Abstract
Neurochemicals that stimulate food foraging and hoarding in Siberian hamsters are becoming more apparent, but we do not know if cessation of these behaviors is due to waning of excitatory stimuli and/or the advent of inhibitory factors. Cholecystokinin (CCK) may be such an inhibitory factor as it is the prototypic gastrointestinal satiety peptide and is physiologically important in decreasing food intake in several species including Siberian hamsters. Systemic injection of CCK-33 in laboratory rats decreases food intake, doing so to a greater extent than CCK-8. We found minimal effects of CCK-8 on food foraging and hoarding previously in Siberian hamsters, but have not tested CCK-33. Therefore, we asked: Does CCK-33 decrease normal levels or food deprivation-induced increases in food foraging, hoarding and intake? Hamsters were housed in a wheel running-based foraging system with simulated burrows to test the effects of peripheral injections of CCK-33 (13.2, 26.4, or 52.8 μg/kg body mass), with or without a preceding 56 h food deprivation. The highest dose of CCK-33 caused large baseline reductions in all three behaviors for the 1st h post injection compared with saline; in addition, the intermediate CCK-33 dose was sufficient to curtail food intake and foraging during the 1st h. In food deprived hamsters, we used a 52.8 μg/kg body mass dose of CCK-33 which decreased food intake, hoarding, and foraging almost completely compared with saline controls for 1 h. Therefore, CCK-33 appears to be a potent inhibitor of food intake, hoarding, and foraging in Siberian hamsters.
Keywords: appetitive behavior, consummatory behavior, injection, satiety
INTRODUCTION
The physiological mechanisms controlling ingestive behavior are of premier importance given the epic proportion of the obesity epidemic in many countries, especially the United States of America 44. Obesity arises from an imbalance in energy flux, with energy intake outstripping energy expenditure over time leading to the accumulation of excess energy in the form of fat stores. The classical animal models of laboratory rats and mice have yielded considerable insight into the physiological causes of obesity, but they typically do not explore the complete ingestive behavior sequence. That is, ingestive behavior consists of two phases: the appetitive phase, that includes all behaviors related to seeking, obtaining and storing food, and the consummatory phase, the actual intake of the food 12. Most research using laboratory rats and mice has focused upon the consummatory phase of food intake, with limited studies examining the appetitive phase. Siberian hamsters (Phodopus sungorus) offer a species where the two phases of ingestive behavior do not necessarily co-vary as they do in other species, especially laboratory rats and mice (for review see: 6). That is, for several energetic challenges (e.g., food deprivation 5,17, pregnancy and lactation 4) and neurochemical stimulation of the brain [e.g., neuropeptide Y (NPY) 15,19, ghrelin 34, agouti-related protein [AgRP]18] food hoarding, and to a lesser extent food foraging, increases markedly more than food intake (for review see: Keen-Rhinehart, Dailey and Bartness, in press).
We have focused on defining the mechanisms underlying the impressive increases in food hoarding and foraging that accompany refeeding after food deprivation 15,35–37. To date, we have shown that food deprivation increases circulating concentrations of the active form of ghrelin, the largely stomach-derived peptide that stimulates food intake in laboratory rats and mice 34. Ghrelin, in turn, appears to stimulate its receptors (growth hormone secretagogue receptors) some of which are located in the arcuate nucleus of the brain 60 on NPY/AgRP neurons 10,33. These arcuate nucleus neurons send projections to several brain areas including the hypothalamic paraventricular nucleus (PVH) and perifornical area (PFA) 22,38. Both areas possess NPY receptors 46,47 and parenchymal microinjections of NPY into either area stimulates food hoarding 15 that is reminiscent of the increases in food hoarding seen after systemic ghrelin injections in non-food deprived hamsters 34 or after 3rd ventricular injections of NPY 19. Although this work is far from complete, we have not as actively pursued the factors that terminate food foraging and hoarding. To this end we tested whether 3rd ventricular injections of melanotan II (MTII) a synthetic analog of the natural agonist for the melanocortin 3 and 4 receptors (MC3/4-R), α-melanocyte-stimulating hormone, would affect food deprivation- or ghrelin-induced increases in food deprived or in ad libitum-fed systemic ghrelin injected hamsters 35. MTII did not always completely block food deprivation- or ghrelin-induced increases in food hoarding suggesting that the MC3/4-R are involved in ghrelin- and food deprivation-induced increases in food intake, but other neurochemical systems also are involved in terminating this appetitive ingestive behavior 35,36.
Two of these other possible anorexigenic signals that could help terminate appetitive ingestive behaviors are leptin, the largely white adipose tissue-derived peptide 68, and cholecystokinin (CCK), the largely small intestine-derived peptide. Food deprivation-induced increases in food hoarding are almost completely blocked by 3rd ventricular injections of leptin, whereas peripheral leptin injection treatment is less effective inhibiting food hoarding and intake, and not effecting in inhibiting foraging37. CCK is a peptide released by the small intestine primarily in response to fat or protein intake 39 that also inhibits food intake when given exogenously, mimicking the naturally-occurring satiety sequence 26,27,57,58. Although there is some discussion as to the exact mechanism by which CCK inhibits food intake, it seems clear that a considerable portion of its satiating ability is via stimulation of CCK A receptors on vagal afferents projecting to the central distribution of the gut-brain axis that includes the caudal hindbrain, and hypothalamic and non-hypothalamic forebrain areas that process meal-related stimuli [for review see: 52]. This has led to classification of CCK as a vagally-mediated gastrointestinal satiety signal [for review see: 54].
