Abstract
Truncated recombinant dengue virus envelope protein subunits (80E) are efficiently expressed using the Drosophila Schneider-2 (S2) cell expression system. Binding of conformationally sensitive antibodies as well as x-ray crystal structural studies indicate that the recombinant 80E subunits are properly folded native-like proteins. Combining the 80E subunits from each of the four dengue serotypes with ISCOMATRIX® adjuvant, an adjuvant selected from a set of adjuvants tested for maximal and long lasting immune responses, results in high titer virus neutralizing antibody responses. Immunization of mice with a mixture of all four 80E subunits and ISCOMATRIX® adjuvant resulted in potent virus neutralizing antibody responses to each of the four serotypes. The responses to the components of the tetravalent mixture were equivalent to the responses to each of the subunits administered individually. In an effort to evaluate the potential protective efficacy of the Drosophila expressed 80E, the dengue serotype 2 (DEN2-80E) subunit was tested in both the mouse and monkey challenge models. In both models protection against viral challenge was achieved with low doses of antigen in the vaccine formulation. In non-human primates, low doses of the tetravalent formulation induced good virus neutralizing antibody titers to all four serotypes and protection against challenge with the two dengue virus serotypes tested. In contrast to previous reports, where subunit vaccine candidates have generally failed to induce potent, protective responses, native-like soluble 80E proteins expressed in the Drosophila S2 cells and administered with appropriate adjuvants are highly immunogenic and capable of eliciting protective responses in both mice and monkeys. These results support the development of a dengue virus tetravalent vaccine based on the four 80E subunits produced in the Drosophila S2 cell expression system.
Keywords: Dengue, Envelope, Subunit, Vaccine
1. Introduction
Dengue fever is a leading cause of morbidity and mortality worldwide, with an estimated 100 million infections occurring annually [1–4]. Despite urgent need, the technical requirements created by complex interactions of the four dengue virus serotypes have prevented the development of an effective dengue vaccine approved for human use. In recent years the incidence of dengue fever has increased at an alarming rate, due primarily to the spread of the mosquito vectors and increased worldwide travel [3–5]. The life threatening forms of the viral infection, dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), are also occurring with increasing frequency during dengue outbreaks, with an estimated 200,000 to 500,000 cases per year [2,6].
Dengue fever is caused by any one of four viral serotypes (DENV-1, DENV-2, DENV-3, and DENV-4). Infection by any serotype creates life-long immunity against that serotype. However, there is a strong association between a second infection with a different viral serotype and the more severe forms of the disease, DHF/DSS [1,2,7]. There are two primary hypotheses for the association of DHF/DSS with secondary infections. One theory suggests that antibodies induced during primary infection do not recognize the second serotype well enough to neutralize the virus, however they bind to virus and facilitate an increase in cell entry and replication for the second infecting dengue virus [1]. The second theory is that the immunity to the first serotype misdirects the initial immune response to the second serotype via the phenomenon of “original antigenic sin”, allowing an initial period of greater viral replication [8–10]. In both cases a higher level of viral replication occurs and results in exacerbated disease. These hypotheses have important implications for vaccine development. Namely, the development of a dengue vaccine must be tetravalent to simultaneously immunize against all four serotypes. This will not only prevent disease induced by infection with any one of the four virus serotypes, but also will insure the presence of specific neutralizing antibodies and specific memory cells that should provide a rapid, and specific, response to a serotype even during a secondary infection and minimize the risk of enhancement and exacerbated disease.
Efforts to develop effective vaccines to prevent dengue disease have included both traditional and molecular approaches [reviewed in 11–13]. A number of groups have focused on development of live-attenuated viral strains [14–22; reviewed in 23–25]. However, to date these efforts have failed to yield a formulation which induces properly balanced tetravalent immunity while maintaining an acceptable safety profile. Recent efforts to produce live attenuated vaccines have utilized molecular techniques to generate chimeric or genetically engineered strains [15–17; reviewed in 26,27]. In these efforts, preliminary data with monovalent and tetravalent formulations appear promising, but the challenge of producing a balanced tetravalent formulation which can be delivered on an attractive schedule remains. While killed viral vaccines have been shown to be quite effective in preclinical studies, their practicality may be limited by the low virus yields typically achieved with dengue viral culture [28–30]. Additional molecular approaches have included virally-vectored, recombinant subunit, and naked DNA vaccines [reviewed in 11–13]. While some level of success has been achieved with all these approaches, an economically feasible, safe, and effective solution for a balanced tetravalent immunization has yet to emerge.
Recombinant subunit-based vaccines may offer significant advantages over other approaches currently being pursued for development of a dengue vaccine. The lack of a replicating virus helps to ensure the safety of the product as there is no possibility for inadequate attenuation or reversion in the context of live virus approaches or inadequate inactivation in the context of killed virus vaccines. Furthermore, in the context of a tetravalent formulation the ability to induce a balanced immune response may be more easily manipulated through dose adjustments using recombinant subunits compared to four replicating viruses. In terms of yields and cost effectiveness for a vaccine targeting primarily developing areas of the world, a high yielding, highly immunogenic, recombinant subunit may offer an attractive alternative to vaccines based on virus replication (live attenuated or killed) where yields may be lower than required.
Antibodies directed against particular epitopes contained within the dengue envelope protein are capable of viral neutralization, i.e., the inhibition of virus infection of susceptible cells in vitro. These epitopes have been mapped to several domains of the envelope protein using sets of monoclonal antibodies for dengue virus [31] and West Nile virus [32,33]. Neutralizing antibodies targeting domain III are often specific for each virus and do not cross-neutralize other viruses (or other serotypes of the same virus if multiple serotypes exist). In contrast, neutralizing antibodies targeting domains I or II tend to be of lower avidity and cross neutralize several flaviviruses [33]. While not perfect, a high titer of virus neutralizing antibodies is generally accepted as the best in vitro indicator of possible in vivo protection against productive viral infection.
We describe here the expression of recombinant 80E subunits from all four DENV serotypes in the Drosophila S2 cell expression system. These subunits are expressed at high levels (10–50 mg/L) and have been shown to exhibit native-like conformation [34,35]. This is in marked contrast to previous expression efforts where recombinant flavivirus products were plagued by either low level expression, improper conformation, or both [36–40]. Immunization of mice with low doses of S2 cell expressed recombinant products in combination with appropriate adjuvants results in induction of potent tetravalent virus neutralizing antibody responses. The high levels of expression and the low doses needed to achieve potent immune responses suggest that an effective, economically feasible, tetravalent vaccine based on recombinant protein subunits can be developed. To further evaluate the Drosophila S2 cell expressed 80E subunits as vaccine candidates, the potential of 80E subunits to elicit a protective response in mice and monkeys was tested. In some experiments non-structural protein 1 (NS1 from DEN2) was included in the vaccine formulation. The purpose of including NS1 is to enhance the protective potential of the vaccine via humoral and/or cell-mediated immune responses. The same expression system used for production of recombinant envelope proteins was used for the production of NS1. We report here the evaluation of the immunogenicity and efficacy of recombinant 80E subunits (with or without NS1) at various doses and with several adjuvants in mice and with one adjuvant in monkeys. Protection of mice and monkeys from virus challenge was achieved with low doses of antigen, thus demonstrating the potential of the subunits for use in a vaccine for dengue virus.
2. Materials and methods
2.1 Expression plasmid construction
The expression plasmid pMttbns (derived from pMttPA [41]) was kindly provided by Dr. Allan Shatzman, (GlaxoSmithKline, King of Prussia, PA). The plasmid pMttΔXho was created by excising a 14 base pair BamHI fragment from the pMttbns to leave a unique XhoI site. The pMttΔXho plasmid allows for directional cloning using the unique BglII and XhoI sites. Dengue expression cassettes including sequences encoding the full-length pre-membrane (prM) protein and the E molecule truncated at amino acid 395 of the envelope protein (393 for DEN3) were introduced into the pMttΔXho vector using the unique BglII and XhoI sites. The expression of this prM-E sequence results in the secretion of a truncated envelope subunit protein (DEN-80E) with a native N-terminus resulting from the cellular processing of the prM-E junction. Dengue gene fragments were generated by RT-PCR or PCR using primers with appropriate restriction endonuclease sites and included two stop codons immediately following the last selected envelope protein codon. The identity of all expression plasmids were confirmed by restriction digestion and sequencing.
DENV-1 strain 258848 virus (kindly provided by Dr. Dennis Trent) was grown in C6/36 cells and was used as a source for viral RNA for RT-PCR. The prM80E cDNA fragment generated for DENV-1 encompasses nucleotides 422 to 2102 on the genomic map of reference strain D1 Thailand AHF82-80 (GenBank accession number D00502). The RT-PCR product was digested with BglII and XhoI (encoded by oligonucleotides) and cloned into pMttΔXho. Upon expression in the S2 cells the product is efficiently processed resulting in secretion of DEN1-80E with native N- and C-termini.
