Abstract
OBJECTIVE
To determine whether dietary restrictions enhance the specificity of guaiac-based fecal occult blood tests (FOBTs) when screening for colorectal cancer.
DATA SOURCES
PubMed-MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, and Cochrane databases were searched.
STUDY SELECTION
English-language case series, cohort studies, randomized controlled trials (RCTs), and meta-analyses were selected. Studies that did not include dietary manipulation or the use of guaiac-based FOBTs available in North America were excluded.
SYNTHESIS
Ten case series, 5 cohort studies, 4 RCTs, and 1 meta-analysis were critically appraised. All studies used Hemoccult, Hemoccult II, or Hemoccult SENSA tests. Data from case series involving challenge diets showed no increase in positive FOBT results from high-peroxidase vegetables, but results varied with red-meat challenges depending on the amount of meat consumed and the test used. Case series, cohort studies, and RCTs comparing FOBT results during restricted versus unrestricted diets consistently showed no differences in positive FOBT results.
CONCLUSION
Most of the evidence evaluating the effect of dietary restrictions on FOBT results is dated and of suboptimal quality. However, 4 RCTs and a meta-analysis of these data do not support dietary restrictions when screening for colorectal cancer. Because patient adherence can be an issue with FOBTs, and dietary restrictions can affect adherence in some populations, it is reasonable to abandon these recommendations without fear of substantially affecting specificity.
Résumé
OBJECTIF
Déterminer si les restrictions alimentaires accroissent la spécificité des tests au gaïac pour la recherche du sang occulte dans les selles (RSOS) lors du dépistage du cancer colorectal.
SOURCES DES DONNÉES
On a consulté PubMed-MEDLINE, le Cumulative Index to Nursing and Allied Health Literature et la base de données Cochrane.
CHOIX DES ÉTUDES
On a retenu les séries de cas, études de cohorte, essais cliniques randomisés (ECR) et méta-analyses de langue anglaise. Les études qui n’incluaient pas de modification alimentaire ou qui n’utilisaient pas les tests au gaïac pour la RSOS disponibles en Amérique du Nord ont été exclues.
SYNTHÈSE
On a fait une évaluation critique de 10 séries de cas, 5 études des 4 ECR et 1 méta-analyse. Toutes les études utilisaient les tests Hemocult, Hemocult II ou Hemocult SENSA. Les données des séries de cas portant sur des régimes types n’ont montré aucune augmentation de résultats positifs pour la RSOS avec les légumes à haute teneur en peroxydase, mais les résultats variaient pour les régimes riches en viande, selon la quantité de viande consommée et le test utilisé. Les séries de cas, études de cohorte et ECR qui comparaient les résultats de la RSOS en présence d’un régime avec ou sans restriction montraient invariablement l’absence de différence du taux de résultats positifs.
CONCLUSION
Les données concernant l’effet des restrictions alimentaires sur les résultats de la RSOS sont pour la plupart désuètes et de qualité sub-optimale. Toutefois, 4 ECR et une méta-analyse de ces résultats ne supportent pas l’utilisation de restrictions alimentaires lors du dépistage du cancer colo-rectal. Puisque l’observance du patient peut être problématique lors de la RSOS et que les restrictions alimentaires peuvent affecter l’observance dans certaines populations, il paraît raisonnable d’abandonner ces recommandations sans crainte de modifier la spécificité du test.
Screening for colorectal cancer (CRC) is recommended for all Canadians aged 50 to 741 and should be considered in healthy older individuals as well. Although there are several options for screening, the clearest evidence for reducing mortality related to CRC supports fecal occult blood tests (FOBTs) every 1 to 2 years. Unfortunately, no screening method for CRC has been shown to reduce absolute mortality.2 This is likely because most CRC is diagnosed in the seventh decade and beyond, an age group in which all-cause mortality is already high and the contribution of one specific cause would be negligible. Whether all-cause mortality is affected in younger age groups is unknown.
All CRC screening methods are limited owing to imperfect sensitivities and specificities, risks, costs, burdens on resources, and patient nonadherence. Because we have no data demonstrating the superiority of one screening method over another, the method chosen is less important than completing CRC screening in a way that is acceptable to our patients.
Fecal occult blood testing is often limited by a lack of patient adherence. The barriers to adherence have been explored in many studies and include the recommended dietary restrictions.3 The recommendations for dietary restrictions have remained largely unchanged since the introduction of FOBTs in the 1950s. Most FOBT methods rely on the activity of peroxidases to induce a colour change when a substrate becomes oxidized. Heme found in blood possesses pseudoperoxidase activity that induces such changes. Early FOBTs, such as Occultest, Hemastix, and Hematest, were based on an ortho-toluidine substrate that was extremely sensitive to peroxidase activity. Because that was the case, dietary restrictions limiting red meats and high-peroxidase vegetables and fruits, such as turnips, horseradish, and melon, were recommended.
The shift to tests based on a guaiac substrate, such as the Hemoccult test, resulted in greater specificity but a loss in sensitivity. With newer tests such as Hemoccult II and the more sensitive Hemoccult SENSA, the balance between specificity and sensitivity has improved. However, manufacturers of all guaiac-based FOBTs available in Canada continue to recommend some dietary restrictions in their patient instructions.4–8
Any peroxidase-based test will be limited by a loss of specificity as sensitivity improves. Many methods have attempted to improve this balance, with varying success. Rehydration of samples before development markedly improves sensitivity but at the cost of unacceptably poor specificity.9 Delaying the development of guaiac-based tests by 2 or 3 days might improve specificity with an acceptable loss of sensitivity.10 Dietary restrictions are still recommended in some screening guidelines to improve specificity, although the evidence for this is limited.