CCK is found in the circulation in various bioactive forms from the parent molecule: CCK-8, CCK-22, CCK-33 and CCK-58, among others [for review see: 13,40]. In humans and dogs, it is thought that CCK-58 is the predominant form 23,24, whereas in rodents, either CCK-33 41,61 or CCK-58 50 seems to predominate. In laboratory rats, it appears that CCK-58 is more potent than CCK-8 in inhibiting food intake 28. It is unknown which amino acid length of CCK is the principal form in Siberian hamsters. Peripheral injection of CCK-8 inhibits food intake in a wide range of species from humans to rodents [for review see: 6]. Relevant to the proposed work, we have found that CCK-8 inhibits food intake in Siberian hamsters 7. Given the increased potency of CCK-33 versus CCK-8 in inhibiting food intake in laboratory rats 21 and given the lack of availability of CCK-58 commercially at this time, we chose to test the effects of CCK-33 in inhibiting foraging, hoarding and food intake by Siberian hamsters. Because CCK inhibits feeding after food consumption has started 51,55 and because during the first hour of re-feeding after food deprivation or injection of orexigenic peptides (ghrelin, NPY, AgRP) Siberian hamsters eat after foraging (although they eat more later 2–24 h post-food deprivation 15,18,19,34–36), then food eaten during the first few hours post-food deprivation could provide the gastrointestinal opportunity for stimulation of CCK release and subsequent effects on foraging/hoarding, as well as food intake. Therefore, we asked two questions: Does CCK-33 inhibit daily food foraging, hoarding, and intake? and Does CCK-33 inhibit food deprivation-induced increases in food foraging, hoarding, and intake?
MATERIALS AND METHODS
Animals and Housing
Adult male Siberian hamsters, ~3 months old and weighing between 32–50 g, were obtained from our breeding colony. The breeding colony was established in 1988 and outbred with wild-caught individuals, as previously described 9. Hamsters were group housed in a summer-like photoperiod (16:8-h light-dark cycle [light onset at 0030]) from birth. Room temperature was maintained at 21 ± 2 °C, with relative humidity at ~50 ± 10 %. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in compliance with the Public Health Service and United States Department of Agriculture guidelines.
At the start of the experiment, animals were transferred to the foraging/hoarding room and singly housed in polypropylene cages (290 × 180 × 130 mm) for two weeks to acclimate to the new light onset (2000) and single housing. The test diet [75 mg pellets, Dustless Precision Pellets (protein 18.8%, fat 5.0%, fiber 4.6%, ash 4.4%, moisture < 5.0%, carbohydrate 61.5%, caloric value: 3.68 kcal/gm; BioServ, Frenchtown, NJ) and tap water were available ad libitum throughout unless otherwise noted. Siberian hamsters were then placed into the foraging/hoarding apparatuses for two weeks of acclimation, as previously described 16. Briefly, hamsters lived in housing where two cages were connected via polyvinyl-chloride tubing system (38.1 mm inner diameter and ~1.5 m long), with vertical climbs and horizontal runs to facilitate movement between cages. The top, or “food” cage, was 456 × 234 × 200 mm (length × width × height) and equipped with a water bottle, running wheel, and food pellet dispenser. The bottom, or “burrow” cage, was 290 × 180 × 130 mm (length × width × height) and contained Alpha-Dri (Specialty Papers, Kalamazoo, MI) bedding and one cotton nestlet (Ancare, Belmore, NY). The bottom cage was covered to simulate the darkness of a burrow.
Acclimation to Foraging/Hoarding Apparatus and Foraging Treatments
During the first two days of the acclimation/training period all animals were given free access to food and were able to earn food by completing 10 revolutions on the running wheel. Wheel revolutions were counted using a magnetic detection system and pellets were delivered using computer-based hardware/software interface (Med Associates, Georgia, VT). On the third day and the remainder of the acclimation/training period, free food was removed and food was delivered solely on a response-contingent condition of one pellet for every 10 wheel revolutions. Animals were then separated into three foraging effort groups that were matched for percent body mass change during the acclimation period and average hoard size over the last week of the acclimation/training period. The three foraging effort groups were: 1) 10 wheel revolutions per pellet (10REV), 2) free wheel/free food (FW; 300 pellets of food were available each day in a non-contingent manner with an active running wheel), and 3) blocked wheel/free food (BW; 300 pellets of food were available each day in a non-contingent manner and animals did not have access to a working running wheel). The last two groups served as important controls. Specifically, data from the FW group tested for non-specific treatment effects on locomotor activity. That is, increased wheel revolutions by the FW group would indicate non-specific stimulation of locomotor activity that, without this group, would be interpreted as an increase in the motivation to obtain food by the 10 revolutions/pellet group. In addition, this group allowed us to test if decreased foraging was due to non-specific/malaise effects on locomotor activity or whether they were due to a bona fide suppression in the motivation to earn food. The BW group was equivalent to a sedentary control condition in exercise experiments and was included to account for the locomotor activity-induced changes in all dependent variables.
Measurement of Food Foraging, Hoarding, and Intake
Food foraging, hoarding and intake were assessed each day of the experiment between 0900 and 1100. Food foraged (pellets earned) was defined as the number of pellets delivered into the top cage of the 10REV group hamsters and calculated as wheel revolutions ÷ 10. Wheel revolutions were recorded continuously in 5 minute bins of activity. Hoarded food was removed each day and was defined as the number of pellets found in the bottom cage, as well as that removed from hamsters’ check pouches. Surplus food was removed each day and defined as the food pellets that remained in the top cage that were neither ingested nor hoarded. Food intake in the 10REV group was determined by: [pellets earned – (surplus food + food hoard)]. In the FW and BW groups, food intake was defined as: [300 − (surplus food + food hoard)]. An electronic balance was used to weigh the food pellets and was set to “parts” measurement; thus, one 75-mg food pellet was equal to 1 pellet, with fractions of a pellet calculated as well.
Experimental Protocol
Experiment 1: Does CCK-33 inhibit daily food foraging, hoarding, and intake?