All DENV-2 sequences were derived from the strain PR159/S1 [42]. All prM and E sequences were generated by PCR from the original cDNA clone, pC8 [43]. A DNA fragment derived from pC8 that represents nucleotides 439 to 2421 of the DENV-2 genome and that encodes the full-length prM (166 amino acids) and full-length E (495 amino acids) was cloned in the pBluescript (Stratagene, La Jolla, CA) using XbaI to create the clone pBSprM100%E. The primer used to amplify the amino terminus of the prM-E sequence included additional codons immediately preceding the first codon (phenylalanine) of the prM coding sequence. The clone p29D280E encodes amino acids 1–395 of E (nucleotide 937 to 2121 of the DEN2 genome) that was cloned into the NheI site of pBR322. To generate the DENV-2 clone representing the truncated version of the envelope protein, prM80E, a 794 bp BamHI-SalI fragment (BamHI is an internal DEN2 site, SalI is an oligonucleotide encoded site), representing the carboxy-terminal end of E, was removed from pBSprM100%E and replaced with the corresponding 431 bp BamHI-SalI fragment from p29D280E, encoding the 80% truncation of E, to generate the plasmid pBSprM80E. The DENV-2 prM80E sequence in the pBSprM80E plasmid was released by BglII and SalI digestion and ligated into the pMttΔXho vector. A PCR induced mutation at amino acid 61 of the 80E molecule was repaired by replacing an AflIII fragment containing the mutation with the homologous fragment from the original pC8 clone. The resulting plasmid, pMttD2prM80E(Ile61), possesses a single silent mutation at nucleotide 2001 and produces an 80E polypeptide with the parental PR159/S1 DENV-2 E protein sequence. Upon expression in the S2 cells the product is efficiently processed resulting in secretion of DEN2-80E with native N- and C-termini.
For the construction of the DEN2-NS1 plasmid, RT-PCR was utilized to generate the NS1 sequence. DENV-2 strain PR159/S1 virus was propagated in C6/36 cells and used as the source for viral RNA. The full length NS1 cDNA fragment generated encompasses nucleotides 2422 to 3477 on the DENV-2 PR159/S1 genomic map [43]. The primer used to amplify the amino terminus of NS1 included a sequence encoding for an additional amino acid proceeding the first codon of NS1 (aspartic acid). The amplified cDNA product was digested with BglII and SalI (encoded by oligonucleotide primers) and cloned into the yeast expression vector pLS6. The NS1 BglII and SalI fragment was excised from pLS6 and cloned into pMttΔXho. Upon expression in the S2 cells the N-terminus of the recombinant DEN2-NS1 begins with the sequence GARSRVPGT-DSGCVV (sequence in italics is non-dengue sequence). The product is efficiently secreted into the culture medium.
DENV-3 strain CH53489 was propagated in C6/36 cells and was used as the source for viral RNA. The prM80E cDNA fragment generated for DENV-3 encompasses nucleotides 437 to 2113 on the genomic map of reference strain D3H87 [44]. The RT-PCR product was digested with BglII and SalI (encoded by oligonucleotides) and cloned into pMttΔXho. Upon expression in the S2 cells the product is efficiently processed resulting in secretion of DEN3-80E with native N- and C-termini.
DENV-4 strain H241 (kindly provided by Dr. Dennis Trent) was propagated in C6/36 cells and was used as the source for viral RNA. The prM80E cDNA fragment generated for DENV-4 encompasses nucleotides 441 to 2120 on the genomic map of the reference strain Dominica [45]. The RT-PCR product for DENV-4 was digested with BglII and XhoI and cloned directly into the Drosophila expression plasmid pMttΔXho. Sequence analysis of the expression plasmid revealed that this strain of DENV-4 contained only a single glycosylation site in the envelope sequence (N67), as opposed to the two sites normally found in the other DENV-4 strains and other serotypes. The second glycosylation site (N153) was restored in the expression plasmid by site-directed oligonucleotide mutagenesis using the pAlter system from Promega (Madison, WI) using double stranded plasmid DNA as template. An oligonucleotide spanning the Ile155 codon (ATA) of the envelope protein was designed to change it to a Thr codon (ACA). A second mutagenic oligonucleotide was used to remove a unique KpnI restriction endonuclease site in the vector for screening purposes. Upon expression in the S2 cells the product is efficiently processed resulting in secretion of DEN4-80E with native N- and C-termini.
2.2 S2 cell growth and maintenance
The Drosophila S2 cell line [46] was obtained from ATCC (Manasas, VA). Cells were grown at 26°C in Schneider's medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum (FBS; Hyclone, Logan, UT). S2 cells were co-transformed with the pMttΔXho-based expression plasmids and the pCoHygro selection plasmid which encodes hygromycin resistance (kindly provided by Dr. Allan Shatzman, GlaxoSmithKline, King of Prussia, PA; [47]) utilizing the calcium phosphate co-precipitation method (Invitrogen, Carlsbad, CA; [48]) according to the manufacturer's recommendations. Cells were co-transformed with 20 µg total DNA with a 20:1 ratio of expression plasmid to selection plasmid. Transformants were selected with hygromycin B (Roche, Indianapolis, IN) at 300 µg/mL in Schneider's medium supplemented with 10% FBS. Following selection, cells were adapted to growth in the serum free medium IPL-41, supplemented with lipids, yeastolate, and pluronic F-68 (Invitrogen, Carlsbad, CA). For expression studies, cells were grown in supplemented IPL-41, 300 µg/mL hygromycin, and induced with 200 µM CuSO4. In all media, the cells were seeded at a density of 2 × 106 cells/mL and allowed to grow for 6–7 days. Under optimal conditions, cell densities of 1–2 × 107 cells/mL were achieved after 6–7 days of growth.
2.3. Subcloning transformed S2 cells
Lines of cells expressing the highest amounts of recombinant DEN-80E were obtained by subcloning. Subcloning was done using a removable feeder layer of homologous cells (Anopore membrane tissue culture inserts, Nunc, Naperville, IL) to allow the cells to survive at low cell densities. Cells were plated in supplemented IPL-41 medium containing 10% FBS and 150 µg/mL hygromycin B in 96 well plates. The first round of subcloning was conducted at 30 cells/well. Feeder layers were removed once a confluent cell layer was achieved. Once the subclones were expanded, expression was evaluated by induction in separate 96 well plates and immunoprobing of culture supernatant transferred to nitrocellulose in a dot blot format. Several selected high expressers were expanded and evaluated by SDS-PAGE and Western blot analysis using serotype specific anti-dengue hyperimmune mouse ascitic fluid.
2.4 Immunoaffinity purification of 80E subunits
The secreted recombinant DEN-80E subunits were purified from clarified culture medium by immunoaffinity chromatography (IAC) using the conformationally sensitive monoclonal antibodies (mAb) 9D12 [49] and 4G2 [50]. S2 cell culture medium was filtered (0.22 µm) and applied to a 5 mL column of mAb immobilized on a Sepharose solid support (HiTrap, Amersham Pharmacia Biotech, Piscataway, NJ). Unbound material was removed by washing with phosphate-buffered saline and the retained protein eluted using 100 mM glycine, pH 2.5. In-line monitoring of optical density at 280 nm ensured complete washing and facilitated accurate collection of eluted peaks. Eluted material was immediately neutralized with one tenth volume of 1 M Tris, pH 8, and then buffer-exchanged with phosphate-buffered saline, pH 7.2, utilizing ultra-filtration (Centricon 30, Millipore, Bedford, MA). Column efficiency was assessed by SDS-PAGE followed by Coomassie staining and Western blotting of original, flow-through, wash and eluted fractions. Purified recombinant products were quantified by UV spectroscopy.
2.5 Evaluation of glycosylation
To evaluate the glycosylation status of the recombinant proteins, purified DEN-80E products were digested with endoglycosidase H (EndoH) or Peptide-N-glycosidase F (PNGase F) (New England Biolabs, Beverly, MA). The protein preparations were denatured by boiling for 5 minutes in 0.5% SDS and 1% β-mercaptoethanol prior to digestion. The Endo H and PNGase F digestions were then conducted in 50 mM sodium citrate, pH 5.5, or 50 mM sodium phosphate pH 7.5 plus 1% NP-40, respectively, for 1 hour at 37°C. The shift in mobility caused by the removal of sugar residues was assessed by separation in SDS-PAGE gels and Coomassie blue staining.
2.6. Formulation, Preparation and Immunization of Mice using Various Adjuvants
All work with animals was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition. All procedures were reviewed and approved by an Institutional Animal Care and Use Committee.
The ability of IAC purified DEN2-80E to elicit virus neutralizing antibody responses in combination with a variety of adjuvant formulations was tested in mice. Adult female Balb/c mice in groups of 5 each were used for all immunizations. The adjuvants tested were MF59 (Chiron, Emeryville, CA), MF-75 with threonyl-MDP (Syntex Adjuvant Formulation; Chiron, Emeryville, CA), Ribi700 (MPL+TDM emulsion, Corixa, Hamilton, MT), ISCOMATRIX® adjuvant (CSL Limited, Melbourne, Australia), Rehydragel (Aluminum hydroxide; Reheis, Berkeley Heights, NJ) and Freund’ s complete/incomplete adjuvant (Sigma, St. Louis, MO). The adjuvants were used according to the recommendations of the manufacturers. For all adjuvants except ISCOMATRIX® adjuvant, the immunization schedule consisted of a 25 µg DEN2-80E antigen priming dose followed by two boosts of 12.5 µg each of DEN2-80E antigen administered at three week intervals. For ISCOMATRIX® adjuvant the immunization schedule consisted of two doses of 10 µg of DEN2-80E antigen, each administered four weeks apart. All immunizations were subcutaneous, except for Freund’ s adjuvant which was administered intraperitoneally. Control groups received antigen administered subcutaneously in saline (no adjuvant) or saline alone subcutaneously (negative control). The control groups each received a total of three immunizations. Following the course of immunization the mice were euthanized and exsanguinated. Virus neutralizing antibodies in the serum of immunized mice were determined by plaque reduction neutralization test (PRNT) as described below.
