Abstract
Background
The Raynaud’s Condition Score (RCS) is a validated outcome measure for Raynaud’s phenomenon (RP). Our objective was to assess the minimally important difference (MID) and Patient Acceptable Symptom State (PASS) for RCS in patients with RP.
Subjects and Methods
Patients with active RP (N=162) [mean RCS > 25 (0–100 VAS)] participated in a placebo-controlled, cross over randomized clinical trial (RCT). Data from the 2 treatment groups were combined for this analysis. We administered retrospective and prospective anchors during the RCT. MID groups were defined as the group who reported being somewhat better (anchor#1) and a 1-step change from “unbearable” to “very severe” etc. (anchor#2). We considered patients as achieving PASS if they rated their Raynaud’s condition as ‘very mild’ or ‘mild’ at the last study visit.
Results
The mean age of participants was 48.9 years and the mean baseline RCS score was 46.4. The RCS change score for the MID improvement group ranged from − 13.9 to − 14.3 points and PASS estimate was 34.0 points.
Conclusion
The MID and PASS estimates for RCS are 14–15 points for improvement and 34 points, respectively on a 0–100 scale in a large RCT of patients with active RP. This information can aid in interpreting RCS in future RP trials.
Keywords: Systemic sclerosis, Patient Acceptable Symptom State, minimally important differences, Rayanud’s phenomenon
Introduction
Raynaud’s phenomenon (RP) is a common disorder that affects 3–5% of the general population and approximately 90% of patients with scleroderma (1;2). The Raynaud’s Condition Score (RCS) is a validated outcome measure used to assess the level of difficulty experienced due to RP each day (anchored from “no difficulty” to “extreme difficulty”)(3;4). It is administered either as a visual analog scale (VAS, 0–100) or on an 11-point Likert scale. Patients complete the scale every day and average scores are taken over 1 or 2-week period.
As future clinical trials and observational studies in RP are likely to include the RCS, it is important to estimate the minimally important difference (MID) — the smallest improvement in the score that patients perceive as beneficial and that may lead to a change in the patient's disease management (5). MID assesses the ‘changed’ score (improvement/worsening) at a group level over time. The OMERACT has also proposed using a Patient Acceptable Symptom State (PASS), defined as an absolute value beyond which the patients consider themselves well (6). The PASS complement MID as it assesses well being (feeling good) at a certain time point (6).
Our objectives were to estimate the MID and PASS scores for RCS in a large RCT using 2 patient-reported anchors.
Methods
The primary results of the randomized controlled trial (clinicaltrials.gov number NCT00577304) have not been published. In brief, the study comprised of patients (15–70 years) with active RP as determined by a history of cold sensitivity with pallor or cyanosis of the digits or an observed event by the physician (N=162). More details of the study are provided as a Supplementary Material.
Analysis
For this analysis, the data was pooled and analyzed without the knowledge of treatment group. The patients completed the RCS daily and were administered 2 anchors every week. The MID estimates were assessed using an anchor-based approach (7). Anchor #1 was a retrospective anchor: “Consider all the ways that RP affects you (such as number of attacks, duration of attacks, pain, numbness, and tingling). Compared to the 2-week period when you were not using medication, how would you rate your overall Raynaud’s since the last visit? Much worse, somewhat worse, about the same, somewhat better, or much better.” The minimally changed group was defined as the group who reported being somewhat better or somewhat worse for anchor #1. Anchor #2 was a prospective anchor: “Consider all the ways that Raynaud’s phenomenon affects you (such as number of attacks, duration of attacks, pain, numbness, and tingling). Since the last visit, how severe was your overall Raynaud’s? Very mild, mild, somewhat severe, moderately severe, very severe, and unbearable.” For anchor #2, the group who had a 1-step change from “unbearable” to “very severe” or “very severe” to moderately severe”, etc. was considered to be the MID group.
To assess PASS, we assigned patients who considered their Raynaud’s condition as ‘very mild’ or ‘ mild’ at week 6 (last visit) as achieving the PASS and those who considered their Raynaud’s condition as ‘somewhat severe’ to ‘unbearable’ as not achieving PASS. PASS cut-off points were identified with the 75th percentile estimation(6). More details of the analyses for MID and PASS is provided as a Supplementary Material.