At the end of the acclimation/training period hamsters were divided into their groups (10REV: n = 14, FW: n = 13, BW: n = 13). Intraperitoneal injections consisted of one of three tyrosine sulfated CCK-33 (Peptide Institute, Inc, Osaka, Japan) doses (13.2, 26.4, and 52.8 μg/kg body mass) or vehicle (0.3ml of 0.09 % sterile saline). The doses of CCK-33 were selected based on those used in laboratory rat food intake experiments 21. Each animal received all possible injections in a counterbalanced schedule to control for possible injection order effects. A washout period of four days separated each injection.
On injection days, each animal was provided with a clean burrow cage and access to food was occluded by blocking access to the top cage two h before light offset. At light offset, each animal was injected and access to the food cage was returned. Wheel revolutions and food surplus, hoarding and intake were measured 1, 2, 4, and 24 h post-injection.
Experiment 2: Does CCK-33 inhibit food deprivation-induced increases in food foraging, hoarding, and intake?
After all animals had received the four treatments in Experiment 1, a final washout period of three days occurred. At that time the treatment groups were balanced using baseline food intake and percent body mass change over the course of Experiment 1. They then were food deprived for 56 h (IACUC approved), a time shown previously to optimally increase food hoarding 5. Foraging treatments remained the same, but no food was available.
Before refeeding at light offset, half of the animals received intraperitoneal injection of CCK-33 (52.8 μg/kg body mass), whereas the other half received 0.3 ml sterile saline vehicle. This dose of CCK-33 was chosen because it yielded the greatest decrease in food hoarding in Experiment 1 (see below). Wheel revolutions and food surplus, hoarding and intake were measured 1, 2, 4 and 24 h post-injection.
Statistical Analyses
For Experiment 1, raw data was transformed into percent change from saline before statistical analyses according to the formula: [((X-Saline)/Saline)*100]. The percent change values were analyzed using a repeated measures two-way ANOVA (4 × 3 × 4; time point × foraging effort group × drug dose). An a priori analysis was run on the percent change values for the first hour alone using 2-way ANOVA (3 × 4; foraging effort group × drug dose) using NCSS version 2000 (Kaysville, UT). Post hoc analysis was done with Tukey-Kramer Multiple-Comparison Tests when appropriate.
For Experiment 2, raw data were transformed in the same manner as in Experiment 1. A repeated measures two-way ANOVA was used to analyze the full data set (4 × 3 × 2; time point × foraging effort group × drug dose); an a priori second analysis was also run: two-way ANOVA (3 × 2; foraging effort group × drug dose) using only the data from the first hour post-refeeding. Post hoc analysis was done with Tukey-Kramer Multiple-Comparison Tests when appropriate.
For both Experiments, differences among groups were considered statistically significant if P<0.05. Exact probabilities and test values have been omitted for simplification and clarity of the presentation of the results. A “1” was added to all values to allow for calculation of the percent change from control values when the control value was “0”.
RESULTS
Experiment 1: Does CCK-33 inhibit daily food foraging, hoarding, and intake?
CCK-33 statistically significantly inhibited food intake and hoarding (Ps<0.05), but not foraging, during the first hour of testing; no differences were found at any time point subsequent (1–2 h, 2–4 h, and 4–24 h; data not shown). Because of the restriction of significance to the 1st h post injection, we report data from this time point only.
Wheel Revolutions
Wheel running in the FW group was not affected by any dose of CCK-33 compared to saline injections, indicating that there were no non-specific effects of the treatments on locomotor activity (data not shown).
Food Foraged
Food foraging was not affected by any dose CCK-33 compared to saline during (data not shown).
Food Intake
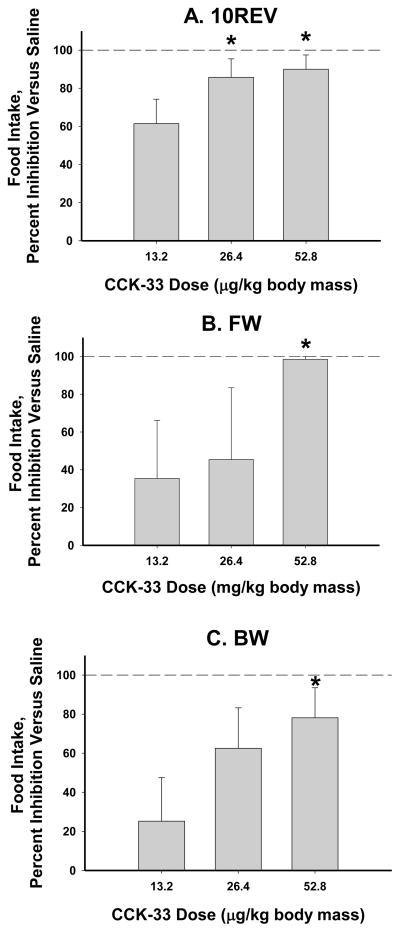
The percent inhibition of saline treatment food intake was nearly complete for the highest CCK-33 dose (52.8 μg/kg body mass) regardless of the foraging effort treatment (Ps<0.05; Fig. 1). The intermediate dose of CCK-3 (26.4 μg/kg body mass) led to a significant percent inhibition in food intake compared with the saline-injected animals only in the 10REV group (P<0.05; Fig. 1A). The lowest CCK-33 dose (13.2 μg/kg body mass) did not result in a significant inhibition of food intake compared to saline-injected animals in any foraging treatment (Fig. 1).
Figure 1.
Mean ad libitum food intake as affected by three doses of CCK-33 (13.2, 26.4, and 52.8 μg/kg body mass), for one hour post-injection, expressed as a percent inhibition from the saline controls ± SEM: A) 10 revolutions per pellet group (saline: 1.57 ± 0.48 pellets eaten), B) Free Wheel/Free Food group (saline: 2.15 ± 0.43 pellets eaten), and C) Blocked Wheel/Free Food group (saline: 2.62 ± 0.61 pellets eaten). * P<0.05 vs saline injections.