To assess the durability of the immune response induced by immunization, groups of adult female Balb/c mice immunized with the recombinant DEN2-80E formulated with 10 µg ISCOMATRIX® adjuvant (N=10), 200 µL Ribi700 (N=10), and 100 µL MF75 with or without 40 µg threonyl-MDP (N=5 for each formulation) were followed for a period of 6 months. Briefly, the immunization schedule for Ribi700, and MF75 with or without threonyl-MDP consisted of a 25 µg priming dose of DEN2-80E followed by two boosts of 12.5 µg each administered at three week intervals, and the schedule for ISCOMATRIX® adjuvant consisted of a priming dose of 10 µg DEN2-80E followed by a single booster dose of 10 µg DEN2-80E four weeks later. The neutralizing antibody response was tested monthly for a period of six months. All mice were given a final boost after the sixth month and then sacrificed and exsanguinated ten days post boost. Virus neutralizing antibodies were assayed as described below.
To establish the immunogenicity of each of the four vaccine components, a dose response analysis was conducted for each antigen by immunizing groups of ten adult female Balb/c mice by subcutaneous injection with various doses of each of the individual purified 80E subunits with 10 µg ISCOMATRIX® adjuvant. A priming dose was followed by an equivalent boost at 4 weeks. The mice were sacrificed ten days following the boost and blood collected. Immunogenicity was assessed by virus neutralizing antibody assay as described below.
To evaluate the immunogenicity of a tetravalent 80E formulation, groups of ten adult female Balb/c mice were immunized by subcutaneous injection with a cocktail of 10 µg each of the four 80E subunits with 10 µg ISCOMATRIX® adjuvant. As controls, groups of mice were immunized in the same experiment with each of the four 80E subunits individually at 10 µg and 10 µg ISCOMATRIX® adjuvant. The priming dose was followed by an equivalent boost at 4 weeks. The mice were sacrificed ten days following the boost and serum collected for virus neutralizing antibody analysis.
2.7 Plaque Reduction Neutralization Test
The presence of virus neutralizing antibodies in the serum of immunized mice was determined using a plaque reduction neutralization test (PRNT) [51,30]. Briefly, serial two-fold dilutions of heat-inactivated mouse antisera were mixed with approximately 100 plaque forming units (pfu) of a homologous serotype virus and incubated for 1 hour at 37°C. The virus-antisera mixture was then plated onto Vero cell monolayers, allowing 1 hour for the virus to bind. A virus only control was also prepared for each assay. The cell sheet was then overlaid with medium containing 0.9% agarose and plaque development was allowed to proceed for five days. Virus plaques were stained with 0.012% neutral red and counted. The number of plaques obtained for each dilution of antisera was compared to the virus only control and the percent reduction in plaque number determined. The highest dilution which resulted in at least 80% reduction in plaque numbers was reported as the PRNT80 titer. For DENV-4, four-fold serial dilutions were utilized and the PRNT50 titer was determined.
2.8. Mouse Challenge Study
For mouse protection studies, 10 to 13 day old weanling Balb/c mice were immunized by subcutaneous injection with either 1 or 5 µg of IAC purified recombinant DEN2-80E formulated with 2 µg ISCOMATRIX® adjuvant. A second equivalent dose was administered two weeks later. Control animals included groups of mice immunized with mouse adapted live DENV-2 or PBS only. One week following the second dose, the mice were challenged by intracranial injection with 100 LD50 of live DENV-2, New Guinea C. Morbidity and mortality was monitored for 17 days post-challenge.
2.9. Immunogenicity of DEN2-80E in Primates
Rhesus monkeys (healthy, young adult, 3–7 kg, Macaca mulatta, of either sex, born in captivity in the U.S. and screened to eliminate any animals positive for simian retroviruses, SIV, STLV, or antibodies to dengue serotypes-1, 2, 3, or 4, Yellow Fever, Japanese encephalitis, or St. Louis encephalitis viruses) were immunized with various doses of IAC purified DEN2-80E formulated with 50 µg of ISCOMATRIX® adjuvant. Two monkeys received 100 µg doses of DEN2-80E, two monkeys received 25 µg doses of DEN2-80E, and two monkeys received 5 µg doses of DEN2-80E. One additional monkey received a 100 µg dose of DEN2-80E formulated with Alum. The doses were administered subcutaneously on day 0, day 34, and day 97 of the study. Control animals included one monkey inoculated with DENV-2 purified inactivated virus (PIV) [28,29] formulated with Alum, and three monkeys that were administered PBS and ISCOMATRIX® adjuvant only. Approximately one month following the final vaccination (Day 132) the monkeys were challenged by subcutaneous injection with 104 pfu of live DENV-2 (strain S16803). Neutralizing antibody responses were monitored throughout the course of the experiment. The PRNT assays were conducted essentially as described above with the following modifications. Guinea pig complement was included in all assays and four-fold serial dilutions were used instead of two-fold dilutions. Plaque counts were compared to a non-flavivirus immune human serum pool and the PRNT50 titer was determined by probit analysis [30].
Protection from viral challenge was monitored by determining the level of virus in the blood for 12 days post-challenge. Sera (0.1 mL) were inoculated onto mosquito (C6/36) cell cultures, which were incubated for 14 days at 28°C to amplify any virus present. The cultures were re-fed on day 7. Virus was detected by plaque assay of 0.2 mL of C6/36 cell culture fluid on Vero cell monolayers [30].
2.10. Immunogenicity and Protective Efficacy of a Tetravalent Formulation in Non-Human Primates
The immunogenicity and protective efficacy of candidate DEN-80E tetravalent formulations were tested in a small, pilot Rhesus monkey vaccine study. Two groups of two monkeys each were immunized with a low dose mixture of 1 µg 80E from each of the four serotypes plus 0.1 µg DEN2-NS1 formulated with 60 µg ISCOMATRIX® adjuvant or with a moderate dose mixture of 5 µg 80E from each serotype plus 0.5 µg DEN2-NSI with 60 µg ISCOMATRIX® adjuvant. The animals were boosted at 1, 2 and 3 months with the same doses of antigens and bled 28 days post each vaccination for PRNT analysis as described [30]. Exogenous complement was not included in these PRNT assays.
Five months after administration of the fourth dose of vaccine, one monkey in each of the vaccinated groups as well as a naïve control monkey were challenged with live DENV-2 strain S16803. The other monkey in each vaccinated group and a naïve control monkey were challenged with DENV-4 strain 341750 Carib. These strains are both heterologous variants compared to the vaccine components. Serum was obtained from each monkey for each of 10 days post challenge and viremia was measured as previously described [30] except initial amplification of virus in the serum was performed in cultures of Vero cells.
3. Results
3.1 Expression and secretion of 80E subunits for all four DENV serotypes
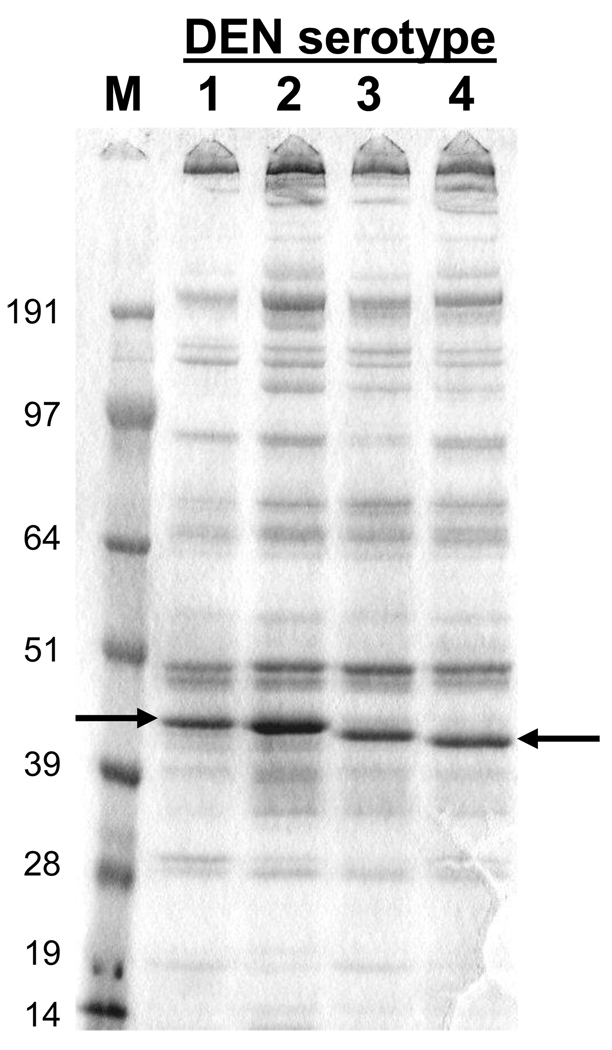
S2 cells were co-transformed with the DEN1, 2, 3, or 4 prM80E expression plasmids and the pCoHygro plasmid and transformants selected by outgrowth in medium containing hygromycin B. Expression was induced with CuSO4 and cells and medium were harvested and analyzed for expression by SDS-PAGE and Western blotting. Comparison of the amount of DEN-80E in the cell associated fraction and in the culture medium demonstrated that all four recombinant 80E molecules were efficiently secreted (data not shown). The DEN1-, DEN2-, and DEN3-80E proteins formed single, discrete bands on SDS-PAGE gels. The DEN4-80E subunit (from the H241 strain) formed two distinct bands, which were slightly different in size. Analysis of the cloned DENV-4 H241 sequence encoding the envelope protein determined that the codon for the amino acid at position 155 of the E protein which typically codes for a Thr residue in most DENV-4 strains as well as in other serotypes, instead codes for an Ile residue due to a single nucleotide change. This Thr to Ile change abolishes the glycosylation site at Asn153. This same mutation was reported in the H241 strain sequenced by Lanciotti et al. [52], but is not present in the sequence of DENV-4 H241 published by Kawano et al. [53]. As the majority of DENV-4 strains have an intact glycosylation site at Asn153 [52], site directed mutagenesis was used to restore the glycosylation site in our clone prM80E sequence from the H241 strain. Restoring the Thr codon resulted in the secretion of product that runs as a single protein band on an SDS-PAGE gel.
