Abstract
The semicircular canals measure head rotations, providing information critical for maintaining equilibrium. The canals of cetaceans (including whales, dolphins and porpoises) are extraordinarily small, making them unique exceptions to the allometric relationship shared by all other vertebrates between canal size and animal mass. Most modern cetaceans have shorter and less flexible necks than those of their ancestors, an adaptation hypothesized to have led to exaggerated head movements during locomotion. These movements are thought to have necessitated a decrease in the size and sensitivity of the canals, increasing their operating range to accommodate increased head motion. We tested whether the size of the semicircular canals in cetaceans is related to their head movements by comparing the rotational head velocities, frequencies and accelerations of the bottlenose dolphin (Tursiops truncatus) and a terrestrial relative, cattle (Bos taurus), using an array of three orthogonal head-fixed miniaturized rotational ratemeters. We collected data during typical locomotion (swimming; trotting) and during behaviors with enhanced head movements (rapid spiraling underwater; bucking). Cattle head movements always exceeded those of dolphins. Maximum head velocities were 528 deg. s−1 in dolphins and 534 deg. s−1 in cattle; maximum head frequencies were 2.86 Hz in dolphins and 3.45 Hz in cattle; and maximum head accelerations were 5253 deg. s−2 in dolphins and 10,880 deg. s−2 in cattle. These results indicate that accentuated head movements cannot explain the reduced size and sensitivity of cetacean semicircular canals. The evolutionary cause for their reduced canal size remains uncertain.
Keywords: cetacean, vestibular, head movement, Artiodactyl, semicircular canal, evolution
INTRODUCTION
The semicircular canals provide information about rotation of the head that is vital for orientation in space and for stabilizing gaze and maintaining posture during movement in both terrestrial and aquatic animals (Angelaki and Cullen, 2008; Day and Fitzpatrick, 2005; Howland, 1971). Most vertebrate species share a common allometric relationship between semicircular canal radius and body mass. The only exceptions to this are the cetaceans (whales, dolphins and porpoises), whose canals are significantly smaller than allometry would predict (Boenninghaus, 1903; Gray, 1907-1908; Hunter, 1787; Hyrtl, 1845; Jones and Spells, 1963; Muller, 1999; Spoor et al., 2002; Spoor and Thewissen, 2008; Watt, 1924). The canals of the bottlenose dolphin, for example, are approximately equal in size to those of a mouse and the canals of the blue whale are comparable to those of humans (Spoor et al., 2002).
Cetaceans evolved from artiodactyls, two-toed terrestrial mammals represented by contemporary species such as cattle, deer, giraffes, pigs and hippopotami (Gingerich et al., 2001). Together, these groups of mammals are known as cetartiodactyls (Montgelard et al., 1997). The cervical vertebrae of cetaceans are foreshortened and sometimes even partially fused (Gingerich et al., 1994). Limited flexibility of the cetacean neck is hypothesized to have rendered it unable to stabilize the head in space and significantly increased the angular rotations of the head during locomotion. If this increased motion exceeded the operating range of the canals, then smaller, less sensitive canals may have been required to maintain normal vestibular function (Spoor et al., 2002).
We tested the hypothesis that decreased canal size in cetaceans is related to increased head motion by comparing the angular head velocities, frequencies, and accelerations of cetaceans to those of a terrestrial artiodactyl, cattle, using head-fixed rotational ratemeters.
MATERIALS AND METHODS
An array of three orthogonal micromachined rotational ratemeters (Trirate 200S100, MemSense, Rapid City, SD, USA) with a range of ±1200 deg. s−1 and a sensitivity of 1.25 mV deg.−1 s−1 was used to measure head movements. The accuracy of the ratemeter was confirmed by comparing its output to the tachometer of a custom-designed rate table (SHOT Scientific, Greenville, IN, USA). Output from the ratemeters was recorded at a sampling rate of 100 Hz on a 10-bit datalogging device (Logomatic, Sparkfun Electronics, Boulder, CO, USA). The ratemeter and datalogging device were powered by two 9-V batteries, mounted together on a 24 cm×4 cm Delrin platform. Head movement recordings were made by securing the platform inside a PVC tube that was sealed at both ends with a threaded cap. The tube was 5 cm in diameter and 40.6 cm long, weighed 790 g (giving it a slight positive buoyancy), and was balanced at its midpoint. The device was designed to avoid influencing locomotion of the animal (Skrovan et al., 1999).
All experiments were performed with the approval of the Washington University Animal Studies Committee. Two captive-born bottlenose dolphins (Tursiops truncatus Montagu 1821) were provided by the Indianapolis Zoo for use in this study. The female was 8 years old, weighed 169 kg and was 216 cm in length. The male was 7 years old, weighed 153 kg, and was 247 cm in length. Recording sessions were conducted in an elliptical pool 41.5 m long, 17.4 m wide and 8.2 m deep, enclosing 5299.6 m3 of water. Each animal was trained to perform two tasks: normal swimming (with vertical movements of the tail in the sagittal plane) at maximum velocity, and a more vigorous ‘spinning’ (spiraling, corkscrewing or ‘barrel-rolling’) motion through the water.