Food Hoarding
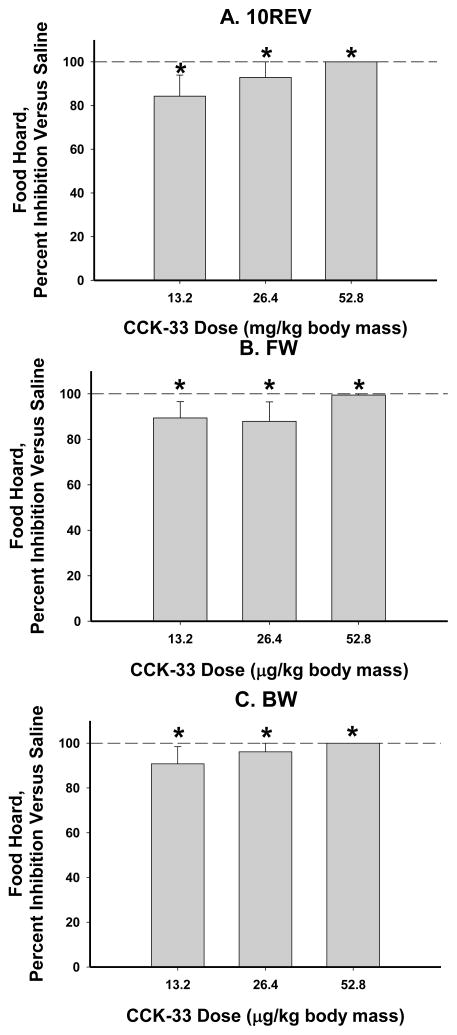
All three doses of CCK-33 inhibited food hoarding equally compared with the saline-injected control animals in each foraging treatment (Ps<0.05; Fig. 2).
Figure 2.
Mean ad libitum food hoarding as affected by three doses of CCK-33 (13.2, 26.4, and 52.8 μg/kg body mass), for one hour post-injection, expressed as a percent inhibition from the saline controls ± SEM: A) 10 revolutions per pellet group (saline: 1.73 ± 0.44 pellets hoarded), B) Free Wheel/Free Food group (saline: 2.25 ± 1.08 pellets hoarded), and C) Blocked Wheel/Free Food group (saline: 1.46 ± 0.41 pellets hoarded). * P<0.05 vs saline injections.
Experiment 2: Does CCK-33 inhibit food deprivation-induced increases in food foraging, hoarding, and intake?
Post-injection/refeeding in food foraging, food intake and hoarding were significantly inhibited within the first hour (Ps<0.05); no differences were found at any subsequent time point subsequent (1–2 h, 2–4 h, and 4–24 h) for all behaviors measured (data not shown). Because of the restriction of significance to the 1st h post injection, we report data from this time point only.
Wheel Revolutions
Wheel running in the FW group was not affected by CCK-33 (52.8 μg/kg body mass) or saline treatment, indicating that there were no non-specific effects of the treatments on locomotor activity (data not shown).
Food Foraged
Food deprivation-induced foraging was significantly inhibited by CCK-33 treatment compared with saline treated animals (P<0.05; 93.6 ± 5.0 % inhibition; data not shown).
Food Intake
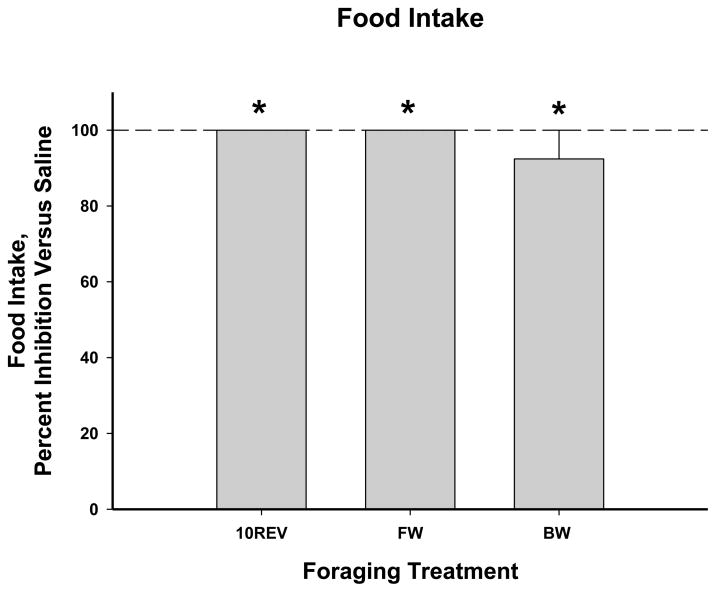
After food deprivation, CCK-33 (52.8 μg/kg body mass) resulted in nearly complete inhibition of food intake for all foraging treatments compared with saline treatment (Ps<0.05; Fig. 3).
Figure 3.
Mean food intake post-food deprivation as affected by CCK-33 (52.8 μg/kg body mass), for one hour post-injection, expressed as percent inhibition from saline controls ± SEM: A) 10 revolutions per pellet group (saline: 4 ± 2.49 pellets eaten), B) Free Wheel/Free Food group (saline: 7.3 ± 0.67 pellets eaten), and C) Blocked Wheel/Free Food group (saline: 4.4 ± 0.93 pellets eaten). * P<0.05 vs saline injections.