The culture medium from S2 transformants representing each of the four DEN-80E subunits was analyzed by SDS-PAGE under non-reducing conditions and the protein bands were visualized by staining with Coomassie Blue. The results are presented in Figure 1. The differences observed in the apparent molecular weights of the four 80E molecules presumably reflect differences in the amino acid sequences and ionic charges of these molecules as the proteins were run under non-reducing conditions. Based on comparison with purified E protein standards, the yield of the 80E subunits was estimated to be in the range of 10–50 mg/L. The recombinant DEN-80E proteins were also probed with serotype specific hyperimmune mouse sera on Western blots. The presence of single reactive bands on the Western blots (see supplemental material Fig. 1 available on-line) for each recombinant 80E protein, and the failure to detect bands representing the prM-80E precursor proteins, demonstrates that processing of the secreted product at the prM-80E junction was efficient. Binding of conformationally sensitive anti-E mAb’s to the non-reduced 80E subunit proteins and not to reduced 80E subunit proteins suggest that the S2 expressed DEN-80E products maintain a native-like conformation (data not shown). The native-like structure of the DEN2- and DEN3-80E proteins have been further verified by X-ray crystallographic studies [34, 35].
Figure 1.
SDS-PAGE Analysis of Secreted Recombinant DEN 80E Products. Ten µl of unconcentrated culture medium from induced S2 cell cultures was separated on a 4–12% SDS-PAGE gel under non-reducing conditions and the proteins stained with Coomassie blue. Size of molecular weight markers in kilodaltons is indicated on the left. Position of DEN-80E bands are indicated by arrows.
3.2 Evaluation of the glycosylation status of the recombinant 80E proteins
To determine whether the glycosylation patterns of the recombinant DEN-80E subunits expressed in Drosophila S2 cells resembled those of native, virion-associated E proteins, immunoaffinity purified recombinant DEN-80E proteins were subjected to digestion with Endoglycosidase H (Endo H) and Peptide-N-glycosidase F (PNGase F), Endo H only cleaves high mannose and some hybrid N-linked oligosaccharides but not mannose-3 type glycan structures. PNGase F cleaves between the innermost N-acetylgalactosamine (GlcNAc) and asparagine residues of high mannose, hybrid, complex N-linked oligosaccharides and mannose-3 type glycan structures. The secreted products were found in all cases to be insensitive to digestion with Endo H, whereas digestion with PNGase F created a detectable increase in mobility (supplemental material Fig. 2, available on-line). This result is consistent with a mannose-3 type glycan structure, which is typical for glycoproteins produced in Drosophila cells where there are typically limited or no high mannose structures. The X-ray crystallography analysis of the DEN3-80E [35] also confirmed the mannose-3 type glycan structure at Asn153 in support of the results obtained by enzymatic analysis.
3.3 Immunogenicity of Purified DEN2-80E Formulated with Different Adjuvants
The purified recombinant DEN2-80E antigen formulated with different adjuvants was tested for its ability to induce virus neutralizing responses in mice. All formulations induced high titers of serum antibody measured by ELISA (data not shown). However, the ability of the different formulations to induce virus neutralizing antibodies varied greatly as shown in Table 1. The highest PRNT80 titers, between 2000 and 4000, were obtained with formulations containing ISCOMATRIX® adjuvant or MF75 with threonyl-MDP. A formulation containing Ribi700 adjuvant induced an intermediate neutralizing PRNT80 titer of approximately 300, while formulations containing aluminum hydroxide, MF59 or Freund’ s adjuvant induced much weaker neutralizing PRNT80 titers of less than 30. These results demonstrate that the ability of the DEN2-80E antigen to induce high-titer virus neutralizing antibody was adjuvant dependent. In comparing the relative potency of the adjuvants to produce neutralizing titers, it should be noted that two doses of ISCOMATRIX® adjuvant administered with 10 µg of antigen are compared here with titers induced by three doses of the other adjuvants formulated with 12.5 or 25 µg of antigen. Despite the lower doses of antigen and fewer immunizations, ISCOMATRIX® adjuvant formulated with 80E antigen produced the highest titers of neutralizing antibodies.
Table 1.
Virus Neutralizing Immune Response in Mice Immunized with DEN2-80E Formulated with Various Adjuvants
| Antigen | Adjuvant | Geometric Mean PRNT80 Titer |
|---|---|---|
| DEN2-80E | MF59 | 26 |
| DEN2-80E | MF75+ThrMDP | 2297 |
| DEN2-80E | Ribi | 333 |
| DEN2-80E | Alum | 24 |
| DEN2-80E | Freund’s | <10 |
| DEN2-80E | ISCOMATRIX® | 4000 |
| DEN2-80E | no Adjuvant | 10 |
| PBS | no Adjuvant | <10 |
3.4 Duration of Immunity to DEN2-80E in Mice
To examine the durability of the immune response to these soluble protein subunits, mice were immunized as described above with DEN2-80E antigen formulated with ISCOMATRIX® adjuvant, Ribi700, MF75 or MF75 with Threonyl-MDP and the resultant virus neutralizing antibody responses were assessed at monthly intervals for 6 months (data available as supplemental material Table 1 on-line). High neutralizing PRNT80 titers of 3000 to 8000 were elicited following immunization with each adjuvant formulation and the PRNT80 titers remained high at levels of approximately 1000 to 4000 for at least 6 months. The antibody titers induced with the MF75 + Threonyl-MDP and ISCOMATRIX® formulations were similar to the results reported in the previous study. However, the titers induced by the Ribi formulation were noticeably higher than the previous experiment. The source of this variability is not clear. It may reflect the variability of the assay which could have been amplified by the small number of animals used per group in these experiments, although highly variable responses is generally not expected with inbred mice. Alternatively, it may reflect lack of consistency in the formulation preparation. At 6 months the mice were reimmunized with the same formulation to test for a memory immune response. Virus neutralizing antibody titers were maintained or increased when tested at one month following this booster immunization. These results demonstrate that DEN2-80E formulated with ISCOMATRIX® adjuvant or Ribi700 adjuvant generated high virus neutralizing antibody responses that persisted in mice with a good memory component for at least 6 months.
3.5 Protection of Mice from Viral Challenge
The protective efficacy of the DEN2-80E recombinant subunit antigen formulated with ISCOMATRIX® adjuvant was assessed in a mouse challenge model. Groups of weanling mice were immunized with 1 and 5 µg doses of DEN2-80E antigen. Following immunization the mice were challenged intracranially with 100 LD50 of mouse-adapted DENV-2, then observed daily for illness and death for 17 days (data available as supplementary material Fig. 3 on-line). While 7 of the 10 unimmunized mice died upon the viral challenge, complete protection from morbidity and mortality was observed with both doses of recombinant antigen. Mice immunized with live DENV-2, the positive control immunogen in this experiment, were also protected.
3.6 Virus neutralizing antibodies induced by the four different serotypes of 80E subunits in mice
DEN1, 2, 3, and 4 recombinant 80E subunits purified by IAC were formulated with ISCOMATRIX® adjuvant and administered individually to adult mice at various doses to determine the effective immunizing dose for each recombinant protein. Virus neutralizing antibody responses to the homologous serotype were determined by PRNT50 analysis of mouse sera. The results are summarized in Table 2. While the neutralizing antibody titers at equivalent antigen doses varied among the dengue serotypes, all of the recombinant immunogens induced a potent virus neutralizing antibody response and a clear dose response effect was evident. Maximum neutralizing antibody titers were attained at protein doses in the range of 3 to 10 µg of 80E for each of the four serotypes. It is not possible to determine if the differences in titers between serotypes represented differences in specific antigenic potency of each individual subunit preparation or reflected variation in the ability of the independent PRNT assays to produce equivalent measures of neutralizing antibodies against the different virus serotypes. Interestingly PRNT titers induced by live attenuated viruses also often exhibit lower anti-DENV-4 titers which may suggest that DENV-4 is inherently less immunogenic than the other serotypes [23].