Results
The mean age of participants was 48.9 years, 80.2% Caucasian, 92.6% of patients were women, and the mean (SD) baseline RCS score was 46.4 (16.6; 0–100 mm; Table 1); 45 patients had primary RP and 117 patients had secondary RP.
Table 1.
Demographics of the participants (N=162)
| Age (years) | |
|---|---|
| Mean (S.D.) | 48.9 (12.5) |
|
Race | |
| American Indian/Alaska Native | 1 (0.6%) |
| Asian/Pacific Islander | 2 (1.2%) |
| Non-Hispanic White | 130 (80.2%) |
| Black | 17 (10.5%) |
| Hispanic | 9 5.6%) |
| Other | 3 (1.9%) |
|
Gender | |
| Male | 12 (7.4%) |
| Female | 150 (92.6%) |
|
Oral Vasodilator Use | |
| Yes | 73 (45.1%) |
| No | 89 (54.9%) |
|
Disease Type | |
| Primary Raynaud’s Phenomenon | 45 (27.8%) |
| Secondary Raynaud’s Phenomenon | 117 (72.2%) |
| --Scleroderma | 96 |
| --Other connective tissue diseases | 21 |
|
Baseline Raynaud’s Condition Score (0–100) | |
| Mean (S.D.) | 46.4 (16.6) |
| Median | 44.9 |
| Min-Max | 0.0–100.0 |
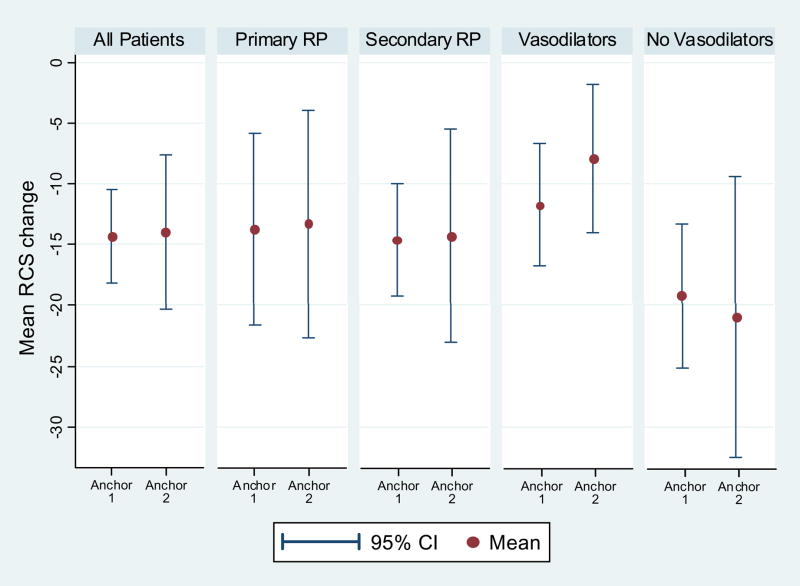
The Spearman correlation between the anchor #1 vs. change in the RCS was 0.42 (p< 0.0001) and between anchor #2 vs. change in the RCS was 0.25 (p=0.002). The RCS change score for MID improvement group ranged from − 13.9 to − 14.3 points and was larger than the “no change” group (− 2.4 to − 9.1; Figure and Table 2). The absolute change for MID improvement group ranged from 13.9% to 14.3% and relative change was 28% to 35%. The MID improvement scores for primary RP were − 13.3 to − 13.8 and − 14.3 to − 14.6 for secondary RP (Figure and Appendix Table 1).
Figure.
Table 2.