Food Hoarding
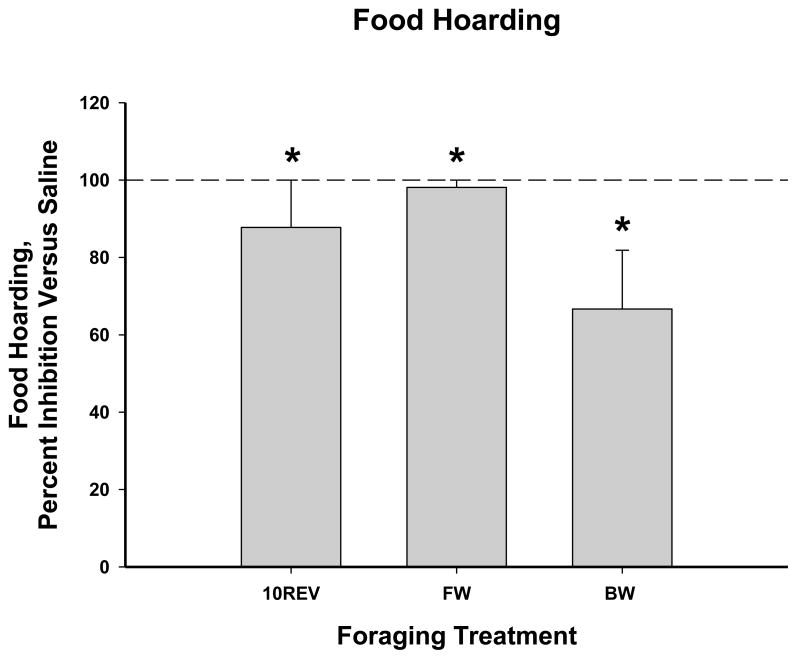
Food deprivation-induced increases in food hoarding were inhibited by CCK-33 administration compared with saline, regardless of the foraging effort treatment (Ps<0.05; Fig. 4).
Figure 4.
Mean food hoarding post-food deprivation as affected by CCK-33 (52.8 μg/kg body mass), for one hour post-injection, expressed as percent inhibition from saline controls ± SEM: A) 10 revolutions per pellet group (saline: 4.7 ± 2.85 pellets hoarded), B) Free Wheel/Free Food group (saline: 10.5 ± 2.81 pellets hoarded), and C) Blocked Wheel/Free Food group (saline: 5.0 ± 3.56 pellets hoarded). * P<0.05 vs saline injections.
DISCUSSION
CCK has been demonstrated to be a potent inhibitor of the consummatory phase of ingestive behavior in several rodent species. Here we show that CCK-33 is an inhibitor of both the consummatory and appetitive phase of ingestive behavior in Siberian hamsters. Daily food intake and hoarding were significantly inhibited by peripheral CCK-33 injection during the first hour post-treatment, whereas food foraging was not affected by CCK-33 treatment; CCK-33 injection did not alter these behaviors at any time point beyond the first hour. CCK-33 administration after a 56 hr-food deprivation inhibited, almost completely, food intake (97.5 % inhibition), foraging (93.6 % inhibition), and hoarding (83.6 % inhibition) during the first hour of refeeding, but at no subsequent time point.
Previous work in our laboratory tested the effects of CCK-8 on ingestive behavior in Siberian hamsters7 demonstrating a heightened sensitivity to the inhibitory effects of peripheral injections of the peptide on home cage feeding in short photoperiod (winter-like days)-housed female Siberian hamsters. Siberian hamsters naturally decrease their food intake when exposed to short photoperiods 7,8,63 and the mechanism underlying these changes are not precisely known, but the greatly exaggerated effect of the CCK-8 inhibition of food intake in short photoperiods 7 might at least account for some of this response. Using the same methodology as here, we previously examined the effects of CCK-8 on both baseline and food deprivation induced responses of food foraging, hoarding, and intake in Siberian hamsters (M. Dailey, C. Vaughan, and T. Bartness; unpublished observations). CCK-8 (10 and 5 μg/kg, i.p.) was not effective at decreasing baseline food foraging, intake, or hoarding; nor was CCK-8 (10 μg/kg) effective in inhibiting food deprivation induced increases in food foraging or hoarding (M. Dailey, C. Vaughan, and T. Bartness; unpublished observations). Those data, together with the present data suggest that CCK-33 is a more potent inhibitor of ingestive behaviors than CCK-8 in male Siberian hamsters in our foraging/hoarding apparatus. CCK-8 was able to inhibit food intake by female Siberian hamsters at the same doses as above in a standard home cage feeding protocol 7. Gender or testing methodology could be playing a role in these incongruent results, although in laboratory rats and mice, CCK is less rather than more effective in females 59,64. The increased efficacy of CCK-33 found here is not unique to Siberian hamsters as a similar hierarchy of CCK isoforms for food intake inhibition has been previously shown in laboratory rats and mice 21. The possibility that the doses of the two CCK isoforms, CCK-33 (present data) and CCK-8 (M. Dailey, C. Vaughan, and T. Bartness; unpublished observations) used were drastically different and is the cause of our increased efficacy of CCK-33 is slight, and probably not important as the molar quantities used were similar (CCK-8: 4.4 and 8.5 nmol; and CCK-33: 3.5, 7.0, and 13.9 nmol). Other labs have shown that CCK-58, the predominant detectable form of CCK, causes the same magnitude, but longer lasting, inhibition of food intake compared with CCK-8 28. The difference in the length of inhibition is possibly due to the rate of clearance/metabolism of the shorter versus longer forms 29,30,67. CCK-58 remains to be tested in Siberian hamsters or for the inhibition of the appetitive phase of ingestive behavior in any species.