A recent joint guideline from the American Cancer Society; the US Multi-Society Task Force on Colorectal Cancer, represented by the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy; and the American College of Radiology recommended that dietary restrictions were “prudent.”11 The Ontario Colorectal Cancer Screening Program, however, recently recommended against imposing dietary restrictions.12 Alberta’s Toward Optimized Practice program guidelines also observed that dietary restrictions have an insignificant effect on FOBT results.13
This systematic review explores the evidence for the effects of diet on the sensitivity and specificity of guaiac-based FOBT results.
DATA SOURCES
Articles were obtained from PubMed-MEDLINE using the key words occult blood, fecal occult blood, or faecal occult blood and either the MeSH headings colonic neoplasms/prevention and control or diet or the key words meat, vegetables, or peroxidase. The search was limited to English-language articles involving humans. This search resulted in 307 articles. All titles and abstracts were reviewed. Articles were selected for further review if they included a clinical trial that involved manipulation of diet. A total of 24 articles fell into this category and were reviewed in depth. References from these studies were also evaluated, resulting in 3 additional articles. A similar process using the Cochrane databases and the Cumulative Index to Nursing and Allied Health Literature revealed no further studies. Final selection included only those studies involving guaiac-based tests currently available in North America. This resulted in 20 relevant studies, all of which involved Hemoccult, Hemoccult II, or Hemoccult SENSA tests.
SYNTHESIS
Case series
Ten case series studied the effects of diet on FOBT results (Table 1).14–23 Six of these evaluated the effects of challenge diets on Hemoccult-based FOBT results. In a letter to the editor, Broderick and Harris14 reported that eating 2 raw turnips daily for 9 days resulted in no positive Hemoccult results, although they did not clarify the number of subjects tested. They also noted that, when boiled, crushed turnip applied directly to the test strip did not produce a positive result. Sinatra et al22 challenged 61 healthy volunteers with 750 g of peroxidase-rich fruits and vegetables daily for 3 days. One volunteer had positive results with Hemoccult and Hemoccult SENSA when the tests were developed within 24 hours. There were no positive results when development of nonrehydrated samples was delayed for 48 hours. Rose et al16 challenged 49 healthy volunteers with 3 cups of raw peroxidase-rich fruits and vegetables daily for 6 days. There were no positive results for nonrehydrated samples using Hemoccult II or Hemoccult SENSA tests developed within 72 hours.
Table 1.
Case series of FOBT results with diet manipulation
| STUDY | N | POPULATION | FOBT TEST | INTERVENTION | REHYDRATION STATUS | DELAY TO DEVELOPMENT | RESULTS | COMMENTS |
|---|---|---|---|---|---|---|---|---|
| Broderick and Harris,14 1982 | NC | NC | Hemoccult | Challenge diet: 2 raw turnips daily for 9 d | NC | NC | 0 positive results | No reaction to boiled turnip directly applied to FOBT strip |
| Macrae et al,15 1982 | 156 | Healthy volunteers, mean age 22.1 y | Hemoccult II | 250 g rare beef daily for 6 d, 6 servings high-peroxidase fruit and vegetables daily for 6 d, both combined, exclusion diet | Nonrehydrated and rehydrated | Within 5 d | 1.9% of nonrehydrated samples produced positive results with full challenge (NS) | 16.7% of rehydrated samples produced positive results with full challenge diet |
| Rose et al,16 1989 | 49 | Healthy volunteers, 16–30 y | Hemoccult II, Hemoccult SENSA | 6 d each of exclusion diet, 250 g rare beef daily, and 3 cups high-peroxidase fruit and vegetables daily | Nonrehydrated | Within 72 h | Hemoccult II: 4% positive with meat challenge (NS); Hemoccult SENSA: 8% positive with meat challenge (NS) | 1 positive result on exclusion diet for both tests; 0 positive for vegetable challenge |
| Thomas et al,17 1989 | 18925 | Participants in the Nottingham trial, 50–74 y | Hemoccult | Unrestricted diet followed by low-peroxidase* dietary restriction if participant had a positive test result | Nonrehydrated | NC | 3.4% positive results for unrestricted diet, of which 77.0% were false positive for CRC and adenoma | Only 463/18 925 subjects (2.4%) were shown to have false-positive results on an unrestricted diet |
| Feinberg et al,18 1990 | 46 | Healthy medical students | Hemoccult II, Hemoccult SENSA | Challenge diet: 350–450 g cooked red meat daily for 3 d, then abstain from red meat for 7 d during testing | Nonrehydrated | NC | Hemoccult II positive results: 15.4% (day 1), 5.5% (day 3), 0% (day 4–7); Hemoccult SENSA positive results: 46.2% (day 1), 8.3% (day 3), 0% (day 4–7) | Percentages represent tests rather than subjects, which were not reported |