Each dolphin completed several trials of each task. At the beginning of each trial, a dolphin trainer placed the PVC tube into the animal's mouth approximately 10 cm posterior to the rostrum so that the ratemeters were aligned with a coordinate system defined by a dorsal plane joining the animal's rostrum and its interocular line, a sagittal plane bisecting the head vertically, and a transverse plane perpendicular to the other planes (Fig. 1). Rotations in the dorsal plane were recorded as yaw motions, in the sagittal plane as pitch motions, and in the transverse plane as roll motions. Each animal was trained to hold the tube securely in place without allowing it to move in the mouth. Each trial was videotaped to ensure that the tube did not shift during the trial and trainers visually confirmed its position at the end of each trial.
Fig. 1.
Recording device in a dolphin's mouth.
One bull (Bos taurus Linnaeus 1758), bred to perform in professional rodeo bull-riding competitions, was used. The bull was 3 years old and weighed approximately 560 kg. For these experiments, the data collection platform was placed inside a 10.2 cm diameter PVC tube, 61.0 cm long and closed at both ends with a threaded cap, weighing 1700 g. The tube was secured to the head immediately posterior to the horns and oriented so that the ratemeter recording yaw rotations was earth-horizontal with the head held in the animal's customary posture. The bull was initially restrained in a standard chute designed for use in competition. On release from the chute, the bull produced bucking motions for approximately 30 s before trotting back to its corral. The tasks for each animal were chosen to measure both head rotations associated with typical locomotion and head rotations associated with more vigorous behavior likely to elicit greater head rotations.
Video recordings of the performing animal were synchronized with the output from the rotational ratemeters to allow the data tracings of the animal's head movements to be correlated precisely with its behavior during each trial. Data were downloaded to a computer and analyzed using custom-written software in the MATLAB (Natick, MA, USA) working environment. Analytic techniques were similar to those described previously (Armand and Minor, 2001). Velocity tracings from each of the ratemeters were divided into transients, defined as periods over which head velocity did not change sign. Transients less than 20 ms or involving a change in head position of less than 10 deg. were eliminated. The velocity of a transient was defined as the maximum velocity of the head during the transient. The power spectrum of each transient was determined by padding and windowing each transient with a cosine window before performing a Fourier analysis (Armand and Minor, 2001; Harris, 1998; Harris et al., 1990). The frequency of the transient was defined as the point below which 99.9% of the power of the transient was located. Head acceleration was determined by differentiating the velocity tracing using a 40 ms sliding window. Head position was determined by integrating velocity data with a constant of integration equal to zero.
RESULTS
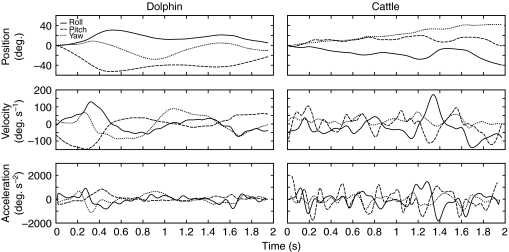
One dolphin performed 18 trials and the other performed 16 trials. The data for the two dolphins were subjectively comparable and were pooled for further analysis. The bull performed 7 trials. Sample data tracings are shown in Fig. 2. The dolphin demonstrated broad, smooth head movements whereas those of cattle changed more abruptly.
Fig. 2.
Sample data tracings showing angular head position, velocity and frequency of two cetartiodactyl species. Solid line, roll plane; dashed line, pitch plane; dotted line, yaw plane.
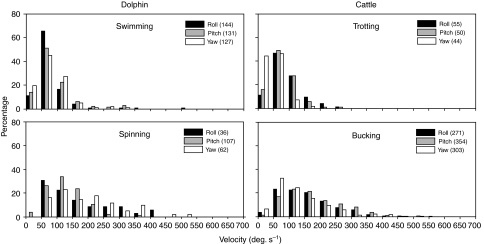
Distributions of the head velocities of the dolphins and cattle are shown in Fig. 3. During swimming, the angular velocities of the dolphins in all three planes were approximately equal, with the mode falling between 50 and 100 deg. s−1 and the distribution skewed heavily to the right. Although much of the dolphin's head motion was in the pitch plane (Fig. 2), Fig. 3 shows that the animals made similar numbers of high-velocity motions in the other planes as well. The mode of head motions during the more vigorous spinning task fell between 50 and 150 deg. s−1 in all planes. A greater proportion of head rotations during the more vigorous spinning task extended to higher velocities than during the swimming task.
Fig. 3.
Distribution of head velocities of dolphin and cattle. Black bars, roll; gray bars, pitch; white bars, yaw. Number of transients in each plane for each task is given in parentheses.
The distribution of the angular velocities of cattle while trotting was similar to that of dolphins while swimming. The modes of the cattle head velocities fell between 50 and 100 deg. s−1, with relatively little right skew. Head movements during the bucking task showed some similarities to those of the dolphin during the spinning task. The mode of the distributions of the head velocities in all planes and in both species was between 50 and 150 deg. s−1, although the distribution of cattle head velocities showed a much greater proportion of high head velocities than the dolphins.