Although this is the first demonstration of CCK affecting the appetitive behaviors of food foraging and hoarding per se, others have reported that CCK can alter other appetitive behaviors. For example, in seminal work regarding non-food related effects of CCK, mice injected with CCK-8 at doses typically used to produce satiety to food explore less and show what would be considered more anxiety-like behaviors than their vehicle controls, without producing deleterious effects on general locomotor behavior 14. Similarly, we found no decrease in wheel running by the FW group that did not have to earn their food by running, but CCK-33 significantly decreased foraging for food by the 10REV group indicating a specific decrease in the motivation to earn food. Intravenously injected CCK-8 also inhibits operant responding for food, water, sucrose solution and heat by food-deprived, water deprived, non-deprived and cold exposed pigs 2 and operant responding for food in laboratory rats 1. The inhibitory effect of CCK-8 on operant responding for food is greatest when bar pressing results in food delivery (i.e., when CCK-8 is interacting with food stimuli) than during extinction bar pressing [i.e., when food is withheld; 31). The inhibition of operant responding (i.e., appetitive ingestive behavior) may be mediated via peripheral CCK A receptors, at least in pigs, as intravenous injection for this receptor agonist, but not central injection nor peripheral or central injection of CCK B receptor agonist, inhibit operant food responses 48. Collectively, these data suggest that CCK-8 can inhibit appetitive ingestive behaviors as well as a wider range of appetitive behaviors than those for food alone 2,14. Note, however, that this partially contrasts with a small effect of CCK-8 on runway performance with food as the reinforcer at the end of the runway. These data are consistent, however, with an ability of CCK-8 to inhibit runway performance if administered following a prefeeding meal that is given before the access to the runway 11. Thus, it seems likely that the ability of CCK-33 to inhibit the appetitive ingestive behaviors of foraging and hoarding here possibly occurred because of the pattern of ingestive behaviors seen in these animals where they forage for food, eat some of it as well as hoarding it, and then continue this sequence (Day and Bartness, unpublished observations).
Food deprivation in Siberian hamsters results in increases in food foraging and hoarding to a much greater extent than intake after refeeding 5,20 -- a phenomenon unlike other rodents commonly used in food intake studies [for review see: 6]. CCK-33 was able to inhibit the food deprivation-induced increases in food foraging and hoarding during the first hour of refeeding, but not subsequently. Food intake also was inhibited by CCK-33 administration during the first hour of refeeding, but did not inhibit food intake beyond that first hour. The rapid waning of the inhibitory affects on food intake by CCK is not unique to these two experiments, as work in both laboratory rats and mice have suggested that CCK acts to signal satiety on a meal-to-meal basis and not in the long term 25,65,66. In Syrian hamsters, the ability of exogenous CCK-8 to inhibit food intake during refeeding after food deprivation is gone 2 h post-refeeding 32.
CCK-33 (present study) and leptin 37 are the only two endogenous anorexigenic compounds that inhibit food hoarding tested to date. Unlike the early and short duration inhibition of food deprivation-induced food hoarding by CCK-33 in the present experiment, peripherally administered leptin had a more protracted effect lasting as long as 48 hr 37. In addition, CCK-33 inhibited the food deprivation-induced increases in foraging and hoarding as well as the small food deprivation-induced increases in food intake, whereas leptin did not affect food deprivation-induced increases in foraging, but did do so for the increases in food hoarding and the small increases in food intake upon refeeding 37. Interestingly, leptin and CCK can act synergistically to inhibit food intake in laboratory rats and mice as shown by subeffective doses of each peptide not altering food intake singly, but doing so when injected together 3,42. Such an interaction has not been tested for the inhibitory effects of these peptides on appetitive and consummatory ingestive behaviors in Siberian hamsters. Previous research has shown that peripherally injected CCK-8 and -33 act via vagal afferents to inhibit food intake, but also in part through pyloric CCK receptor inhibition of gastric emptying 49,53. The primacy of vagal afferents in mediating the inhibitory effect of CCK on food intake is seen by the complete blockade of this effect with selective gastric vagotomy 56. Relative to hamster species, complete subdiaphragmatic vagotomy blocks the ability of exogenously administered CCK to inhibit food intake in Syrian hamsters 43. The effects of selective vagotomies on the satiety effect of CCK have not been explored in any hamster species to our knowledge. Interruption of the gut-brain signaling pathway does not always result in a loss of CCK functionality [e.g., 21 including in Syrian hamsters 43]. High doses of CCK (i.e., 26.4 and 52.8 μg/kg in rats and ≥8 μg/kg in Syrian hamsters) are able to inhibit food intake when the vagus nerve is cut below the diaphragm 21,43. One possible explanation for this effect is that high doses of CCK are able to enter circulation and thereby gain access to the brain, although some contend that CCK cannot cross the blood-brain barrier 45,62.
Collectively, our findings demonstrate that CCK-33 is a potent inhibitor of both the appetitive and consummatory phases of ingestive behavior acutely, 1-hour post injection in ad libitum-fed Siberian hamsters and during the same time in food deprived-refed hamsters. Specifically, CCK-33 is able to inhibit food intake and hoarding in ad libitum-fed animals and food foraging, intake, and hoarding after a 56 hr food deprivation upon refeeding. These findings are unique as they demonstrate a system that can be manipulated to not only decrease food intake, but also decrease food seeking/storing behaviors. This type of effect could lead to alternative interventions to treat human obesity rather than targeting the consumption of food only which has been less than spectacularly effective to date.