| Robinson et al,19 1993 | 2 series (347 and 574) | Consecutive participants in the Nottingham trial, 50–74 y, with ≥ 1 positive test result | Hemoccult | Low-peroxidase* dietary restriction over 6-d testing period | Nonrehydrated | NC | If results of ≥ 5 of 6 tests were positive, 88.1% (series 1) remained positive with dietary restriction | In the second series, all subjects with ≥ 5 positive results proceeded directly to colonoscopy |
| Robinson et al,20 1995 | 35260 | Participants in the Nottingham trial, 50–74 y | Hemoccult | Unrestricted diet followed by low-peroxidase* dietary restriction if participant had a positive test result | Nonrehydrated | NC | 3.4% positive results for unrestricted diet, of which 88.4% were false positive for CRC | Only 1061/35 260 subjects (3.0%) were shown to have false-positive results on an unrestricted diet |
| Rozen et al,21 1995 | 527 | CRC screening and follow-up service attendees, 95% asymptomatic (27% were following up adenomas or cured CRC), mean age 59 (SD 12) y | Hemoccult SENSA | Low-peroxidase* dietary restriction for 2 d, increased to 3 d for the last 100 subjects | Nonrehydrated | Within 1 d of final application, increased to 72 h after final application for last 100 subjects | Positive results in 17% in first group; 7% in last 100 subjects (P = .02) | Most patients evaluated with sigmoidoscopy or colonoscopy to determine false-positive rate of 15%, but results combined for all 527 subjects |
| Sinatra et al,22 1999 | 61 | Healthy volunteers, 18–30 y | Hemoccult, Hemoccult SENSA | Challenge diet: 750 g high-peroxidase fruit and vegetables daily for 3 d | Nonrehydrated | 24 hours, 48 hours, 72 hours | Hemoccult: 1.6% positive at 24 h; Hemoccult SENSA: 1.6% positive at 24 h | 0 positive when development delayed 48 or 72 h |
| Fludger et al,23 2002 | 10 | Healthy volunteers <35 y | Hemoccult | Challenge diet: one 7-oz black (blood) pudding | NC | NC | 40% of results were positive | Local delicacy in Bury, England |
CRC—colorectal cancer, FOBT—fecal occult blood test, NC—not clarified in publication, NS—no statistically significant difference between groups, SD—standard deviation.
Low-peroxidase diet involved eating no red meats and no high-peroxidase fruits or vegetables (horseradish, turnip, melon, etc).
While vegetable- or fruit-derived peroxidases have minimal effects on Hemoccult results, the effects of red meat are less clear in challenge-diet studies. Rose et al16 also challenged their 49 volunteers with 250 g (8.8 oz) of rare beef daily for 6 days. Hemoccult II and Hemoccult SENSA test results using nonrehydrated samples were positive within 72 hours for 2 and 4 subjects, respectively. This was not a statistically significant difference, however, from results obtained when these same volunteers were subjected to a low-peroxidase diet free of red meat. Macrae et al15 challenged 156 healthy volunteers with 250 g (8.8 oz) of rare beef along with 6 servings of peroxidase-rich fruits and vegetables daily for 6 days. Results of Hemoccult II tests with nonrehydrated samples beginning on the third day of the challenge were positive for only 3 subjects (1.9%). Feinberg et al18 challenged 46 medical students with 350 to 450 g (12 to 16 oz) of cooked red meat for 3 days, followed by meat restriction during 7 days of Hemoccult testing. Results of Hemoccult II and Hemoccult SENSA tests (both using nonrehydrated samples) were positive for 15.4% and 46.2% of samples, respectively, on the first day of testing. Unfortunately, the number of medical students with positive test results was not stated. Positive results dropped to 5.5% and 8.3% by the third day and to 0 by the fourth day after completing the red meat challenge. Fludger et al23 challenged 10 healthy volunteers with 7 oz of black pudding, a pudding consisting of congealed pigs’ blood produced locally in Bury, England. Four subjects who had previously had negative results using the Hemoccult test developed positive test results. Hydration status and timing of development were not clarified.
Four case series evaluated the effect of restriction diets on FOBT results. Rozen et al21 evaluated the rate of positive results for Hemoccult SENSA tests among 527 subjects prescribed a low-peroxidase diet free of red meat. A total of 95% of subjects were asymptomatic; 42% underwent flexible sigmoidoscopy; and 59% underwent colonoscopy, including all patients with positive FOBT results. With a 2-day dietary restriction, and slide development 1 day after the final stool application, the rate of positive results was 17% among the first 427 subjects. With a 3-day dietary restriction, and a delay in slide development of 6 to 7 days after the final stool application, the rate of positive results fell to 7% among the subsequent 100 subjects (P = .02). It was unclear whether this reduction resulted from extending the dietary restriction to 3 days, extending the delay in slide development to 6 or 7 days, or a combination of both. The false-positive rate determined by endoscopic evaluation was 15% for the 2 groups combined but was not reported separately for the 2 interventions.