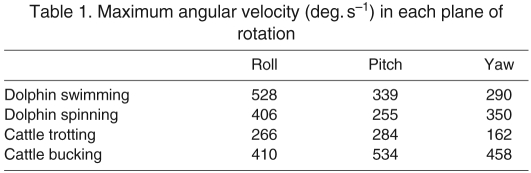
The maximum angular head velocity is critical to determining if head movements exceed the operating range of the canals. The maximum head velocities of the dolphins were seen in the roll plane during both tasks. The maximum head velocities of cattle were seen during the pitch plane in both tasks. The maximum velocity of cattle exceeded the maximum velocity of dolphins (Table 1).
Table 1.
Maximum angular velocity (deg. s−1) in each plane of rotation
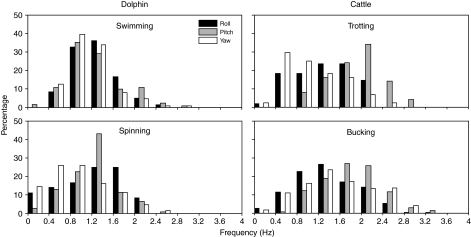
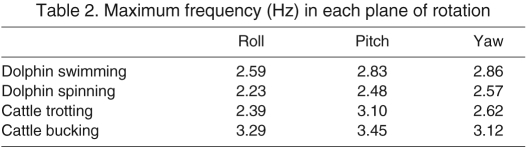
The response of a semicircular canal to a head rotation depends on the frequency content of the rotation as well as its angular velocity (Fernandez and Goldberg, 1971; Steinhausen, 1931). Distributions of the frequency content (in Hz) of transient head movements for dolphins performing each task are shown in Fig. 4. The frequency distributions were less right-skewed than those for velocity, and in all cases had maximal values of 2–3 Hz in all planes (Table 2). These values fall within the range previously reported for Tursiops species (Fish et al., 2003; Lang and Norris, 1966). The frequencies of head motion in cattle while trotting were dictated by the animal's gait. In each gait cycle, the animal pitched its head twice but yawed it only once (Fig. 4). The dolphins were not similarly constrained, so their frequencies of motion were more even across all three planes during normal swimming. The peak frequencies of head movement in dolphins and cattle are shown in Table 2. The peak values for the dolphins were lower than for cattle, consistent with the broad sinusoidal movements of the dolphin and the abrupt oscillations of cattle shown in Fig. 2.
Fig. 4.
Distribution of head frequencies of dolphin and cattle. Black bars, roll; gray bars, pitch; white bars, yaw. Number of transients in each plane for each task is given in parentheses in Fig. 3.
Table 2.
Maximum frequency (Hz) in each plane of rotation
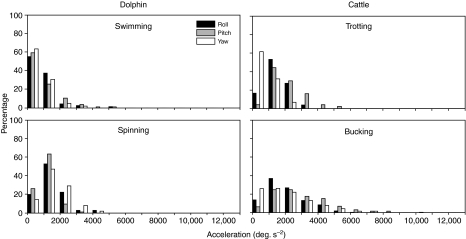
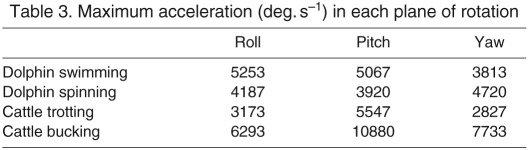
The acceleration of the head during a transient is dependent both on the transient's velocity and its frequency content. In both animals and all the test conditions, the acceleration distributions were right-skewed, with cattle extending a higher proportion of head movements to higher accelerations (Fig. 5). The maximal accelerations of cattle bucking were higher than the dolphin (Table 3).
Fig. 5.
Distribution of head accelerations of dolphin and cattle. Black bars, roll; gray bars, pitch; white bars, yaw. Number of transients in each plane for each task indicated in parentheses in Fig. 3.
Table 3.
Maximum acceleration (deg. s−1) in each plane of rotation
DISCUSSION
The extraordinarily small size of the semicircular canals of cetaceans has been hypothesized to be related to the development of particularly vigorous head motions as their ancestors evolved from terrestrial artiodactyls into an aquatic habitat. We tested this by determining if the head motions of the bottlenose dolphin (Tursiops truncatus) were greater than those of a close terrestrial relative, cattle (Bos taurus). We found that the head movements of dolphins do not exceed those of cattle, suggesting that factors other than head kinematics favored the reduction in size of the semicircular canals during cetacean evolution.
Canal responses in cattle and dolphins
The radius of curvature of a semicircular canal has been shown experimentally to have a positive linear correlation to its sensitivity and a negative linear correlation to its operating range (Yang and Hullar, 2007). This relationship takes into account the additional influence on sensitivity of the cross-sectional area of the canal, which scales in tandem with its radius of curvature and is also reduced in cetaceans (Jones and Spells, 1963). The radius of curvature of the semicircular canals of cattle is 3.2 mm and of dolphins is 1.1 mm (Spoor et al., 2002). The canals of cattle are therefore about 2.9 times more sensitive, and have an operating range correspondingly narrower, than those of dolphins. If the size and operating range of cetacean canals changed in tandem with increases in head velocity (Spoor et al., 2002), the head velocities of dolphins would be expected to be greater than those of cattle by nearly three times. The data presented here show instead that the distributions of head rotational velocities of dolphins are qualitatively similar to those of cattle and that the maximum rotational velocities of cattle and dolphins are almost identical. This is far different from the large disparity predicted by their relative canal sizes.