Acknowledgments
The authors thank Bruce Smith Jr. and Dominique Okoduwa for assistance in data collection. This work was supported, in part, by NIH R01 DK 78358 to TJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babcock AM, Livosky M, Avery DD. Cholecystokinin and bombesin suppress operant responding for food reward. Pharmacol Biochem Behav. 1985 May;22(5):893–895. doi: 10.1016/0091-3057(85)90543-x. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin BA, Cooper TR, Parrott RF. Intravenous cholecystokinin octapeptide in pigs reduces operant responding for food, water, sucrose solution or radiant heat. Physiol Behav. 1983 Mar;30(3):399–403. doi: 10.1016/0031-9384(83)90143-9. [DOI] [PubMed] [Google Scholar]
- 3.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997 Sep;94(19):10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartness TJ. Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am J Physiol. 1997;272:R118–R125. doi: 10.1152/ajpregu.1997.272.1.R118. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- 6.Bartness TJ, Demas GE. Comparative studies of food intake: Lessons from non-traditionally studied species. In: Stricker EM, Woods SC, editors. Food and Fluid Intake. New York: Plenum; 2004. pp. 423–467. [Google Scholar]
- 7.Bartness TJ, Morley JE, Levine AS. Photoperiod-peptide interactions in the energy intake of Siberian hamsters. Peptides. 1986;7:1079–1085. doi: 10.1016/0196-9781(86)90137-3. [DOI] [PubMed] [Google Scholar]
- 8.Boss-Williams KA, Bartness TJ. NPY stimulation of food intake in Siberian hamsters is not photoperiod dependent. Physiol Behav. 1996;59:157–164. doi: 10.1016/0031-9384(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 9.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003 Feb;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 11.Cox JE. Cholecystokinin interacts with prefeeding to impair runway performance. Behav Brain Res. 1986 Jul;21(1):29–36. doi: 10.1016/0166-4328(86)90057-4. [DOI] [PubMed] [Google Scholar]
- 12.Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15(4):731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 14.Crawley JN, Hays SE, Paul SM, Goodwin FK. Cholecystokinin reduces exploratory behavior in mice. Physiol Behav. 1981 Sep;27(3):407–411. doi: 10.1016/0031-9384(81)90324-3. [DOI] [PubMed] [Google Scholar]
- 15.Dailey MJ, Bartness TJ. Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R877–R892. doi: 10.1152/ajpregu.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool. 2001;289:162–171. [PubMed] [Google Scholar]
- 17.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav. 2003;78:655–668. doi: 10.1016/s0031-9384(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 18.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol. 2004;286:R38–R45. doi: 10.1152/ajpregu.00284.2003. [DOI] [PubMed] [Google Scholar]
- 19.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005 Jul;289(1):R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 20.Day DE, Mintz EM, Bartness TJ. Diet self-selection and food hoarding after food deprivation by Siberian hamsters. Physiol Behav. 1999;68:187–194. doi: 10.1016/s0031-9384(99)00167-5. [DOI] [PubMed] [Google Scholar]
- 21.Eisen S, Phillips RJ, Geary N, Baronowsky EA, Powley TL, Smith GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol. 2005 Aug;289(2):R456–R462. doi: 10.1152/ajpregu.00062.2005. [DOI] [PubMed] [Google Scholar]
- 22.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998 Dec;402(4):442–459. [PubMed] [Google Scholar]
- 23.Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem. 1987 Jan;262(1):214–217. [PubMed] [Google Scholar]
- 24.Eysselein VE, Eberlein GA, Schaeffer M, Grandt D, Goebell H, Niebel W, Rosenquist GL, Meyer HE, Reeve JR., Jr Characterization of the major form of cholecystokinin in human intestine: CCK-58. Am J Physiol. 1990 Feb;258(2 Pt 1):G253–G260. doi: 10.1152/ajpgi.1990.258.2.G253. [DOI] [PubMed] [Google Scholar]
- 25.Gallmann E, Arsenijevic D, Williams G, Langhans W, Spengler M. Effect of intraperitoneal CCK-8 on food intake and brain orexin-A after 48 h of fasting in the rat. Regul Pept. 2006 Jan;133(1–3):139–146. doi: 10.1016/j.regpep.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs J, Smith GP. Gut peptides and food in the gut produce similar satiety effects. Peptides. 1982 May;3(3):553–557. doi: 10.1016/0196-9781(82)90125-5. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 28.Glatzle J, Raybould HE, Kueper MA, Reeve JR, Jr, Zittel TT. Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr Neurosci. 2008 Apr;11(2):69–74. doi: 10.1179/147683008X301432. [DOI] [PubMed] [Google Scholar]
- 29.Gores GJ, LaRusso NF, Miller LJ. Hepatic processing of cholecystokinin peptides. I. Structural specificity and mechanism of hepatic extraction. Am J Physiol. 1986 Mar;250(3 Pt 1):G344–G349. doi: 10.1152/ajpgi.1986.250.3.G344. [DOI] [PubMed] [Google Scholar]
- 30.Gores GJ, Miller LJ, LaRusso NF. Hepatic processing of cholecystokinin peptides. II. Cellular metabolism, transport, and biliary excretion. Am J Physiol. 1986 Mar;250(3 Pt 1):G350–G356. doi: 10.1152/ajpgi.1986.250.3.G350. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao S, Deupree D. Cholecystokinin and bombesin effects on rewarded and nonrewarded operants. Peptides. 1983 Jan;4(1):1–3. doi: 10.1016/0196-9781(83)90155-9. [DOI] [PubMed] [Google Scholar]
- 32.Jones JE, Corp ES, Wade GN. Effects of naltrexone and CCK on estrous behavior and food intake in Syrian hamsters. Peptides. 2001 Apr;22(4):601–606. doi: 10.1016/s0196-9781(01)00370-9. [DOI] [PubMed] [Google Scholar]