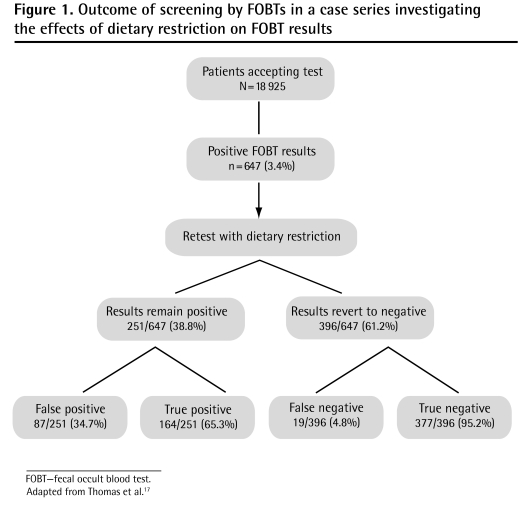
Three studies addressed the effect of retesting subjects with dietary restrictions if there was an initial positive FOBT result obtained when patients were following unrestricted diets. All 3 arose from the Nottingham study,24 a large randomized controlled trial evaluating the effectiveness of FOBT screening for CRC, initiated in 1981. Subjects were initially tested with Hemoccult tests while on unrestricted diets. Slides were not rehydrated; delay to slide development was not clarified. All subjects with positive results were retested while consuming a low-peroxidase diet free of red meat. Thomas et al17 reported initial positive FOBT results in 647 (3.4%) of 18 925 subjects on unrestricted diets. Subsequent follow-up of these patients showed that 463 (71.6%) of these initially positive results were false positive for CRC and adenomas (Figure 1).17 Retesting subjects with initial positive FOBT results while they were following a restricted diet reduced the proportion of false-positive results to 48.6% (122 of 251 whose results remained positive on retesting). The authors recommended limiting endoscopic evaluation to those who remained positive on retesting, but following up with subjects who reverted to negative FOBT results on retesting, in order to eliminate false-negative results. When looking at the whole study population, however, false-positive results were relatively rare, even on an unrestricted diet: only 463 of 18 925 subjects (2.4%) had false-positive results. Such results are rare enough to suggest that any positive FOBT result warrants further investigation.
Figure 1.
Outcome of screening by FOBTs in a case series investigating the effects of dietary restriction on FOBT results
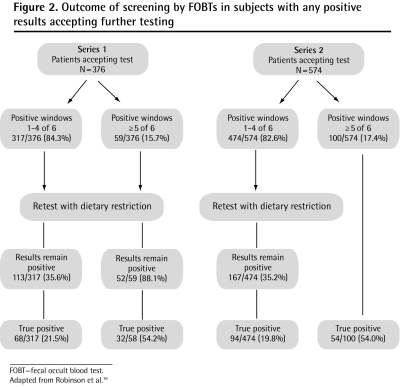
Robinson et al19 evaluated 2 series of 376 and 574 consecutive patients with positive Hemoccult results to determine the positive predictive value of FOBTs relative to the number of positive samples provided by each subject. The authors found that among subjects on an unrestricted diet with positive results on 5 or more of 6 test smears, 88.1% in the first series still had positive results with dietary restrictions. In the second series, all patients with 5 or more positive smear results on a normal diet proceeded directly to colonoscopy (Figure 2).19 In both series, when patients had 5 or 6 smears that produced positive results on a normal diet, more than half were shown to have adenomas or cancer. Owing to the low false-positive rate in this group, the authors concluded that when 5 or 6 test smears for one subject produce positive results, retesting with dietary restrictions is unnecessary and immediate evaluation is recommended.
Figure 2.
Outcome of screening by FOBTs in subjects with any positive results accepting further testing
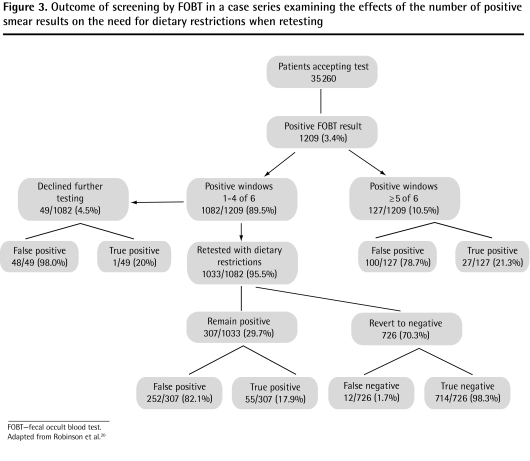
In a second study, Robinson et al20 reported initial positive FOBT results in 1209 (3.4%) of 35 260 subjects following unrestricted diets. Subsequent follow-up of these patients showed that 1114 (92.1%) of these initially positive results were false positive for CRC; adenomas were not included (Figure 3).20 The actual results were unknown for 168 subjects who declined further evaluation but who were presumed to be falsely positive because no CRC was reported within 2 years, so the proportion of false-positive results might actually have been lower. Subjects with fewer than 5 of 6 positive smears were offered retesting with dietary restrictions. Of the 29.7% of subjects whose results remained positive, CRC was subsequently diagnosed in 17.9%. However, CRC was also diagnosed in 12 subjects (1.7%) whose results reverted to negative on retesting. The authors concluded that a policy of restricting evaluation to subjects with a persistent positive FOBT result on retesting during dietary restrictions improves the rate of unnecessary endoscopy but can result in a small but important loss in screening sensitivity. Again, overall false-positive results were relatively rare: of those patients on unrestricted diets, only 1114 of 35 260 (3.2%) were shown to have false-positive results.
Figure 3.
Outcome of screening by FOBT in a case series examining the effects of the number of positive smear results on the need for dietary restrictions when retesting
Cohort studies
Five nonrandomized cohort studies compared FOBT results among subjects assigned dietary restrictions with those of subjects following unrestricted diets (Table 2).10,25–28 Only the earliest study, for which Greegor25 used homemade slides incorporating Hemoccult filter paper that was produced in 1959, reported a difference between the 2 cohorts, with a total proportion of positive results of 23% on an unrestricted diet and 5% on a meat-free diet (P < .01). Rehydration status and delay to development were not reported.