The same point may be illustrated by estimating the firing rates of primary vestibular nerve afferents in both species. The operating range of the semicircular canals is determined by the maximum firing rates of afferent neurons, which are likely to fall between 400 and 500 deg. s−1 (Sadeghi et al., 2007b). Published estimates of the relationship of canal size and velocity of head rotations to vestibular afferent firing rate allow the maximum firing rate of the vestibular afferents of the animals studied here to be predicted (Yang and Hullar, 2007). A typical rotation of each animal's head to a maximum velocity of 500 deg. s−1, consistent with the movements reported here, would be expected to increase the firing rate of cattle afferents up to approximately 567 spikes s−1 and the dolphin afferents up to only about 223 spikes s−1 (given a baseline firing rate of about 50 spikes s−1), far less than would be expected if the canal sizes of dolphins were tuned to their head movements.
The response of a semicircular canal to a head rotation also depends on the rotation's frequency and acceleration content. The effect of frequency on canal response is minor from 0.1 to 4 Hz, but at higher frequencies the sensitivity of the canal steadily increases and its operating range decreases (Baird et al., 1988; Fernandez and Goldberg, 1971; Hullar et al., 2005; Steinhausen, 1931). The distributions describing the frequency content of cattle and dolphin head rotations are comparable, falling largely between 0.5 and 2.5 Hz. This range is consistent with previous reports of swimming frequencies in bottlenose dolphin (Tursiops truncatus), false killer whale (Pseudorca crassidens), beluga whale (Delphinapterus leucas) and killer whale (Orcinus orca) (Fish, 1998). The maximum frequency of head movements in cattle exceeds that in dolphins by about 20%.
Rotational head accelerations are a critical parameter for evaluating the responses of the semicircular canals because they embody both velocity and frequency information and serve as the fundamental hydrodynamic stimuli for the semicircular canals (Steinhausen, 1931). The distributions of head accelerations are similar in cattle and dolphins, but the maximal head acceleration of cattle is more than twice that of dolphins.
The head movements of captive dolphins reported here could underestimate the head movements of animals in the wild (Fish, 2002; Maresh et al., 2004), but this difference is unlikely to affect the conclusions of this study for at least two reasons. First, even extraordinarily rapid angular head movements performed by wild cetaceans are matched by animals with much larger canals. The highest recorded rotational velocity of a wild cetacean is seven revolutions per second (Fish et al., 2006) but humans, despite having larger canals with an operating range approximately half that of dolphins (Spoor et al., 2002), can reach the same angular rate during triple Axel figure skating jumps (King, 1997). Second, it is likely that any difference between captive and wild dolphin head movements would be matched by similar differences between the head movements of captive and wild terrestrial artiodactyls.
The data reported here are the first detailed recordings of the angular head movements of cetaceans through the use of head-fixed rotational ratemeters. This technique is different from that used in previous studies, which calculated head motion from behaviors recorded on videotape by measuring the speed of a point on the animal's body (the center of gravity, or the tip of the rostrum, respectively) moving along a curved arc through the water (Fish, 2002; Maresh et al., 2004). That technique measures the vector sum of the point's linear velocity (Youm et al., 1978), but does not quantify the angular velocities of the head as was done here (Domenici, 2001). The use of head-fixed rotational ratemeters also avoids possible inaccuracies inherent to video techniques such as parallax, digitization errors and limitations due to frame rate.
A comparison between species of closer mass than cattle and bottlenose dolphin would have been preferable for this study, but the difference in mass should have only a small effect on this study's conclusions. Cattle have a typical mass of approximately 290 kg and their semicircular canals have radii of 3.2 mm, whereas bottlenose dolphins have a mass of about 190 kg and semicircular canal radii of 1.1 mm (Silva and Downing, 1995). If the cetacean species used here had been the same mass as the cattle, their canal size would be expected to be larger by only about 10% (Spoor et al., 2002). This difference is only a small fraction of the 290% difference in canal size and sensitivity between the two species.
A related consideration is that the bull used here was almost twice the mass of typical cattle as reported in the literature (Silva and Downing, 1995). Previous work has shown that there is little mass-dependent intraspecific variation in the size of the semicircular canals of mammals, suggesting that the canals of the bull used here were not enlarged despite its greater mass (Welker et al., 2009). If the bull's canals had in fact been enlarged proportional to its mass, they would have had a radius of approximately 3.6 mm (Spoor et al., 2002), making them correspondingly more sensitive with a narrower operating range and providing even further strength to the conclusions presented here.
