asn.0000061774.90975.25. [DOI] [PubMed] [Google Scholar]
- 55.Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Neph. 2002;13:1230–1237. doi: 10.1097/01.asn.0000013300.11876.37. [DOI] [PubMed] [Google Scholar]
- 56.Reed BY, McFann K, Reza Bekheirnia M, et al. Variation in age at ESRD in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:173–183. doi: 10.1053/j.ajkd.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nature Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 58.Wilson PD. Curr Top Dev Biol. New York: Elsevier, Inc; 2008. Mouse Models of Polycsytic Kidney Disease; pp. 311–350. [DOI] [PubMed] [Google Scholar]
- 59.Lu W, Fan X, Basora N, et al. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nature Genet. 1999;21:160–161. doi: 10.1038/5944. [DOI] [PubMed] [Google Scholar]
- 60.Wu G, D’Agati V, Cai Y, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 61.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- 62.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest. 1997;99:194–199. doi: 10.1172/JCI119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type 1. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 65.Jiang ST, Chiou YY, Wang E, et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168:205–220. doi: 10.2353/ajpath.2006.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 67.Happé H, van der Wal AM, Leonhard WN, Breuning MH, De Heer E, Peters DJM. Inhibition of the Raf/MEK/ERK pathway reduces new cyst formation but increases cyst growth in murine PKD. J Am Soc Nephrol. 2009;20:494–495A. [Google Scholar]
- 68.Wu G, Tian X, Nishimura S, et al. Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet. 2002;11:1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 69.Pei Y, Paterson AD, Wang KR, et al. Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet. 2001;68:355–363. doi: 10.1086/318188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watnick T, Lan Z, Wang K, et al. Mild renal cystic disease associated with PKD1 missense mutation. J Am Soc Nephrol. 2007;18:366A. [Google Scholar]
- 71.The ADPKD Mutation Database (PKDB) 2009 http://pkdb.mayo.edu.
- 72.Consugar MB, Wong WC, Lundquist PA, et al. Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int. 2008;74:1468–1479. doi: 10.1038/ki.2008.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Gonzalez MA, Jones JG, Allen SK, et al. Evaluating the clinical utility of a molecular genetic test for polycystic kidney disease. Mol Genet Metab. 2007;92:160–167. doi: 10.1016/j.ymgme.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan YC, Blumenfeld JD, Anghel R, et al. Novel method for genomic analysis of PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease. Hum Mutat. 2009;30:264–273. doi: 10.1002/humu.20842. [DOI] [PubMed] [Google Scholar]
- 75.Rossetti S, Kubly VJ, Consugar MB, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris P, Rossetti S. Molecular diagnostics of autosomal dominant polycystic kidney disease (ADPKD) Nature Reviews Nephrology. 2009 doi: 10.1038/nrneph.2010.18. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian F, Boletta A, Bhunia AK, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1- associated mutations. Proc Natl Acad Sci U S A. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dedoussis GV, Luo Y, Starremans P, et al. Co-inheritance of a PKD1 mutation and homozygous PKD2 variant: a potential modifier in autosomal dominant polycystic kidney disease. Eur J Clin Invest. 2008;38:180–190. doi: 10.1111/j.1365-2362.2007.01913.x. [DOI] [PubMed] [Google Scholar]
- 79.Sandford RN. The diversity of PKD1 alleles: implications for disease pathogenesis and genetic counseling. Kidney Int. 2009;75:765–767. doi: 10.1038/ki.2009.17. [DOI] [PubMed] [Google Scholar]
- 80.Reed B, McFann K, Kimberling WJ, et al. Presence of de novo mutations in autosomal dominant polycystic kidney disease patients without family history. Am J Kidney Dis. 2008;52:1042–1050. doi: 10.1053/j.ajkd.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossetti S, Strmecki L, Gamble V, et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001;68:46–63. doi: 10.1086/316939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Connor A, Lunt PW, Dolling C, et al. Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am J Transplant. 2008;8:232–237. doi: 10.1111/j.1600-6143.2007.02030.x. [DOI] [PubMed] [Google Scholar]