Table 2.
Nonrandomized cohort studies of FOBT results with diet manipulation
| STUDY | N | POPULATION | FOBT TEST | INTERVENTION | REHYDRATION STATUS | DELAY TO DEVELOPMENT | RESULTS | COMMENTS |
|---|---|---|---|---|---|---|---|---|
| Greegor,25 1971 | 1028 | Unrestricted diet cohort: asymptomatic adults Restricted diet cohort: population NC | Hemoccult paper on homemade slides | Unrestricted diet vs no red meat, chicken, or fish beginning ≥ 1 d before first test | NC | NC | 5% positive for restricted diet; 23% positive for unrestricted diet | False-positive rate for CRC or adenoma 3% on restricted diet; NC for restricted diet |
| Bassett and Goulston,26 1980 | 60 | Inpatients and outpatients, 78% with endoscopic diagnosis of GI pathology | Hemoccult | Unrestricted diet vs low-peroxidase diet* beginning ≥ 48 h before first test | NC | NC | 5% false-positive results for restricted diet; 25% false-positive results for unrestricted diet (NS) | False-positive results established by demonstrating < 1.5 mL blood/g feces using labeled RBCs |
| Daron and Goldman27 1981 | 100 | Men referred for inguinal hernia repair | Hemoccult II | Unrestricted diet vs low-peroxidase diet* beginning 3 d before first test | NC | NC | 23% false-positive results for restricted diet; 27%† false-positive results for unrestricted diet (NS) | False-positive results established with barium enema and colonoscopy for all subjects with positive test results |
| Norfleet,28 1986 | 288 | Outpatients referred for colonoscopic evaluation | Hemoccult II | Unrestricted diet vs low-peroxidase diet* | Nonrehydrated | NC | 20.8% false-positive results for restricted diet; 25.6% false-positive results for unrestricted diet (P = .34) | False-positive results established with colonoscopy; 67% of total had adenomas or CRC |
| Rozen et al,10 1999 | 901 | CRC screening and follow-up service attendees, 97% asymptomatic (24.5% following up adenoma or cured CRC) | Hemoccult SENSA | Unrestricted diet vs low-peroxidase diet* beginning 3 d before first test | Nonrehydrated | 4–14 d after application | 7.2% false-positive rate for restricted diet; 5.5% false-positive rate for unrestricted diet (NS) | False-positive results determined by endoscopic evaluation in 377 restricted-diet and 524 unrestricted-diet subjects |
CRC—colorectal cancer, FOBT—fecal occult blood test, GI—gastrointestinal, NC—not clarified in publication, NS—no statistically significant difference between groups, RBC—red blood cell.
Low-peroxidase diet involved eating no red meats and no high-peroxidase fruits or vegetables (horseradish, turnip, melon, etc).
Incorrectly reported in original article.
The remaining 4 studies investigating false-positive results showed no statistically significant reductions when diets were restricted. Using Hemoccult tests, Bassett and Goulston26 compared 40 inpatients on unrestricted diets with 20 patients on low-peroxidase dietary restriction. Twenty-five percent of patients on the unrestricted diet demonstrated false-positive results compared with 5% of patients following the restricted diet (P = .12). False-positive results were established by demonstrating that patients’ samples contained less than 1.5 mL of blood per gram of stool using labeled red blood cells. Norfleet28 compared positive Hemoccult II results in 288 patients referred for colonoscopy and found that 25.6% of subjects on unrestricted diets demonstrated false-positive results compared with 20.8% of subjects on low-peroxidase diets (P = .34). Daron and Goldman27 determined false-positive rates using barium enema and colonoscopy for 100 subjects presenting for inguinal hernia repair. For those subjects on unrestricted diets, the false-positive rate was 27% (incorrectly reported in the article) compared with 23% for those on a low-peroxidase diet (P = .98). Finally, Rozen et al10 showed no differences in false-positive rates, as determined by colonoscopy, in 901 subjects on restricted or unrestricted diets, using Hemoccult SENSA tests. They delayed slide development by 4 to 14 days and used nonrehydrated slides. The false-positive rate was reported as 5.5% of patients with unrestricted diets and 7.2% when the diet was restricted in red meat and high-peroxidase vegetables and fruits (P = .26), a reversal of the results from the other cohort studies.
Randomized controlled trials
Four RCTs29–32 looked at the effects of diet on guaiac-based testing (Table 3).3,29–32 These all compared FOBT results of patients following dietary restrictions with those of subjects on unrestricted diets. All studies restricted red meats, and 3 restricted fruits or vegetables containing peroxidase as well. Two studies used Hemoccult and the others used Hemoccult II. Only 1 study specified that they used nonrehydrated samples, and none specified the time delay between sample collection and development. With unrestricted diets, 2.1% to 5.8% of patients had positive test results. With dietary restrictions, positive rates ranged from 2.2% to 7.7%, differences which were not statistically significant in any of the 4 studies. Two of the studies showed that when positive FOBT results were evaluated with colonoscopy, abnormalities were equally distributed between the 2 groups. Pignone et al3 performed a meta-analysis of these RCTs that confirmed that there was no difference in the rates of positive results between subjects following restricted and unrestricted diets.
Table 3.