Minimal Important Difference (MID) Estimates for the RCS VAS (0–100)
| Patient-rated RCS | Mean RCS Change | 95% CI | Effect Size | p-value* | ||
|---|---|---|---|---|---|---|
| Anchor | ||||||
| 1 | Visit #1 | much better (n = 13) | −17.91 | −27.37, −8.44 | −0.92 | 0.002 |
| somewhat better (n = 52) | −14.33 | −18.23, −10.43 | −1.05 | <.001 | ||
| about the same (n = 76) | −2.44 | −6.09, 1.2 | −0.14 | - | ||
| somewhat worse (n = 10) | −1.77 | −10.55, 7 | −0.11 | 0.899 | ||
| much worse (n = 0) | ||||||
| Visit #2 | much better (n = 13) | −2.89 | −7.78, 2 | −0.16 | 0.891 | |
| somewhat better (n = 52) | −2.53 | −6.07, 1.01 | −0.12 | 0.967 | ||
| about the same (n = 72) | −2.44 | −5.09, 0.21 | −0.10 | - | ||
| somewhat worse (n = 7) | 6.03 | −3.4, 15.46 | 0.25 | 0.060 | ||
| much worse (n = 2) | 2.29 | −180.81, 185.39 | 0.15 | 0.566 | ||
| Anchor | ||||||
| 2 | Visit #1 | improve >=2 (n = 28) | −14.45 | −22.19, −6.72 | −0.71 | 0.139 |
| improve 1 (n = 41) | −13.93 | −20.28, −7.57 | −0.71 | 0.147 | ||
| same (n = 63) | −9.07 | −12.54, −5.6 | −0.44 | - | ||
| worse 1 (n = 19) | −0.68 | −7.9, 6.54 | −0.04 | 0.025 | ||
| worse >=2 (n = 1) | −9.29 | |||||
p-value denotes comparison of “about the same” or "same" group to 4 other groups
For participants on oral vasodilators, the MID improvement scores were − 19.2 to − 21.0 and for those not on vasodilators − 7.9 to − 11.7 (Figure and Appendix Table 2).
The number of participants who worsened were too few to make any definitive conclusions. For anchor#1, MID estimates were consistently smaller at time2 vs. time1 (Appendix Tables 1–2). We chose time 2 for anchor#1 because of cross over design of the study.
For analysis involving PASS, patients considered their Raynaud’s condition satisfactory if their average RCS score was ≤ 34.0 mm (n=71). For primary and secondary RP, PASS estimates were 32.0 (N=14) and 42.5 (N=40), respectively. For participants on oral vasodilators, the PASS estimate was 32.0 (N=23) and 44.7 (N=31) for those not on vasodilators.
We assessed MID scores for improvement and PASS for RCS scores stratified by “less severe” Raynaud’s condition (RCS < 49) and “more severe” group (≥ 49) at baseline. Patients with “more severe” baseline RCS scores required a larger change to be minimally improved (Appendix Table 3). Similarly, patients with “less severe” RCS considered 24.7 (n=45) as achieving PASS whereas patients with “more severe” RCS considered 46.4 (n=26) as satisfactory.
Discussion
Raynaud’s Condition Score (RCS) is a validated outcome measure for RP(3;4) and has been used as the primary outcome measure for clinical trials for RP(3;8;9). We show that a change of 14–15 points (0–100 VAS) is the MID for improvement and achieving a score of 34 points is the PASS in patients with active RP.
Prior literature has shown that a change of 10 mm (on a 0–100 VAS) is generally accepted as a MID(10;11) and a score of 30–35mm is PASS(6). Our analyses show that a 14–15 points change is the MID for improvement in this RCT. This may be part due to the study design that recruited patients with active RCS (active symptoms and RCS score> 25) who are likely to improve on active treatment or due to “placebo effect.” The higher than usual MID is also reflected by the magnitude of effect size (0.71 to 1.05; Table 2) in this study; previous studies have shown that an effect size 0.20 to 0.50 constitutes a MID (10;12). Tubach(6) and Heiberg(13) assessed PASS estimates for different VAS in osteoarthritis and rheumatoid arthritis and reported estimates between 30–35 mm. Although we did not estimate the PASS for RCS using the conventional question that asks a patient to rate their current state as “satisfactory”, our PASS estimate (34mm) is similar to published literature providing confidence in our results.
When we assessed MID at time2 for anchor#1 (at the cross over period), the MID estimates were smaller than at time1 for anchors#1 and #2. This is likely due to a floor effect since majority of patients already noticed an improvement in their RP during the first phase of the study (either due to active treatment or placebo effect). For this analysis, we chose to use MID estimates at time1 since time 2 estimates were very small and likely not clinically meaningful. In addition, the MID estimates were similar to “no change” group (at time 2) making estimates unreliable(14). Therefore, we chose estimates at time 1.
Our MID estimates for patients on oral vasodilators were greater compared to patients not on vasodilators (Figure). We were unable to discern the reason for this difference as the mean baseline RCS scores were similar in 2 groups (45.4 on vasodilators vs. 46.3 not on vasodilators). Of the 117 with secondary RP, 60 (52.3%) were on oral vasodilators.