Mechanisms of the vestibular system to tolerate vigorous head motions
Fundamental principles of the physiology and anatomy of the vestibular system are consistent with the conclusion that a reduction in canal size in cetaceans need not be a consequence of increases in head motion. The neuronal substrate of the vestibular system is extraordinarily adaptable to changing stimuli. Efferent fibers leading from the central vestibular system to the periphery could be able to modulate the responses of the semicircular canals (Highstein, 1992; Sadeghi et al., 2009). This suggests that they may be able to widen the operating range during rapid head motions while preserving the signal processing advantages of large, high-sensitivity canals (Hullar, 2008; Sadeghi et al., 2007a; Spoor et al., 2007; Yang and Hullar, 2007). Central vestibular interneurons, located postsynaptically to primary vestibular afferents, are able to change their sensitivity in response to changes in peripheral input (Smith and Curthoys, 1988a; Smith and Curthoys, 1988b; Straka et al., 2005). The population of vestibular afferents contains fibers with a wide range of sensitivities. The proportions of these fibers could adjust to changes in peripheral vestibular input, offering another means to modify the overall sensitivity of the system that does not invoke osteologic modifications (Sadeghi et al., 2007b).
The geometric relationship of the semicircular canals helps reduce the possibility that any particular head rotation exceeds the operating range of the semicircular canal system. Every rotation can be represented by a vector whose direction is related to the axis of rotation by the right-hand rule and whose length is proportional to the angular velocity. The length of the projection of this vector on the plane of each canal determines the response of the canal to the rotation. The canals on one side are not precisely orthogonal and those on opposite sides are not precisely parallel (Blanks et al., 1985; Blanks et al., 1975; Calabrese and Hullar, 2006; Curthoys et al., 1975; Ezure and Graf, 1984). This dramatically widens the operating range of the system by ensuring that any rotation is measured by several canals simultaneously, each with a different sensitivity to the rotation (Hullar and Williams, 2006; Rabbitt, 1999; Yang and Hullar, 2007).
Stabilization of the head in cetaceans
The finding that cetacean head movements are not increased above those of terrestrial animals is unsurprising given the basic requirements of their sensory and locomotory systems. Particularly vigorous head motions would be likely to disrupt the flow of critical auditory information both directly through movement of the head itself and indirectly through disturbing the nearby water (Au, 1993; Fish et al., 2003; Weihs, 2002). They would also compromise the ability of the animal to maintain the head as a stable reference frame for coordinating and interpreting information provided by the otolith organs and semicircular canals (Angelaki et al., 2004; Fitzpatrick et al., 2006).
Wide deflections of the head would require large amounts of energy and lead to an increased chance of disruption of the boundary layer surrounding the animal, increasing drag (Lighthill, 1971; Weihs, 1972; Weihs, 2002). The potential disadvantages of increased head movements are emphasized by the presence of several anatomical adaptations that passively contribute to stabilization of the head. These include reduced flexibility of the body and flippers, anterior displacement of the center of mass, location of flippers and tail far from the center of mass, and shape of the flippers (Fish et al., 2003; Long et al., 1997). Cetaceans also use an active mechanism to stabilize the head. By arcing the rostrum and tail in the same direction during each cycle of locomotion, the head is prevented from moving excessively in the sagittal plane (Fish et al., 2003).
Function of the semicircular canals in cetaceans
If rapid head movements do not explain the reduction in canal size among cetaceans, what does? One possible explanation is that the head motions of the ancestors of modern cetaceans might have been increased and their canal sizes decreased during their early evolution, with the small size of the canals remaining phylogenetically constrained despite the animals returning later to less agile behaviors. Given the disadvantage of small canals (Spoor et al., 2007) and the finding that a broad tail evolved with stabilizing flippers (Gingerich et al., 2009), this seems unlikely. An alternative proposed explanation is that the cetacean vestibular system has become profoundly hypofunctional to help prevent motion sickness (Ketten, 2000). This hypothesis is supported by the low numbers of axons found in the vestibular nerves of small cetaceans (Gao and Zhou, 1995). Reduced vestibular function might also help prevent potentially profoundly disorienting illusions resulting from cross-coupling among linear and rotational motions (Holly, 2004). This seems unlikely, given that other animals that live in a complex three-dimensional environment do not have a similar reduction in canal size (Gray, 1907-1908; Jones and Spells, 1963).
A markedly hypofunctional vestibular system would be unlikely, given its critical importance for cervical and spinal reflexes (Fetter, 2007), posture and locomotion (Fitzpatrick et al., 2006), body orientation (Friedmann, 1970), path integration (Glasauer et al., 2002), autonomic control (Yates and Bronstein, 2005), and skeletal development (Sahlstrand and Petruson, 1979). Input from the semicircular canals is also critical for a cetacean to determine if the apparent movement of an acoustic target was due to motion of the target, motion of the animal, or both. One unifying explanation might be that the attenuated semicircular canals of cetaceans represent a moderate reduction in sensitivity of the vestibular system. The presence of a vestibulo-ocular reflex in cetaceans demonstrates the system is functional (Butterworth et al., 2004), but its role in maintaining equilibrium and orientation may have been supplanted in part by auditory cues or the velocity of water passing over the skin (Ridgway and Carder, 1990).