Table 2.
Virus Neutralizing Antibody Titers in Mice Immunized with Various Doses of Dengue Virus Recombinant 80E Proteins Formulated with 10 µg ISCOMATRIX® Adjuvant.
| DEN 80E Antigen Dose |
Geometric Mean PRNT50 Titer |
|||
|---|---|---|---|---|
| DEN1 | DEN2 | DEN3 | DEN4 | |
| 30 µg | NT* | NT | 1741 | 728 |
| 10 µg | 1450 | 10,556 | 1550 | 526 |
| 3 µg | 1053 | 8574 | 1908 | 278 |
| 1 µg | 504 | 4595 | 1414 | 144 |
| 0.3 µg | 35 | 2096 | 400 | 28 |
| 0.1 µg | <20 | 590 | NT | NT |
| PBS | <10 | <10 | <10 | <10 |
NT - Not tested
To demonstrate that the four 80E subunits could be combined in an effective tetravalent formulation that elicits neutralizing antibodies against all four virus serotypes, the PRNT50 titers elicited against the virus of each serotype was compared when mice were immunized using the individual 80E subunit components alone or in a tetravalent mixture of the components. In the same experiment, groups of mice were immunized with 10 µg of each serotype of 80E alone formulated with ISCOMATRIX® adjuvant, and a group of mice was immunized with a mixture of 10 µg of each of DEN1, 2, 3, and 4 80E subunits formulated with ISCOMATRIX® adjuvant. The results are summarized in Table 3. Mice immunized with the tetravalent formulation exhibited a similar virus neutralizing antibody titer for a given dengue virus serotype to that which was obtained in mice immunized with the same dose of a single serotype recombinant 80E subunit alone. The differences in the relative PRNT50 levels in the assays for neutralization of the serotype viruses persisted in these sera from the tetravalent immunizations. Thus, there was no evidence of antigenic interference or dominance that might prevent the production of a potent, balanced tetravalent immune response in animals vaccinated with a tetravalent 80E subunit formulation.
Table 3.
Homologous Virus Neutralizing Antibody Titers in Mice Immunized with Monovalent or Tetravalent Dengue Virus Recombinant 80E Proteins Formulated with ISCOMATRIX® Adjuvant
| Immunogen | Geometric Mean PRNT50 Titer |
|||
|---|---|---|---|---|
| DEN1 | DEN2 | DEN3 | DEN4 | |
| Monovalent* | 2759 | 3031 | 381 | 174 |
| Tetravalent+ | 1589 | 2639 | 564 | 159 |
| PBS | <20 | <20 | <20 | <20 |
4 groups of mice were immunized with 10 µg of each of the 4 monovalent 80E subunits. Each group was tested for homologous serotype virus neutralizing antibody titers.
1 group of mice was immunized with a tetravalent mixture containing 10 µg of each of the four 80E subunits. The group was tested individually for virus neutralizing antibody titers for each of the dengue serotypes.
3.7 Immunization and Protection of Monkeys from Viral Challenge
The ability of the DEN2-80E recombinant subunit antigen to protect monkeys from virus challenge was also tested. Rhesus monkeys were immunized with different doses of purified DEN2-80E antigen formulated with ISCOMATRIX® adjuvant or aluminum hydroxide adjuvant. The animals were vaccinated at 0, 1 and 3 months and then challenged with a near wild-type dengue virus approximately one month after the last dose. Virus neutralizing antibodies were measured in sera collected over the course of the immunization schedule and after the virus challenge. The neutralizing antibody titers obtained are shown in Table 4. All recombinant DEN2-80E formulations elicited virus neutralizing antibodies measured two weeks after the second immunization. There was no correlation between the dose of the recombinant 80E antigen and the dengue virus neutralizing antibody titers obtained in this experiment. The protective efficacy of the vaccine formulations was evaluated by testing serum samples collected daily after challenge for the presence of live dengue virus (viremia) using virus isolation on C6/36 mosquito cells followed by plaque assay on Vero cells. The results are shown in Table 5. All unvaccinated controls became viremic for an average of 9 days. The control animal vaccinated with DEN2 purified inactivated virus (PIV) vaccine was fully protected. All animals vaccinated with 80E subunit formulations exhibited some degree of protection from viremia. Interestingly, there was no detectable viremia in animals that received the lowest dose of recombinant antigen of 5 µg formulated with ISCOMATRIX® adjuvant, while animals immunized with higher doses of vaccine of 25 µg or 100 µg showed moderate levels of viremia. Due to the small number of animals in this experiment, comparisons of the number of viremic animals between vaccinated groups and controls do not achieve statistical significance (P = 0.10, comparing unvaccinated controls (3/3 viremic animals) and the 5 µg 80E vaccinated group (0/2 viremic animals); Fisher exact probability test.) However, if one compares the sum of the number of viremic days in all animals within groups, the differences become significant. Thus, 0/24 viremic days in the 5 µg 80E vaccinated group compared to the control group (27/36 viremic days), the 25 µg 80E vaccinated group (6/24 viremic days), and the 100 µg 80E vaccinated group (5/24 viremic days), yields p values (2-tailed) of <0.0001, 0.0219, and 0.0496, respectively (Fisher exact probability test). The vaccinated animals also exhibited large increases in dengue virus neutralizing antibody titers following challenge. This anamnestic antibody response demonstrates intact immunological memory as a result of immunization with the recombinant DEN2-80E.
Table 4.
DEN2 Virus Neutralizing Antibody in Sera from Rhesus Monkeys Immunized with Recombinant DEN2- 80E Formulations
| ID | Vaccine | PRNT50 Antibody Titer on Study Day: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 Vac |
Day 34 Vac |
Day 49 | Day 69 | Day 97 Vac |
Day 113 | Day 132 Virus |
Day 159 | ||
| G617 | 100 µg 80E ISCOMATRIX® adjuvant |
<10 | 10 | 180 | 460 | 230 | 920 | 400 | >12,800 |
| B7487 | 100 µg 80E ISCOMATRIX® adjuvant |
<10 | 110 | 480 | 480 | 600 | >640 | >640 | >12,800 |
| F477 | 25 µg 80E ISCOMATRIX® adjuvant |
<10 | <10 | 300 | 230 | 450 | 660 | 470 | >12,800 |
| I613 | 25 µg 80E ISCOMATRIX® adjuvant |
<10 | 90 | 3000 | 1000 | 1100 | >1280 | >1280 | >12,800 |
| I619 | 5 µg 80E ISCOMATRIX® adjuvant |
<10 | 50 | 1600 | 590 | 1900 | >1280 | 1200 | >12,800 |
| H7J | 5 µg 80E ISCOMATRIX® adjuvant |
<10 | 10 | 620 | 760 | 530 | >1280 | >640 | >12,800 |
| F485 | 100 µg 80E alum |
<10 | 60 | 650 | 1200 | 250 | 770 | 600 | >12,800 |
| 517Z | PIV alum |
<10 | <10 | 120 | 320 | 90 | 490 | 50 | 8000 |
| N637 | none | NT* | NT | NT | NT | NT | NT | <10 | 860 |
| N670 | none | NT | NT | NT | NT | NT | NT | <10 | 1600 |
| N816 | none | NT | NT | NT | NT | NT | NT | <10 | 990 |
NT - not tested
Table 5.