Randomized controlled trials and meta-analysis of FOBT results with diet manipulation
| STUDY | N | POPULATION | FOBT TEST | INTERVENTION | REHYDRATION STATUS | DELAY TO DEVELOPMENT | RESULTS | COMMENTS |
|---|---|---|---|---|---|---|---|---|
| Hoogewerf et al,29 1987 | 3554 | BC residents ≥ 45 y presenting to FP with no GI symptoms or history | Hemoccult II | Unrestricted diet vs no red meat | NC | NC | 2.1% positive for restricted diet; 2.2% positive for unrestricted diet (NS) | False-positive rates were not evaluated |
| Joseph30 1988 | 639 | Patients of the medical clinic of the Minneapolis Veterans Administration with outpatient FOBTs ordered by resident | Hemoccult II | Unrestricted diet vs low-peroxidase diet* beginning 48 hours before testing | Nonrehydrated | NC | 5.4% positive for restricted diet; 5.6%† positive for unrestricted diet (NS) | Colonoscopy in 69% of patients with positive results showed false-positive rates for CRC or villous or adenomatous polyps in 2.2% on restricted diet and 2.5% on unrestricted diet |
| Verne et al,31 1993 | 311 | General practice patients in Thame, England, 40–74 y, without CRC symptoms or history | Hemoccult | Unrestricted diet vs low-peroxidase* diet beginning 2 d before testing | NC | NC | 5.8% positive for restricted diet; 2.2%† positive for unrestricted diet (NS) | False-positive rates were not evaluated |
| Robinson et al,32 1994 | 95 | Participants in the Nottingham trial, 50–74 y, excluding those with cancer or serious health problems | Hemoccult | Unrestricted diet vs low-peroxidase* diet beginning 2 d before testing | NC | NC | 7.7% positive for restricted diet; 5.4% for unrestricted diet | All 6 patients with positive results had colonoscopies: 1 had CRC; 1 had a 1.7-cm adenoma (other group NC) |
| Pignone et al,3 2001 | 4599 | Participants in 4 RCTs21–24 | Hemoccult, Hemoccult II | Unrestricted diet vs low-peroxidase* diet | Nonrehydrated or NC | NC | No difference in rates of positive results between unrestricted and restricted diets | None |
CRC—colorectal cancer, FOBT—fecal occult blood test, GI—gastrointestinal, NC—not clarified in publication, NS—No statistically significant difference between groups, RCT—randomized controlled trial.
Low peroxidase diet involves eating no red meats and no high-peroxidase fruits or vegetables (horseradish, turnip, melon, etc).
Incorrectly reported in original article.
DISCUSSION
The literature addressing the role of dietary manipulation in improving the specificity of FOBT screening is limited and somewhat mixed. There are few RCTs, and of the RCTs that do exist, only 2 used Hemoccult II and none used Hemoccult SENSA tests. None reported the delay from sampling to testing, which could play a considerable role in the sensitivity and specificity of the tests.
Challenge diets presented as case series rather than randomized trials consistently show a lack of effect from high-peroxidase fruits and vegetables in the diet. Meat products, however, when eaten in sufficient quantities, can produce false-positive FOBT results. The challenge diets, however, included quantities of meat well above those in the average Canadian diet: per capita daily consumption for red meat averaged 67 g (2.4 oz) in 2007,33 nowhere near the 350 to 450 g (12 to 16 oz) daily in the single challenge diet that produced an increase in positive results. And fortunately, blood pudding, which clearly results in positive FOBT results, is not commonly consumed in Canada. The 4 most recent of 5 nonrandomized cohort studies also consistently showed no increase in false-positive rates when diets were not restricted.
Dietary restrictions might theoretically improve specificity, but strong clinical evidence for this is clearly lacking. Although there might be empiric logic favouring dietary restriction, its lack of effect can be explained: Plant-derived peroxidases tend to break down when passing through the gut, and degrade further with drying. Some peroxidase activity is regained with hydration, in part explaining the loss of specificity with specimen rehydration. Heme from ingested red meat loses its pseudoperoxidase activity as it is broken down to peroxidase-free porphyrins during gut transit, reducing the potential for false-positive FOBT results.
Current guidelines for testing with Hemoccult II and Hemoccult SENSA no longer recommend restricting peroxidase-containing fruits and vegetables, but continue to recommend a 3-day abstinence from red meat.4,5 ColoScreen, Tri-Slide, and Hema Screen recommend a 2-day restriction of red meats and peroxidase-containing fruits and vegetables.6–8 Although compliance might not change as a result of dietary restrictions, as shown in the meta-analysis by Pignone et al,3 there might be individuals in specific cultural groups for whom compliance is affected, as demonstrated in the British population by Robinson et al.32 This has not been studied in Canadian populations since the 1987 study by Hoogewerf et al29 included in the meta-analysis.
Conclusion
There is no current evidence to support the recommendation for restricting diet before FOBT screening for CRC. Most of the evidence, albeit of varying quality, shows no difference in positive FOBT result rates when diet is restricted. Because nonadherence to FOBT screening is an important issue, it might be time we disregarded the dietary recommendations when offering FOBT screening to our patients. Further research might provide greater insight into the role of dietary restrictions on adherence among Canadians, but such restrictions are unlikely to reduce the specificity of FOBT screening in any meaningful way, regardless of their effect on adherence.
EDITOR’S KEY POINTS
One of the barriers to fecal occult blood tests (FOBTs) is the recommended dietary restrictions. These recommendations have remained largely unchanged since the introduction of FOBTs in the 1950s; manufacturers of all the newer guaiac-based FOBTs available in Canada continue to recommend some dietary restrictions in their patient instructions.