Conclusions
We have shown that the head movements of a small cetacean, dolphin (Tursiops truncatus), do not exceed those of a comparison terrestrial artiodactyl (Bos taurus). This indicates that changes in the head movements of cetacean ancestors as they evolved from land into an aquatic habitat do not explain the reduction in size of their semicircular canals during this transition.
ACKNOWLEDGEMENTS
Animals were generously provided by the Indianapolis Zoo (dolphins) and Kirk Shot (cattle). We sincerely appreciate thoughtful comments on the manuscript by Paul E. Nachtigall, Ian S. Curthoys and Philip D. Gingerich. Supported by grant NIH/NIDCD K08 DC006869 (T.E.H.). Preliminary experiments were conducted with the assistance of the National Aquarium in Baltimore. Deposited in PMC for release after 12 months.
REFERENCES
- Angelaki D. E., Cullen K. E. (2008). Vestibular system: the many facets of a multimodal sense. Annu. Rev. Neurosci. 31, 125-150 [DOI] [PubMed] [Google Scholar]
- Angelaki D. E., Shaikh A. G., Green A. M., Dickman J. D. (2004). Neurons compute internal models of the physical laws of motion. Nature 430, 560-564 15282606 [Google Scholar]
- Armand M., Minor L. B. (2001). Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J. Comput. Neurosci. 11, 217-239 [DOI] [PubMed] [Google Scholar]
- Au W. W. (1993). The Sonar of Dolphins New York: Springer-Verlag; [Google Scholar]
- Baird R. A., Desmadryl G., Fernandez C., Goldberg J. M. (1988). The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J. Neurophysiol. 60, 182-203 [DOI] [PubMed] [Google Scholar]
- Blanks R. H. I., Curthoys I. S., Markham C. H. (1975). Planar relationships of the semicircular canals in man. Acta Otolaryngol. (Stockh.) 80, 185-196 [DOI] [PubMed] [Google Scholar]
- Blanks R. H. I., Curthoys I. S., Bennett M. L., Markham C. H. (1985). Planar relationships of the semicircular canals in rhesus and squirrel monkeys. Brain Res. 340, 315-324 [DOI] [PubMed] [Google Scholar]
- Boenninghaus G. (1903). Das Ohr des Zahnwales, zugleich ein Beitrag zur Theorie der Schalleitung. Zool. Jahr. (Anatomie) 17, 189-360 [Google Scholar]
- Butterworth A., Kestin S. C., McBain J. F. (2004). Evaluation of baseline indices of sensibility in captive cetaceans. Vet. Rec. 155, 513-518 [DOI] [PubMed] [Google Scholar]
- Calabrese D. R., Hullar T. E. (2006). MicroCT analysis of the dolphin vestibular labyrinth. Assoc. Res. Otolaryngol. Abs. 29, 447 [Google Scholar]
- Curthoys I. S., Curthoys E. J., Blanks R. H., Markham C. H. (1975). The orientation of the semicircular canals in the guinea pig. Acta Otolaryngol. (Stockh.) 80, 197-205 [DOI] [PubMed] [Google Scholar]
- Day B. L., Fitzpatrick R. C. (2005). The vestibular system. Curr. Biol. 15, R583-R586 [DOI] [PubMed] [Google Scholar]
- Domenici P. (2001). The scaling of locomotor performance in predator-prey encounters: from fish to killer whales. Comp. Biochem. Physiol. 131A, 169-182 [DOI] [PubMed] [Google Scholar]
- Ezure K., Graf W. (1984). A quantitative analysis of the spatial organization of the vestibulo-ocular reflexes in lateral- and frontal-eyed animals. I. Orientation of semicircular canals and extraocular muscles. Neuroscience 12, 85-93 [DOI] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. (1971). Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J. Neurophysiol. 34, 661-675 [DOI] [PubMed] [Google Scholar]
- Fetter M. (2007). Vestibulo-ocular reflex. Dev. Ophthalmol. 40, 35-51 [DOI] [PubMed] [Google Scholar]
- Fish F. (1998). Comparative kinematics and hydrodynamics of odontocete cetaceans: morphological and ecological correlates with swimming performance. J. Exp. Biol. 201, 2867-2877 [PubMed] [Google Scholar]
- Fish F. E. (2002). Balancing requirements for stability and maneuverability in cetaceans. Integr. Comp. Biol. 42, 85-93 [DOI] [PubMed] [Google Scholar]
- Fish F. E., Peacock J. E., Rohr J. J. (2003). Stabilization mechanism in swimming odontocete cetaceans by phased movements. Mar. Mamm. Sci. 19, 515-528 [Google Scholar]
- Fish F., Nicastro A., Weih D. (2006). Dynamics of the aerial maneuvers of spinner dolphins. J. Exp. Biol. 209, 590-598 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R. C., Butler J. E., Day B. L. (2006). Resolving head rotation for human bipedalism. Curr. Biol. 16, 1509-1514 [DOI] [PubMed] [Google Scholar]
- Friedmann G. (1970). The judgement of the visual vertical and horizontal with peripheral and central vestibular lesions. Brain 93, 313-328 [DOI] [PubMed] [Google Scholar]