- 83.Fick GM, Johnson AM, Gabow PA. Is there evidence for anticipation in autosomal-dominant polycystic disease? Kidney Int. 1994;45:1153–1162. doi: 10.1038/ki.1994.153. [DOI] [PubMed] [Google Scholar]
- 84.Geberth S, Ritz E, Zeier M, Stier E. Anticipation of age at renal death in autosomal dominant polycystic kidney disease (ADPKD)? Nephrol, Dialysis, Transpl. 1995;10:1603–1606. [PubMed] [Google Scholar]
- 85.Persu A, Duyme M, Pirson Y, et al. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004;66:2132–2136. doi: 10.1111/j.1523-1755.2004.66003.x. [DOI] [PubMed] [Google Scholar]
- 86.Fain PR, McFann KK, Taylor MR, et al. Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 2005;67:1256–1267. doi: 10.1111/j.1523-1755.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- 87.Paterson AD, Magistroni R, He N, et al. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]
- 88.Baboolal K, Ravine D, Daniels J, et al. Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int. 1997;52:607–613. doi: 10.1038/ki.1997.373. [DOI] [PubMed] [Google Scholar]
- 89.Pereira TV, Nunes AC, Rudnicki M, et al. Influence of ACE I/D gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease: a meta-analysis. Nephrol Dial Transplant. 2006;21:3155–3163. doi: 10.1093/ndt/gfl412. [DOI] [PubMed] [Google Scholar]
- 90.Persu A, Stoenoiu MS, Messiaen T, et al. Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2002;11:229–241. doi: 10.1093/hmg/11.3.229. [DOI] [PubMed] [Google Scholar]
- 91.Walker D, Consugar M, Slezak J, et al. The ENOS polymorphism is not associated with severity of renal disease in polycystic kidney disease 1. Am J Kidney Dis. 2003;41:90–94. doi: 10.1053/ajkd.2003.50027. [DOI] [PubMed] [Google Scholar]
- 92.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 93.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steemers FJ, Gunderson KL. Whole genome genotyping technologies on the BeadArray platform. Biotechnol J. 2007;2:41–49. doi: 10.1002/biot.200600213. [DOI] [PubMed] [Google Scholar]
- 95.Kingsmore SF, Lindquist IE, Mudge J, Gessler DD, Beavis WD. Genome-wide association studies: progress and potential for drug discovery and development. Nat Rev Drug Discov. 2008;7:221–230. doi: 10.1038/nrd2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mardis ER. Next-generation DNA sequencing methods. Annual review of genomics and human genetics. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 99.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 100.Tucker T, Marra M, Friedman JM. Massively parallel sequencing: the next big thing in genetic medicine. Am J Hum Genet. 2009;85:142–154. doi: 10.1016/j.ajhg.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brook-Carter PT, Peral B, Ward CJ, et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease – a contiguous gene syndrome. Nat Genet. 1994;8:328–332. doi: 10.1038/ng1294-328. [DOI] [PubMed] [Google Scholar]
- 102.Sampson JR, Maheshwar MM, Aspinwall R, et al. Renal cystic disease in tuberous sclerosis: Role of the polycystic kidney disease 1 gene. Am J Hum Genet. 1997;61:843–851. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 104.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Sullivan DA, Torres VE, Gabow PA, Thibodeau SN, King BF, Bergstralh EJ. Cystic fibrosis and the phenotype expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:976–983. doi: 10.1016/s0272-6386(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 106.Xu N, Glockner JF, Rossetti S, Babovich-Vuksanovic D, Harris PC, Torres VE. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J Nephrol. 2006;19:529–534. [PubMed] [Google Scholar]
- 107.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:2723–2729. doi: 10.1097/01.asn.0000025282.48298.7b. [DOI] [PubMed] [Google Scholar]
- 109.Tanner GA, Tanner JA. Chronic caffeine consumption exacerbates hypertension in rats with polycystic kidney disease. Am J Kidney Dis. 2001;38:1089–1095. doi: 10.1053/ajkd.2001.28614. [DOI] [PubMed] [Google Scholar]
- 110.Orth SR, Stockmann A, Conradt C, et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926–931. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]