Some Canadian guidelines have recently recommended against imposing dietary restrictions. This systematic review explores the evidence for the effects of diet on the specificity of peroxidase-based FOBT results.
The literature addressing the role of dietary manipulation in improving the specificity of FOBT screening is limited and somewhat mixed, but 4 randomized controlled trials, as well as a meta-analysis of these trials, confirmed that there was no difference in the rates of positive results between subjects following restricted and unrestricted diets. Challenge diets presented as case series consistently show no effect from high-peroxidase fruits and vegetables. Meat products, however, when eaten in sufficient quantity, can produce false-positive FOBT results, although the challenge diets included quantities of meat well above those in the average Canadian diet. The 4 most recent of 5 nonrandomized cohort studies also consistently showed no increase in false-positive rates when diets were not restricted. Dietary restrictions might theoretically improve specificity of FOBTs, but strong clinical evidence for this is lacking.
POINTS DE REPÈRE DU RÉDACTEUR
La recommandation de restrictions alimentaires pour la recherche du sang occulte dans les selles (RSOS) est un obstacle à cet examen. Cette recommandation n’a à peu près pas changé depuis l’introduction de la RSOS, dans les années 1950; les fabricants de tous les tests au gaïac existant au Canada pour la RSOS continuent de recommander certaines restrictions alimentaires dans leurs instructions au patient.
Récemment, certaines directives canadiennes déconseillaient ces restrictions alimentaires. Cette revue systématique examine les preuves que l’alimentation influence la spécificité des tests à la peroxydase pour la RSOS.
La littérature traitant du rôle possible de l’alimentation pour améliorer la spécificité des tests de dépistage pour la RSOS est peu abondante et quelque peu discordante, mais 4 essais cliniques randomisés et une méta-analyse de ces essais ont confirmé que les taux de résultats positifs ne différaient pas avec ou sans restrictions alimentaires. Les régimes types présentés sous forme de série de cas montraient invariablement que les fruits et légumes riches en peroxydase n’avaient pas d’effet. Toutefois, la consommation d’une quantité suffisante de viande peut entraîner des faux positifs, quoique les régimes à l’étude contenaient des quantités de viande bien supérieures à la consommation canadienne moyenne. De même, sur 5 études de cohortes non randomisées, les 4 plus récentes ne montraient pas d’augmentation du taux de faux positifs en absence de restrictions. En théorie, les restrictions alimentaires pourraient améliorer la spécificité de la RSOS, mais les preuves solides à cet effet sont insuffisantes.
Footnotes
This article has been peer reviewed.
Competing interests
None declared
References
- 1.Canadian Cancer Society, National Cancer Institute of Canada . Canadian cancer statistics 2007. Toronto, ON: Canadian Cancer Society, National Cancer Institute of Canada; 2007. [Google Scholar]
- 2.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–9. doi: 10.1111/j.1572-0241.2008.01875.x. Epub 2008 May 13. [DOI] [PubMed] [Google Scholar]
- 3.Pignone M, Campbell MK, Carr C, Phillips C. Meta-analysis of dietary restriction during fecal occult blood testing. Eff Clin Pract. 2001;4(4):150–6. [PubMed] [Google Scholar]
- 4.Hemoccultfobt.com [website] Patient instructions Hemoccult II slide (test card) Fullerton, CA: Beckman Coulter Inc; 2005. Available from: http://hemoccultfobt.com/docs/English461860.C.pdf. Accessed 2010 Feb 2. [Google Scholar]
- 5.Beckmancoulter.com [website] Hemoccult SENSA product instructions. Fullerton, CA: Beckman Coulter Inc; 2002. Available from: www.beckman-coulter.com/literature/ClinDiag/462489-E.pdf. Accessed 2010 Feb 2. [Google Scholar]
- 6.Bcbio.com [website] ColoScreen: a test for fecal occult blood. Patient’s instructions for using ColoScreen slides. Surrey, BC: BCBiomedical Laboratories Ltd; 2007. Available from: www.bcbio.com/uploadedFiles/Physicians/Physician_Test_Information/Collection_Instructions/INS007-Patient%20Instructions%20-%20ColoScreen%20Occult%20Blood-English.pdf. Accessed 2010 Feb 2. [Google Scholar]
- 7.Cenogenics Corporation . Cenogenics Tri-Slide, single slide and tape stool blood tests. Morganville, NJ: Cenogenics Corporation; 2004. [Google Scholar]
- 8.Immunostics Inc . Hema Screen. Ocean, NJ: Immunostics, Inc; 2004. [Google Scholar]
- 9.Hammes PH, Gnauck R. Does rehydration improve Hemoccult screening for intestinal cancer? [Article in German] Z Gastroenterol. 1985;23(12):676–80. [PubMed] [Google Scholar]
- 10.Rozen P, Knaani J, Samuel Z. Eliminating the need for dietary restrictions when using a sensitive guaiac fecal occult blood test. Dig Dis Sci. 1999;44(4):756–60. doi: 10.1023/a:1026618010312. [DOI] [PubMed] [Google Scholar]