- Gao G., Zhou K. (1995). Fiber analysis of the vestibular nerve of small cetaceans. In Sensory Systems of Aquatic Mammals (ed. Kastelein R. A., Thomas J. A., Nachtigall P. E.), pp. 447-453 Woerden, Netherlands: De Spil; [Google Scholar]
- Gingerich P., Raza S., Arif M., Anwar M., Zhou X. (1994). New whale from the Eocene of Pakistan and the origin of cetacean swimming. Nature 368, 844-847 [Google Scholar]
- Gingerich P., Haq M., Zalmout I., Khan I., Malkani M. (2001). Origin of whales from early artiodactyls: hands and feet of Eocene Protocetidae from Pakistan. Science 293, 2239-2242 [DOI] [PubMed] [Google Scholar]
- Gingerich P. D., Ul-Haq M., von Koenigswald W., Sanders W. J., Smith B. H., Zalmout I. S. (2009). New protocetid whale from the middle Eocene of Pakistan: birth on land, precocial development, and sexual dimorphism. PLoS One 4, e4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer S., Amorim M. A., Viaud-Delmon I., Berthoz A. (2002). Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp. Brain Res. 145, 489-497 [DOI] [PubMed] [Google Scholar]
- Gray A. A. (1907-1908). The Labyrinth of Animals London: Churchill; [Google Scholar]
- Harris C. M. (1998). The Fourier analysis of biological transients. J. Neurosci. Methods 83, 15-34 [DOI] [PubMed] [Google Scholar]
- Harris C. M., Wallman J., Scudder C. A. (1990). Fourier analysis of saccades in monkeys and humans. J. Neurophysiol. 63, 877-886 [DOI] [PubMed] [Google Scholar]
- Highstein S. M. (1992). The efferent control of the organs of balance and equilibrium in the toadfish, Opsanus tau. Ann. NY Acad. Sci. 656, 108-123 [DOI] [PubMed] [Google Scholar]
- Holly J. E. (2004). Vestibular coriolis effect differences modeled with three-dimensional linear-angular interactions. J. Vest. Res. 14, 443-460 [PubMed] [Google Scholar]
- Howland H. (1971). The role of the semicircular canals in the angular orientation of fish. Ann. NY Acad. Sci. 188, 202-216 [DOI] [PubMed] [Google Scholar]
- Hullar T. (2008). Neurometric and psychometric thresholds of the semicircular canals Soc. Neurosci. Abs. 168, 8 [Google Scholar]
- Hullar T. E., Williams C. D. (2006). Geometry of the semicircular canals of the chinchilla (Chinchilla laniger). Hear. Res. 213, 17-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar T. E., Della Santina C. C., Hirvonen T. P., Lasker D. M., Carey J. P., Minor L. B. (2005). Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J. Neurophysiol. 93, 2777-2786 [DOI] [PubMed] [Google Scholar]
- Hunter J. (1787). Observations on the structure and oeconomy of whales. Phil. Trans. Roy. Soc. Lond. 77, 371-450 [Google Scholar]
- Hyrtl J. (1845). Vergleichend-anatomische Untersuchungen über das innere Gehörorgan des Menschen und der Säugethiere Prag: Friedrich Ehrlich; [Google Scholar]
- Jones G. M., Spells K. E. (1963). A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc. R. Soc. Lond. B. Biol. Sci. 157, 403-419 [DOI] [PubMed] [Google Scholar]
- Ketten D. R. (2000). Cetacean ears. In Hearing by Whales and Dolphins (ed. Au W. W. L., Popper P., Fay R. R.), pp. 43-108 Heidelberg: Springer; [Google Scholar]
- King D. (1997). A biomechanical analysis of the Axel: critical parameters for successful jumps. Professional Skater, 10-12 [Google Scholar]
- Lackner J. R., DiZio P. A. (2000). Aspects of body self-calibration. Trends Cogn. Sci. 4, 279-288 [DOI] [PubMed] [Google Scholar]
- Lang T., Norris K. (1966). Swimming speed of a Pacific bottlenose porpoise. Science 151, 588-590 [DOI] [PubMed] [Google Scholar]
- Lighthill J. (1971). Elongated-body theory of fish locomotion. Proc. R. Soc. Lond. B. Biol. Sci. 179, 125-138 [Google Scholar]
- Long J. J., Pabst D., Shepherd W., McLellan W. (1997). Locomotor design of dolphin vertebral columns: Bending mechanics and morphology of Delphinus delphis. J. Exp. Biol. 200, 65-81 [DOI] [PubMed] [Google Scholar]
- Maresh J. L., Fish F. E., Nowacek D. P., Nowacek S. M., Wells R. S. (2004). High performance turning capabilities during foraging by bottlenose dolphins (Tursiops truncatus). Mar. Mamm. Sci. 20, 498-509 [Google Scholar]
- Montgelard C., Catzeflis F. M., Douzery E. (1997). Phylogenetic relationships of artiodactyls and cetaceans as deduced from the comparison of cytochrome b and 12S rRNA mitochondrial sequences. Mol. Biol. Evol. 14, 550-559 [DOI] [PubMed] [Google Scholar]
- Muller M. (1999). Size limitations in semicircular duct systems. J. Theor. Biol. 198, 405-437 [DOI] [PubMed] [Google Scholar]