As previously noted (6;10), the MID and PASS estimates may depend on the baseline scores. This trend was also seen in our analyses where people with higher baseline scores required a larger change in their RCS scores for improvement to be considered as minimally improved and their current state satisfactory. This may be related to again the floor effect (where people near to bottom of the scale are limited by how much they can improve) or may represent difference in interpretation of the scale along the continuum(10).
Although our MID estimates are likely to be applicable in interpreting change in the RCS in active RP, it may be a larger-than-usual estimate in patients with RP who are participating in observational studies, and routine care. Since a single MID estimate is unlikely to be applicable to all patient populations, future studies need to address MID estimates of RCS in other cohorts.
Our study has several strengths. Our MID and PASS estimates are based on a large sample size of patients participating in a RCT. Second, we prospectively incorporated anchors with an a priori aim to calculate MID estimates. Our estimates were similar using the prospective and retrospective anchors giving confidence in our estimates.
Our study has a few limitations. For the current analysis, the RCS was administered as a 0–100 VAS. In the original publication, a 11-point Likert scale (0–10) was used (4). Prior analyses have shown that VAS and Likert responses yield similar results in chronic diseases(15). Second, the RCS was administered on an electronic diary rather than in paper-and-pencil. Electronic diaries have found to reliable and valid when compared to paper diaries (16) but conceivably can affect the MID estimates.
In conclusion, the MID estimates for RCS are between 14–15 points for improvement on 0–100 scale (or 1.4 to 1.5 points on a 0–10 VAS) and PASS is 34 points (on a 0–100 VAS) in patients with active RP. This information can aid in interpreting the RCS in ongoing and future RP RCTs.
Supplementary Material
Acknowledgments
Dr. Puja P. Khanna was supported by National Institutes of Health Award (T32 AR 053463). Dr. Dinesh Khanna was supported by National Institutes of Health Award (NIAMS K23 AR053858-01A1) and a New Investigator Grant from the Scleroderma Foundation. The clinical trial was supported by MediQuest Therapeutics, Inc. who provided the study drug, underwrote the costs of the trial and participated fully with the investigators in protocol design, analysis, and interpretation, but did not influence the decision to submit this manuscript, nor did they in any way contribute or influence the content of the manuscript.
Reference List
- 1.Pope JE. The diagnosis and treatment of Raynaud's phenomenon: a practical approach. Drugs. 2007;67(4):517–525. doi: 10.2165/00003495-200767040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29(2):255–73. vi. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Merkel PA. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr Rheumatol Rep. 2007;9(2):151–157. doi: 10.1007/s11926-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 4.Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud's phenomenon. Arthritis Rheum. 2002;46(9):2410–2420. doi: 10.1002/art.10486. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 6.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis. 2005;64(1):34–37. doi: 10.1136/ard.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 8.Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005;112(19):2980–2985. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 9.Chung L, Shapiro L, Fiorentino D, Baron M, Shanahan J, Sule S, et al. MQX-503, a novel formulation of nitroglycerin, improves the severity of Raynaud's phenomenon: A randomized, controlled trial. Arthritis Rheum. 2009;60(3):870–877. doi: 10.1002/art.24351. [DOI] [PubMed] [Google Scholar]
- 10.Khanna D, Pope JE, Khanna PP, Maloney M, Samedi N, Norrie D, et al. The Minimally Important Difference for the Fatigue Visual Analog Scale in Patients with Rheumatoid Arthritis Followed in an Academic Clinical Practice. J Rheumatol. 2008;35(12):2339–2343. doi: 10.3899/jrheum.080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34(2):280–289. [PubMed] [Google Scholar]
- 12.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally Important Difference in Diffuse Systemic Sclerosis- Results from the D-Penicillamine Study. Ann Rheum Dis. 2006;65(10):1325–1329. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiberg T, Kvien TK, Mowinckel P, Aletaha D, Smolen JS, Hagen KB. Identification of disease activity and health status cut-off points for the symptom state acceptable to patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67(7):967–971. doi: 10.1136/ard.2007.077503. [DOI] [PubMed] [Google Scholar]
- 14.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis. 1987;40(12):1129–1133. doi: 10.1016/0021-9681(87)90080-4. [DOI] [PubMed] [Google Scholar]
- 16.Gaertner J, Elsner F, Pollmann-Dahmen K, Radbruch L, Sabatowski R. Electronic pain diary: a randomized crossover study. J Pain Symptom Manage. 2004;28(3):259–267. doi: 10.1016/j.jpainsymman.2003.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.