- 11.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008. A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. Epub 2008 Mar 5. [DOI] [PubMed] [Google Scholar]
- 12.Rabeneck L, Zwaal C, Goodman JH, Mai V, Zamkanei M. Cancer Care Ontario guaiac fecal occult blood test (FOBT) laboratory standards: evidentiary base and recommendations. Clin Biochem. 2008;41(16–17):1289–305. doi: 10.1016/j.clinbiochem.2008.08.069. Epub 2008 Aug 29. [DOI] [PubMed] [Google Scholar]
- 13.Topalbertadoctors.org [website] Clinical practice guideline: screening for colorectal cancer. Edmonton, AB: Toward Optimized Practice; 2008. Available from: www.topalbertadoctors.org/informed_practice/cpgs/colorectal_cancer.html. Accessed 2010 Feb 2. [Google Scholar]
- 14.Broderick GT, Jr, Harris SM. False-positive Hemoccult test not caused by turnips. N Engl J Med. 1982;307(3):191. doi: 10.1056/NEJM198207153070324. [DOI] [PubMed] [Google Scholar]
- 15.Macrae FA, St John DJ, Caligiore P, Taylor LS, Legge JW. Optimal dietary conditions for Hemoccult testing. Gastroenterology. 1982;82(5 Pt 1):899–903. [PubMed] [Google Scholar]
- 16.Rose IS, Young GP, St John DJ, Deacon MC, Blake D, Henderson RW. Effect of ingestion of hemoproteins on fecal excretion of hemes and porphyrins. Clin Chem. 1989;35(12):2290–6. [PubMed] [Google Scholar]
- 17.Thomas WM, Pye G, Hardcastle JD, Chamberlain J, Charnley RM. Role of dietary restriction in Haemoccult screening for colorectal cancer. Br J Surg. 1989;76(9):976–8. doi: 10.1002/bjs.1800760935. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg EJ, Steinberg WM, Banks BL, Henry JP. How long to abstain from eating red meat before fecal occult blood tests. Ann Intern Med. 1990;113(5):403–4. doi: 10.7326/0003-4819-113-5-403. [DOI] [PubMed] [Google Scholar]
- 19.Robinson MH, Thomas WM, Pye G, Hardcastle JD, Mangham CM. Is dietary restriction always necessary in Haemoccult screening for colorectal neoplasia? Eur J Surg Oncol. 1993;19(6):539–42. [PubMed] [Google Scholar]
- 20.Robinson MH, Moss SM, Hardcastle JD, Whynes DK, Chamberlain JO, Mangham CM. Effect of retesting with dietary restriction in Haemoccult screening for colorectal cancer. J Med Screen. 1995;2(1):41–4. doi: 10.1177/096914139500200111. [DOI] [PubMed] [Google Scholar]
- 21.Rozen P, Knaani J, Papo N. Evaluation and comparison of an immunochemical and a guaiac faecal occult blood screening test for colorectal neoplasia. Eur J Cancer Prev. 1995;4(6):475–81. doi: 10.1097/00008469-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem. 1999;45(1):123–6. [PubMed] [Google Scholar]
- 23.Fludger S, Turner AM, Harvey RF, Haslam N. Controlled prospective study of faecal occult blood screening for colorectal cancer in Bury, black pudding capital of the world. BMJ. 2002;325(7378):1444–5. doi: 10.1136/bmj.325.7378.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardcastle JD, Farrands PA, Balfour TW, Chamberlain J, Amar SS, Sheldon MG. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet. 1983;2(8340):1–4. doi: 10.1016/s0140-6736(83)90001-6. [DOI] [PubMed] [Google Scholar]
- 25.Greegor DH. Occult blood testing for detection of asymptomatic colon cancer. Cancer. 1971;28(1):131–4. doi: 10.1002/1097-0142(197107)28:1<131::aid-cncr2820280125>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Bassett ML, Goulston KJ. False positive and negative Hemoccult reactions on a normal diet and effect of diet restriction. Aust N Z J Med. 1980;10(1):1–4. doi: 10.1111/j.1445-5994.1980.tb03408.x. [DOI] [PubMed] [Google Scholar]
- 27.Daron PB, Goldman LI. Hemoccult screening in selected patients. South Med J. 1981;74(6):676–8. doi: 10.1097/00007611-198106000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Norfleet RG. Effect of diet on fecal occult blood testing in patients with colorectal polyps. Dig Dis Sci. 1986;31(5):498–501. doi: 10.1007/BF01320314. [DOI] [PubMed] [Google Scholar]
- 29.Hoogewerf PE, Hislop TG, Morrison BJ, Burns SD, Sizto R. Patient compliance with screening for fecal occult blood in family practice. CMAJ. 1987;137(3):195–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph A. Compliance with fecal occult blood testing: the role of restrictive diets. Am J Public Health. 1988;78(7):839–41. doi: 10.2105/ajph.78.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verne J, Kettner J, Mant D, Farmer A, Mortenson N, Northover J. Self-administered faecal occult blood tests do not increase compliance with screening for colorectal cancer: results of a randomized controlled trial. Eur J Cancer Prev. 1993;2(4):301–5. doi: 10.1097/00008469-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Robinson MH, Pye G, Thomas WM, Hardcastle JD, Mangham CM. Haemoccult screening for colorectal cancer: the effect of dietary restriction on compliance. Eur J Surg Oncol. 1994;20(5):545–8. [PubMed] [Google Scholar]
- 33.Statistics Canada Food statistics 2007. Catalogue No. 21-020-X. Ottawa, ON: Statistics Canada; 2007. Available from: www.statcan.gc.ca/pub/21-020-x/21-020-x2007001-eng.pdf. Accessed 2010 Feb 4.