- Rabbitt R. D. (1999). Directional coding of three-dimensional movements by the vestibular semicircular canals. Biol. Cybern. 80, 417-431 [DOI] [PubMed] [Google Scholar]
- Ridgway S. H., Carder D. A. (1990). Tactile sensitivity, somatosensory responses, skin vibrations, and the skin surface ridges of the bottlenose dolphin, Tursiops truncatus. In Sensory Abilities of Cetaceans (ed. Thomas J. A., Kastelein R. A.), pp. 163-179 New York: Plenum Press; [Google Scholar]
- Rogers S. (1998). Exploring dinosaur neuropaleobiology: Computed tomography scanning and analysis of an Allosaurus fragilis endocast. Neuron 21, 673-679 [DOI] [PubMed] [Google Scholar]
- Sadeghi S. G., Chacron M. J., Taylor M. C., Cullen K. E. (2007a). Neural variability, detection thresholds, and information transmission in the vestibular system. J. Neurosci. 27, 771-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi S. G., Minor L. B., Cullen K. E. (2007b). Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J. Neurophysiol. 97, 1503-1514 [DOI] [PubMed] [Google Scholar]
- Sadeghi S. G., Goldberg J. M., Minor L. B., Cullen K. E. (2009). Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J. Neurophysiol. 101, 988-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlstrand T., Petruson B. (1979). A study of labyrinthine function in patients with adolescent idiopathic scoliosis. I. An electro-nystagmographic study. Acta Orthop. Scand. 50, 759-769 [DOI] [PubMed] [Google Scholar]
- Silva M., Downing J. A. (1995). CRC Handbook of Mammalian Body Masses Boca Raton, FL: CRC Press; [Google Scholar]
- Skrovan R. C., Williams T. M., Berry P. S., Moore P. W., Davis R. W. (1999). The diving physiology of bottlenose dolphins (Tursiops truncatus). II. Biomechanics and changes in buoyancy at depth. J. Exp. Biol. 202, 2749-2761 [DOI] [PubMed] [Google Scholar]
- Smith P. F., Curthoys I. S. (1988a). Neuronal activity in the contralateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Res. 444, 295-307 [DOI] [PubMed] [Google Scholar]
- Smith P. F., Curthoys I. S. (1988b). Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Res. 444, 308-319 [DOI] [PubMed] [Google Scholar]
- Spoor F., Thewissen J. G. M. (2008). Comparative and functional anatomy of balance in aquatic mammals. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates (ed. Thewissen J. G. M., Nummela S.), pp. 257-284 Berkeley: University of California Press; [Google Scholar]
- Spoor F., Bajpai S., Hussain S. T., Kumar K., Thewissen J. G. (2002). Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature 417, 163-166 [DOI] [PubMed] [Google Scholar]
- Spoor F., Garland T., Jr, Krovitz G., Ryan T. M., Silcox M. T., Walker A. (2007). The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci. USA 104, 10808-10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen W. (1931). Über den Nachweis der Bewegung der Cupula in der intakten Bogengansampulle des Labyrinthes bei der natürlichen rotatorischen und calorischen Reizung. Arch. Ges. Physio. 228, 322-328 [Google Scholar]
- Straka H., Vibert N., Vidal P. P., Moore L. E., Dutia M. B. (2005). Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog. Neurobiol. 76, 349-392 [DOI] [PubMed] [Google Scholar]
- Watt H. (1924). Dimensions of the labyrinth correlated. Proc. R. Soc. Lond. B. Biol. Sci. 96, 334-338 [Google Scholar]
- Weihs D. (1972). A hydrodynamical analysis of fish turning manoeuvres. Proc. R. Soc. Lond. B. Biol. Sci. 182, 59-72 [Google Scholar]
- Weihs D. (2002). Stability versus maneuverability in aquatic locomotion. Integr. Comp. Biol. 42, 127-134 [DOI] [PubMed] [Google Scholar]
- Welker K. L., Orkin J. D., Ryan T. M. (2009). Analysis of intraindividual and intraspecific variation in semicircular canal dimensions using high-resolution x-ray computed tomography. J. Anat. 215, 444-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Hullar T. E. (2007). The relationship of semicircular canal size to vestibular-nerve afferent sensitivity in mammals. J. Neurophysiol. 98, 3197-3205 [DOI] [PubMed] [Google Scholar]
- Yates B. J., Bronstein A. M. (2005). The effects of vestibular system lesions on autonomic regulation: observations, mechanisms, and clinical implications. J. Vestib. Res. 15, 119-129 [PubMed] [Google Scholar]
- Youm Y., McMurthy R. Y., Flatt A. E., Gillespie T. E. (1978). Kinematics of the wrist. I. An experimental study of radial-ulnar deviation and flexion-extension. J. Bone Joint Surg. Am. 60, 423-431 [PubMed] [Google Scholar]