Viremia in Vaccinated Rhesus Monkeys After Challenge with Live DEN2 Virus
| ID | Vaccine | Viremia on Day: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| G617 | 100 μg DEN2-80E ISCOMATRIX® adjuvant |
01 | 0 | 0 | 0 | 0 | 0 | +2 | + | 0 | 0 | 0 | 0 |
| B7487 | 100 μg DEN2-80E ISCOMATRIX® adjuvant |
0 | 0 | 0 | 0 | 0 | + | + | + | 0 | 0 | 0 | 0 |
| F477 | 25 μg DEN2-80E ISCOMATRIX® adjuvant |
0 | 0 | 0 | + | + | + | + | + | 0 | 0 | 0 | 0 |
| I613 | 25 μg DEN2-80E ISCOMATRIX® adjuvant |
0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 |
| I619 | 5 μg DEN2-80E ISCOMATRIX® adjuvant |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H7J | 5 μg DEN2-80E ISCOMATRIX® adjuvant |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F485 | 100 μg DEN2- 80E/Alum |
0 | 0 | 0 | 0 | 0 | 0 | + | + | + | + | 0 | 0 |
| 517Z | PIV/Alum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N637 | none | 0 | + | + | + | + | + | + | + | + | 0 | 0 | + |
| N670 | none | 0 | + | + | F3 | + | + | + | + | + | + | + | 0 |
| N816 | none | 0 | + | + | + | F | + | + | 0 | + | + | 0 | 0 |
0 - no virus plaques
+ - > 5 virus plaques
F - 1–5 virus plaques
3.8 Immunogenicity and Protective Efficacy of Tetravalent Formulations in Monkeys
In a small trial to assess the safety and immunogenicity of a tetravalent 80E vaccine formulation, two groups of two monkeys each were immunized with different doses of tetravalent 80E proteins formulated with ISCOMATRIX® adjuvant. One group received a low dose, 1 µg each 80E, and the second group received a moderate dose, 5 µg of each 80E. Both the low and moderate dose tetravalent formulations tested in this monkey study included DEN2-NS1. Each animal received 4 doses at 0, 1, 2, and 3 months. The animals were observed for local side affects such as redness and swelling and for general side effects such as lethargy and weight loss after each inoculation. Both the low and moderate antigen doses were found to be well tolerated by the monkeys without evidence for any adverse local or systemic reactions (data not shown). As shown in Table 6, all monkeys developed virus neutralizing antibodies to each of the four dengue serotypes, although the neutralizing antibody titers varied among animals. The PRNT50 neutralizing antibody titers were highest for DENV-1 and DENV-2, similar to that observed with mice vaccinated with either monovalent or tetravalent antigens. Lymphocytes from the vaccinated monkeys also exhibited significant responses in T-lymphocyte proliferation assays to antigens from each of the four dengue serotypes (data not shown). As with the PRNT levels, proliferative responses varied among serotypes, but this variation did not correlate with the variation in the PRNT levels with serotype. Immune responses to DEN2-NS1 were also examined in this study and both antibody and cell mediated immune responses were induced against NS1 (data not shown).
Table 6.
Virus Neutralizing Antibody Responses Induced in Rhesus Macaques Following Vaccination with a Tetravalent Recombinant Subunit Vaccine Formulation
| Group/ Vaccine |
Anima l ID |
Dengue Serotype for PRNT Testing |
Day 0 (Dose 1) |
Day 28 (Dose 2) |
Day 67 (Dose 3) |
Day 102 (Dose 4) |
Day 130 |
Day 275 (Challenge) |
Day 293 |
|---|---|---|---|---|---|---|---|---|---|
|
Low dose. 1 µg each 80E + 0.1 µg NS1 + ISCOMATRIX Adjuvant |
AA37 | DENV-1 | <10 | <10 | 68 | 290 | 510 | 72 | 161 |
| DENV-2 | <10 | <10 | 112 | 194 | 604 | 232 | 135 | ||
| DENV-3 | <10 | <10 | 29 | 136 | 127 | 33 | 61 | ||
| DENV-4 | <10 | <10 | 47 | 83 | 300 | 89 | 107 | ||
| FTH | DENV-1 | <10 | <10 | 232 | 1230 | 1361 | 231 | 711 | |
| DENV-2 | <10 | <10 | 105 | 887 | 1969 | 293 | 548 | ||
| DENV-3 | <10 | <10 | <20 | 231 | 305 | 90 | 747 | ||
| DENV-4 | <10 | <10 | 39 | 313 | 894 | 105 | 812 | ||
|
Moderate dose. 5 µg each 80E + 0.5 µg NS1 + ISCOMATRIX Adjuvant |
T206 | DENV-1 | <10 | <10 | 34 | 88 | 193 | 20 | 1300 |
| DENV-2 | <10 | <10 | 53 | 363 | 602 | NA | 630 | ||
| DENV-3 | <10 | <10 | <10 | 75 | 147 | 136 | 6845 | ||
| DENV-4 | <10 | <10 | <10 | 32 | 59 | 17 | 5958 | ||
| AJ14 | DENV-1 | <10 | <10 | 82 | 420 | 326 | 117 | 700 | |
| DENV-2 | <10 | <10 | 340 | 1048 | 2536 | 200 | 9500 | ||
| DENV-3 | <10 | <10 | 157 | 206 | 201 | 355 | 4020 | ||
| DENV-4 | <10 | <10 | 92 | 170 | 282 | 237 | 1889 | ||
NA – no valid result available
To test the ability of the tetravalent 80E vaccine formulation to protect against different dengue serotypes, the vaccinated monkeys, together with unvaccinated dengue naïve controls, were challenged with live dengue viruses five months after the last dose. One monkey from each group was challenged with DENV-2 and the other animal from each group was challenged with DENV-4. Serum was collected daily from each animal for 10 days following challenge to measure viremia. As shown in Table 7, the non-immunized control monkey that received the DENV-2 challenge exhibited viremia lasting for four days and the non-immunized control monkey that received the DENV-4 challenge exhibited viremia lasting for five days. One of the monkeys that received the 5 µg dose of the tetravalent formulation had an anti-DENV-4 PRNT titer of 14 one month before and 17 at the time of DENV-4 challenge and exhibited some breakthrough viremia; however, all other vaccinated monkeys, including those immunized with the 1 µg dose of the tetravalent formulation, developed robust virus neutralizing antibody titers and were fully protected. There is some evidence that a certain threshold level of dengue virus neutralizing antibody is associated with protection [30,54] and these data tend to support this conclusion. In addition, the observation of protection associated with lower doses of antigen is consistent with the DEN2-80E monkey protection study presented above and another DEN2 monkey study [30] that included the S2 expressed DEN2-80E antigen. These data demonstrate that properly formulated tetravalent vaccines based on recombinant 80E subunit proteins can induce protective immunity against more than one dengue serotype.
Table 7.
Live Virus Challenge of Monkeys after Tetravalent Immunization.
| Group | Animal ID |
PRNT50 for each serotype one month before challenge |
Challenge serotype assigned randomly |
Viremia (positive days) |
|---|---|---|---|---|
| Low dose. 1 µg 80E each of four serotypes + 0.1 µg NS1 + ISCOMATRIX® Adjuvant |
AA37 | DEN1: 63 | DEN4 | None |
| DEN2: 139 | ||||
| DEN3: 59 | ||||
| DEN4: 61 | ||||
| FTH | DEN1: 242 | DEN2 | None | |
| DEN2: 309 | ||||
| DEN3: 64 | ||||
| DEN4: 181 | ||||
| Moderate dose. 5 µg 80E each of four serotypes + 0.5 µg NS1 + ISCOMATRIX® Adjuvant |
T206 | DEN1: 49 | DEN4 | Days 4, 5, 6, 7 |
| DEN2: 39 | ||||
| DEN3: 192 | ||||
| DEN4: 14 | ||||
| AJ14 | DEN1: 51 | DEN2 | None | |
| DEN2: 530 | ||||
| DEN3: 270 | ||||
| DEN4: 148 | ||||
| Naive controls | B34Z | <10 for each serotype |
DEN4 | Days 6, 7, 8, 9, 10 |
| B08Z | <10 for each serotype |
DEN2 | Days 7, 8, 9, 10 | |
4. Discussion
Despite more than forty years of sustained effort, an effective dengue vaccine has yet to be developed, primarily because of the complications associated with the need for balanced tetravalent immunity. While a number of promising results have been reported in the effort to develop an economically feasible, safe, and efficacious dengue vaccine, difficulties such as obtaining the proper balance of replicating viruses, over- or under-attenuation of infectivity, and the difficulty to efficiently produce native, immunogenic dengue antigens continue to hamper dengue vaccine development. By using the Drosophila S2 cell system to efficiently produce recombinant dengue envelope proteins with native-like conformation, and formulation of these recombinant proteins with potent, modern adjuvants, we report significant advances toward a safe and effective tetravalent subunit dengue vaccine.
The Drosophila S2 system has only recently been more extensively utilized for recombinant protein expression. Other expression systems, including bacterial, fungal and mammalian cells, have been explored for dengue envelope protein expression. However, in our hands, these systems produced recombinant proteins at levels 10 to 1000 fold lower than the 10 to 50 mg/L achieved with the Drosophila S2 cell system (data not shown), and only the S2 cells produced antigen with native-like conformation as demonstrated by reactivity with conformationally sensitive monoclonal antibodies and X-ray crystal structure analysis [34,35,55]. More importantly, when 80E antigens produced in yeast and in S2 cells were tested for the ability to elicit virus neutralizing antibodies in mice, the S2 cell produced 80E proteins resulted in significantly higher neutralizing titers than the yeast produced 80E protein (data not shown).
To assess the ability of the recombinant DEN2-80E antigen produced in S2 cells to induce dengue virus neutralizing antibodies in mice, formulations with a variety of adjuvants were tested. The results demonstrate the importance of selecting a proper adjuvant to induce dengue virus neutralizing antibodies with recombinant E antigen. The virus neutralizing antibody titers achieved with the most effective adjuvants far exceed those that have been previously reported for other recombinant dengue envelope proteins [36–40]. Importantly, these high antibody titers can be induced by low doses of antigen, as demonstrated in mice by the dose responses for 80E antigen with ISCOMATRIX® adjuvant. High neutralizing antibody titers were maintained for at least 6 months in mice and a strong memory component was demonstrated by the large increase in neutralizing antibody titer following booster vaccination at the end of the 6 month period. The exceptionally high levels of immunogenicity exhibited by the recombinant 80E antigen, combined with high levels of expression, suggest the feasibility of this approach for producing a subunit vaccine for dengue.