- 33.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000 Dec;141(12):4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 34.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 35.Keen-Rhinehart E, Bartness TJ. MTII attenuates ghrelin- and food deprivation-induced increases in food hoarding and food intake. Horm Behav. 2007;52:612–620. doi: 10.1016/j.yhbeh.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol. 2007 Apr;292(4):R1728–R1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keen-Rhinehart E, Bartness TJ. Leptin inhibits food-deprivation-induced increases in food intake and food hoarding. Am J Physiol Regul Integr Comp Physiol. 2008 Dec;295(6):R1737–R1746. doi: 10.1152/ajpregu.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Chen P, Smith MS. Corticotropin releasing hormone neurons in the paraventricular nucleus are direct targets for neuropeptide Y neurons in the arcuate nucleus: an anterograde tracing study. Brain Res. 2000 Jan;854(1–2):122–129. doi: 10.1016/s0006-8993(99)02324-0. [DOI] [PubMed] [Google Scholar]
- 39.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddle RA, Goldfine ID, Williams JA. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- 41.Linden A, Carlquist M, Hansen S, Uvnas-Moberg K. Plasma concentrations of cholecystokinin, CCK-8, and CCK-33, 39 in rats, determined by a method based on enzyme digestion of gastrin before HPLC and RIA detection of CCK. Gut. 1989 Feb;30(2):213–222. doi: 10.1136/gut.30.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matson CA, Wiater MF, Kuijper JL, Weigle DS. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides. 1997;18(8):1275–1278. doi: 10.1016/s0196-9781(97)00138-1. [DOI] [PubMed] [Google Scholar]
- 43.Miceli MO. Abdominal vagus and regulation of ingestive behavior and body weight in golden hamsters. Am J Physiol. 1985;248:R686–R697. doi: 10.1152/ajpregu.1985.248.6.R686. [DOI] [PubMed] [Google Scholar]
- 44.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006 Apr;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 45.Oldendorf WH. Blood-brain barrier permeability to peptides: pitfalls in measurement. Peptides. 1981;2 (Suppl 2):109–111. doi: 10.1016/0196-9781(81)90020-6. [DOI] [PubMed] [Google Scholar]
- 46.Parker R, Herzog H. Localization of Y-receptor subtype mRNAs in rat brain by digoxigenin labeled in situ hybridization. Methods Mol Biol. 2000;153:165–183. doi: 10.1385/1-59259-042-X:165. [DOI] [PubMed] [Google Scholar]
- 47.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999 Apr;11(4):1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 48.Parrott RF. Peripheral and central effects of CCK receptor agonists on operant feeding in pigs. Physiol Behav. 1993 Feb;53(2):367–372. doi: 10.1016/0031-9384(93)90219-6. [DOI] [PubMed] [Google Scholar]
- 49.Raybould HE. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol. 2007 Dec;7(6):570–574. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003 Aug;285(2):G255–G265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- 51.Reidelberger RD. Cholecystokinin and control of food intake. J Nutr. 1994;124:1327S–1333S. doi: 10.1093/jn/124.suppl_8.1327S. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz GJ. Gastrointestinal sensorineural function in the control of food intake. In: Harris RBS, Mattes RD, editors. Appetite and Food Intake: Behavioral and Physiological Considerations. New York: CRC PRess; 2008. pp. 253–266. [Google Scholar]
- 53.Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol. 1998 May;274(5 Pt 2):R1236–R1242. doi: 10.1152/ajpregu.1998.274.5.R1236. [DOI] [PubMed] [Google Scholar]
- 54.Smith GP. Cholecystokinin: A Molecular Negative-Feedback Control of Eating. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. San Diego: Academic Press; 2009. pp. 2527–2540. [Google Scholar]
- 55.Smith GP, Gibbs J. Satiating effect of cholecystokinin. Ann N Y Acad Sci. 1994 Mar;713:236–241. doi: 10.1111/j.1749-6632.1994.tb44071.x. [DOI] [PubMed] [Google Scholar]
- 56.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981 Aug;213(4511):1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 57.Strohmayer AJ, Smith GP. Cholecystokinin inhibits food intake in genetically obese (C57BL/6j-ob) mice. Peptides. 1981;2(1):39–43. doi: 10.1016/s0196-9781(81)80009-5. [DOI] [PubMed] [Google Scholar]
- 58.Strohmayer AJ, Smith GP. Obese male mice (ob/ob) are normally sensitive to the satiating effect of CCK-8. Brain Res Bull. 1986 Oct;17(4):571–573. doi: 10.1016/0361-9230(86)90227-3. [DOI] [PubMed] [Google Scholar]
- 59.Strohmayer AJ, Smith GP. A sex difference in the effect of CCK-8 on food and water intake in obese (ob/ob) and lean (+/+) mice. Peptides. 1987 Sep;8(5):845–848. doi: 10.1016/0196-9781(87)90070-2. [DOI] [PubMed] [Google Scholar]
- 60.Tups A, Helwig M, Khorooshi RM, Archer ZA, Klingenspor M, Mercer JG. Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus) J Neuroendocrinol. 2004 Nov;16(11):922–928. doi: 10.1111/j.1365-2826.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 61.Turkelson CM, Solomon TE. Molecular forms of cholecystokinin in rat intestine. Am J Physiol. 1990 Sep;259(3 Pt 1):G364–G371. doi: 10.1152/ajpgi.1990.259.3.G364. [DOI] [PubMed] [Google Scholar]
- 62.Vanderhaeghen JJ, Lotstra F, De MJ, Gilles C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1190–1194. doi: 10.1073/pnas.77.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight and body composition in Siberian hamsters. Am J Physiol. 1984;246:R26–R30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 64.Wager-Srdar SA, Morley JE, Levine AS. The effect of cholecystokinin, bombesin and calcitonin on food intake in virgin, lactating and postweaning female rats. Peptides. 1986 Sep;7(5):729–734. doi: 10.1016/0196-9781(86)90086-0. [DOI] [PubMed] [Google Scholar]
- 65.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984 May;246(5 Pt 2):R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- 66.West DB, Greenwood MR, Sullivan AC, Prescod L, Marzullo LR, Triscari J. Infusion of cholecystokinin between meals into free-feeding rats fails to prolong the intermeal interval. Physiol Behav. 1987;39(1):111–115. doi: 10.1016/0031-9384(87)90407-0. [DOI] [PubMed] [Google Scholar]
- 67.Wu SV, Harikumar KG, Burgess RJ, Reeve Jr, JR, Miller LJ. Effects of cholecystokinin-58 on Type 1 cholecystokinin receptor function and regulation. Receptor binding, activation, internalization and oligomerization. Am J Physiol Gastrointest Liver Physiol. 2008 Jul; doi: 10.1152/ajpgi.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]