The protective efficacy of the DEN2-80E when combined with ISCOMATRIX® adjuvant was demonstrated with low doses of antigen in both murine and non-human primate models. In the mouse model low doses of 1 and 5 µg recombinant 80E antigen, resulted in complete protection against a lethal virus challenge. In monkeys, antigen doses of 100, 25 and 5 µg all resulted in high PRNT50 titers in the range of several hundred to the low thousands two weeks after a second dose. These neutralizing antibody titers were sustained and a third dose resulted in a further increase in titer. The monkeys vaccinated with 5 µg DEN2-80E exhibited no viremia after live virus challenge while animals vaccinated with the higher doses of antigen exhibited some breakthrough viremia but less than the controls. Viremia in these experiments was measured using a qualitative cell culture based assay rather than a Q-PCR based assay which has the advantage of being quantitative. However, since Q-PCR measures viral genomes rather than infectious virus, the data generated in the context of vaccinees, with circulating virus neutralizing antibodies, can be difficult to interpret [30], while the measurement of non-neutralized, infectious virus using cell-based methods provides unambiguous results. The presence of infectious virus in the blood of some animals, despite the presence of high titer virus neutralizing antibody, suggests that significant viral replication occurred in those animals, and that the antibody present, though demonstrating virus neutralization capacity in vitro, was not of adequate activity, avidity or specificity to control the infection in vivo.
The basis for the superior protection achieved with lower antigen doses remains to be demonstrated and could be linked to a number of factors including antibody avidity, subclass and/or specificity. An effect on the Th1/Th2 cell balance is one possibility, as data from mouse studies suggest that immunization with low doses of antigen, in the context of saponin-based adjuvants, results in a more Th1-type response, as evidenced by a shift in the ratio of IgG2a + IgG2b: IgG1 and higher levels of IFNγ produced by T cells upon in vitro stimulation, compared to results obtained following immunization with higher antigen doses (unpublished data). A shift in the antibody avidity or subclass induced in the Rhesus monkeys could have impacted the efficiency of virus neutralization, in a manner not detected in the classic PRNT assay conducted in Vero cells, but resulting in a demonstrable shift in efficacy in vivo as measured by viremia. The complexities of virus neutralization are elegantly described in the recent review by Pierson and Diamond [56] and highlights the multifactorial nature of neutralization and protection and is consistent with the data presented in this study. Interestingly, this inverse dose effect where lower doses of antigen appear more efficacious may be unique to the specific combination of dengue antigens and saponin-based adjuvants (e.g. ISCOMATRIX® Adjuvant) as this effect has not been observed with alum-based formulations (unpublished data) or the alum-MPL formulation AS04 [30].
Following the live virus challenge in the monkey studies, all vaccinated animals exhibited anamnestic neutralizing antibody responses, suggesting a good memory component in vaccine immunity. However, the presence of an anamnestic immune response, even in animals that developed no detectable viremia, suggests limited or localized viral replication may have occurred in those animals. Since the rhesus monkey model is not a disease model, it is not possible to predict the implication of this finding for humans. However, there is a growing body of evidence which suggests that increased disease severity is correlated with increased levels of viremia in dengue infected individuals [57–60]. Therefore, it is reasonable to expect that a dengue vaccine which significantly limits viral replication should also prevent disease. Data obtained from serological surveys in dengue endemic areas suggest that sterilizing immunity is not required for protection as evidenced by continued antibody boosting, presumably due to multiple re-infection events, but without evidence of overt disease [61]. In any event the induction of sterilizing immunity is a difficult target for any vaccine to achieve, compared to the much more attainable goals of limiting viral replication and thereby preventing disease.
The data presented in both mice and monkeys demonstrates that the immune response induced by the recombinant protein based formulations are durable with antibody titers detected out at 6 months after immunization in mice and protection from challenge at 5 months after immunization in monkeys. As highlighted above, the boost in antibody responses post-challenge further confirms a strong memory component, suggesting that upon natural exposure vaccinated individuals would respond with a rapid, robust, and protective response. This is key in the effort to prevent severe disease associated with immune mediated pathogenic mechanisms.
The production of recombinant dengue 80E proteins in Drosophila S2 cells that are capable of eliciting potent immune responses in mice and non-human primates represents a major achievement in the effort to develop a recombinant dengue vaccine. The S2 cell expression system efficiently produces 80E from all four dengue serotypes. Our data show that co-administration of the subunits from the four serotypes results in a balanced immune response, equivalent to that observed when the four individual components are administered separately. Furthermore this response can be induced in a relatively short period of time (2–3 months). Several live attenuated vaccines are currently in clinical development with promising results from Phase 1 or Phase 2 studies [Reviewed in 62 – 65], with the most advanced candidate, Chimerivax, now being tested in a field efficacy trial in Thailand. While these live attenuated approaches offer significant promise, there are challenges that remain, including the requirement for an extended dosing schedule of up to 12 months [63]. Thus, the development of a safe and effective alternative to live attenuated approaches, with the possibility for a shortened dosing schedule, is an attractive option particularly for a traveler’s vaccine. The tetravalent 80E formulations described in this report demonstrated immunogenicity and efficacy in animal models similar to the results reported with live attenuated vaccine candidates [30, 63, 64] and in a previous head-to-head comparison in rhesus macaques the DENV-2 80E antigen compared favorably with a DENV-2 purified inactivated virus (PIV) vaccine candidate [30]. Moreover, the 80E products described in this publication are efficiently produced, highly immunogenic, inherently safe, and capable of inducing durable, balanced, tetravalent immune responses using an accelerated dosing schedule. These attributes give them significant potential as a safe, effective, affordable alternative to live attenuated dengue vaccines, with the goal of protecting at risk populations.
Supplementary Material
Acknowledgments
The authors thank Dennis Trent formerly at the Food and Drug Administration for providing dengue viral strains and Alan Shatzman at GSK for the Drosophila expression vectors. They also thank Y.S. Hahn for the gift of the plasmid pC8. The authors also thank James Senda, Eric Rohlinger, Beverly Orillo, Timothy Martyak, Michael Thorne, Teri Wong, Milicent Yong, Abu Aslamkhan, Tim Chamberlain, and David Chang for excellent technical assistance. This work was supported by Public Health Service Grants 1 R43AI35401 and 2 R44AI35401, USDA Contract 1890-119, and a grant from the Department of Defense DAMD17-93-C-3128.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed herein are those of the authors and should not be construed as official or to reflect the views of the Department of Defense or the U.S. Government.
References
- 1.Halstead SB. Pathogenesis of Dengue: Challenges to Molecular Biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 2.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP. Dengue: The risk to developed and developing countries. Proc. Natl. Acad. Sci. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Research. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 5.Rigau-Perez JG, Gubler DJ, Vorndam AV, Clark GG. Dengue surveillance- United States, 1986–1992. MMWR. 1994;43:7–19. [PubMed] [Google Scholar]
- 6.Gubler DJ, Trent DW. Emergence of epidemic dengue/dengue hemorrhagic fever as a public health problem in the Americas. Infectious Agents and Dis. 1994;2:383–393. [PubMed] [Google Scholar]
- 7.Hayes EB, Gubler DJ. Dengue and Dengue Hermorrhagic Fever. Pediatr. Infect. Dis. J. 1992;11:311–317. doi: 10.1097/00006454-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J. Biol. Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 9.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 10.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 11.Putnak R. Progress in the development of recombinant vaccines against dengue and other arthropod-borne flaviviruses. In: Kurstak E, editor. Modern Vaccinology. Plenum Medical, NY: 1994. pp. 231–252. [Google Scholar]
- 12.Cardosa MJ. Dengue vaccine design: issues and challenges. Br. Med. Bull. 1998;54:395–405. doi: 10.1093/oxfordjournals.bmb.a011696. [DOI] [PubMed] [Google Scholar]
- 13.Chambers TJ, Tsai TF, Pervikov Y, Monath TP. Vaccine development against dengue and Japanese encephalitis: report of a World Health Organization meeting. Vaccine. 1997;15:1494–1502. doi: 10.1016/s0264-410x(97)00195-3. [DOI] [PubMed]
- 14.Polo S, Ketner G, Levis R, Falgout B. Infectious RNA transcripts from full-length dengue virus type 2 cDNA clones made in yeast. J. Virol. 1997;71:5366–5374. doi: 10.1128/jvi.71.7.5366-5374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinney RM, Butrapet S, Chang GJ, Tsuchiya KR, Roehrig JT, Bhamarapravati N, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 16.Bray M, Men R, Lai CJ. Monkeys immunized with intertypic chimeric dengue viruses are protected against wild-type virus challenge. J. Virol. 1996;70:4162–4166. doi: 10.1128/jvi.70.6.4162-4166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray M, Lai CJ. Construction of intertypic chimeric dengue viruses by substitution of structural protein genes. Proc. Natl. Acad. Sci. USA. 1991;88:10342–10346. doi: 10.1073/pnas.88.22.10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughn DW, Hoke CH, Yoksan S, LaChance R, Innis BL, Rice RM, et al. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine. 1996;14:329–336. doi: 10.1016/0264-410x(95)00167-y. [DOI] [PubMed] [Google Scholar]
- 19.Edelman R, Tacket CO, Wasserman SS, Vaughn DW, Eckels KH, Dubois DR, et al. A live attenuated dengue-1 vaccine candidate (45AZ5) passaged in primary dog kidney cell culture is attenuated and immunogenic for humans. J. Inf. Dis. 1994;170:1448–1455. doi: 10.1093/infdis/170.6.1448. [DOI] [PubMed] [Google Scholar]
- 20.Angsubhakorn S, Yoksan S, Pradermwong A, Nitatpattana N, Sahaphong S, Bhamarapravati N. Dengue-3 (16562) PGMK 33 vaccine: neurovirulence, viremia, and immune responses in Macaca fascicularis. Southeast Asian J. Trop. Med. Public Health. 1994;25:554–559. [PubMed] [Google Scholar]
- 21.Hoke CH, Malinoski FJ, Eckels KH, Scott RM, Dubois DR, Summers PL, et al. Preparation of an attenuated dengue 4 (341750 Carib) virus vaccine. II Safety and immunogenicity in humans. Am. J. Trop. Med. Hyg. 1990;43:219–226. doi: 10.4269/ajtmh.1990.43.219. [DOI] [PubMed] [Google Scholar]
- 22.Gualano RC, Pryor MJ, Cauchi MR, Wright J, Davidson AD. Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J. Gen. Virol. 1998;79:437–446. doi: 10.1099/0022-1317-79-3-437. [DOI] [PubMed] [Google Scholar]
- 23.Innis BI, Eckels KH. Progress in development of a live-attenuated tetravalent dengue virus vaccine by the United States Army Medical Research and Materiel Command. Am. J. Trop. Med. Hyg. 2003;69 Suppl 6:1–4. doi: 10.4269/ajtmh.2003.69.6_suppl.0690001. [DOI] [PubMed] [Google Scholar]
- 24.Edelman R. Dengue and Dengue vaccines. J. Infect. Dis. 2005;191:650–653. doi: 10.1086/427784. [DOI] [PubMed] [Google Scholar]
- 25.Edelman R. Dengue vaccines approach the finish line. Clin. Infect. Dis. 2007;45 Suppl 1:S56–S60. doi: 10.1086/518148. [DOI] [PubMed] [Google Scholar]
- 26.Lai C-J, Monath TP. Chimeric flaviviruses: Novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis. Advances in Viral Research. 2003;61:469–509. doi: 10.1016/s0065-3527(03)61013-4. [DOI] [PubMed] [Google Scholar]
- 27.Halstead SB, Deen J. The future of dengue vaccines. Lancet. 2002;360:1243–1246. doi: 10.1016/S0140-6736(02)11276-1. [DOI] [PubMed] [Google Scholar]
- 28.Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: Immunogenicity and protection in mice and rhesus monkeys. J. Inf. Dis. 1996;174:1176–1184. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 29.Putnak R, Cassidy K, Conforti N, Lee R, Sollazzo D, Truong T, et al. Immunogenic and protective response in mice immunized with a purified, inactivated, dengue-2 virus vaccine prototype made in fetal rhesus lung cells. Am. J. Trop. Med. Hyg. 1996;55:504–510. doi: 10.4269/ajtmh.1996.55.504. [DOI] [PubMed] [Google Scholar]
- 30.Putnak JR, Coller B-A, Voss D, Vaughn DW, Clements D, Houng HS, et al. Recombinant subunit protein and inactivated virus vaccines for dengue type-2 formulated with new adjuvants induce high-titered virus-neutralizing antibodies and confer protection against virus challenge in rhesus macaques. Vaccine. 2005;23:4442–4452. [Google Scholar]
- 31.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 32.Beasley DW, Barrett AD. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol. 2002;76:13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J. Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79(2):1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delenda C, Frenkiel MP, Deubel V. Protective efficacy in mice of a secreted form of recombinant dengue-2 virus envelope protein produced in baculovirus infected insect cells. Arch. Virol. 1994;139:197–207. doi: 10.1007/BF01309465. [DOI] [PubMed] [Google Scholar]
- 37.Putnak R, Feighny R, Burrous J, Cochran M, Hackett C, Smith G, et al. Dengue-1 virus envelope glycoprotein gene expressed in recombinant baculovirus elicits virus-neutralizing antibody in mice and protects them from virus challenge. Am. J. Trop. Med. Hyg. 1991;45:159–167. doi: 10.4269/ajtmh.1991.45.159. [DOI] [PubMed] [Google Scholar]
- 38.Bielefeldt-Ohmann H, Beasley DWC, Fitzpatrick DR, Aaskov JG. Analysis of a recombinant dengue-2 virus-dengue-3 virus hybrid envelope protein expressed in a secretory baculovirus system. J. Gen. Virol. 1997;78:2723–2733. doi: 10.1099/0022-1317-78-11-2723. [DOI] [PubMed] [Google Scholar]
- 39.Staropoli I, Frenkiel M-P, Megret F, Deubel V. Affinity purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine. 1997;15:1946–1954. doi: 10.1016/s0264-410x(97)00128-x. [DOI] [PubMed] [Google Scholar]
- 40.Sugrue RJ, Fu J, Howe J, Chan Y-C. Expression of the dengue virus structural proteins in Pichia pastoris leads to the generation of virus-like particles. J. Gen. Virol. 1997;78:1861–1866. doi: 10.1099/0022-1317-78-8-1861. [DOI] [PubMed] [Google Scholar]
- 41.Culp JS, Johansen H, Hellmig B, Beck J, Matthews TJ, Delers A, Rosenberg M. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Bio/Technology. 1991;9:173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- 42.Eckels KH, Brandt WE, Harrison VR, McCown JM, Russell PK. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976;14:1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn YS, Galler R, Hunkapiller T, Dalrymple JM, Strauss JH, Strauss EG. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988;162:167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- 44.Osatomi K, Fuke I, Tsuru D, Shiba T, Sakaki Y, Sumiyoshi H. Nucleotide sequence of dengue type 3 virus genomic RNA encoding viral structural proteins. Virus Genes. 1988;2:99–108. doi: 10.1007/BF00569739. [DOI] [PubMed] [Google Scholar]
- 45.Zhao B, Mackow E, Buckler-White A, Markoff L, Chanock RM, Lai CJ, et al. Cloning full-length dengue type4 viral DNA sequences: Analysis of genes coding for structural proteins. Virology. 1986;155:77–88. doi: 10.1016/0042-6822(86)90169-8. [DOI] [PubMed] [Google Scholar]
- 46.Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morph. 1972;27:353–365. [PubMed] [Google Scholar]
- 47.Ivey-Hoyle M, Culp JS, Chaikin MA, Hellmig BD, Matthews TJ, Sweet RW, et al. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. PNAS USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigler M, Sweet R, Sim GK, Wold B, Pellicer A, Lacey E, et al. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979;16:777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- 49.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982 Jul;31(4):830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 50.Gentry MK, Henchal EA, McCown JM, Brandt WE, Dalrymple JM. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):548–555. doi: 10.4269/ajtmh.1982.31.548. [DOI] [PubMed] [Google Scholar]
- 51.Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967;99:285–290. [PubMed] [Google Scholar]
- 52.Lanciotti RS, Gubler DJ, Trent DJ. Molecular evolution and phylogeny of dengue-4 viruses. J Gen Virol. 1997;78:2279–2286. doi: 10.1099/0022-1317-78-9-2279. [DOI] [PubMed] [Google Scholar]
- 53.Kawano H, Rostapshov V, Rosen L, Lai CJ. Genetic determinants of dengue type 4 virus neurovirulence for mice. J Virol. 1993;67:6567–6575. doi: 10.1128/jvi.67.11.6567-6575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, et al. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierson TC, Diamond MS. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Exp. Rev. Mol. Medicine. 2008;10:1–13. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue in the early fibrile phase: viremia and antibody responses. J. Infect. Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 58.Vaughn DW, Green S, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Endy TP, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 59.Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 2002;1185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 60.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002;186:1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 61.Chanthavanich P, Luxemburger C, Sirivichayakul C, Lapphra K, Pengsaa K, Yoksan S, Sabchareon A, Lang J. Short report: immune response an occurrence of dengue infection in Thai children three to eight years after vaccination with live attenuated tetravalent dengue vaccine. Am. J. Trop. Med. Hyg. 2006;75:26–28. doi: 10.4269/ajtmh.2006.75.1.0750026. [DOI] [PubMed] [Google Scholar]
- 62.Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect. Dis. 2009;9:678–687. doi: 10.1016/S1473-3099(09)70254-3. [DOI] [PubMed] [Google Scholar]
- 63.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2009 Oct 4; doi: 10.1016/j.vaccine.2009.09.098. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Blaney JE, Durbin AP, Murphy BR, Whitehead SS. Targeted mutagenesis as a rational approach to dengue virus vaccine development. Curr. Top. Microbiol. Immunol. 2010;338:145–158. doi: 10.1007/978-3-642-02215-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr. Top. Microbiol. Immunol. 2010;338:129–143. doi: 10.1007/978-3-642-02215-9_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.