Abstract
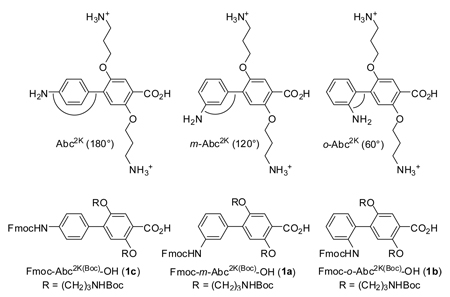
This paper introduces the unnatural amino acids m-Abc2K and o-Abc2K as nanometersized building blocks for the creation of water-soluble macrocycles with well-defined shapes. m-Abc2K and o-Abc2K are homologues of the nanometer-sized amino acid Abc2K, which we recently introduced for the synthesis of water-soluble molecular rods of precise length. [J. Am. Chem. Soc. 2007, 129, 7272]. Abc2K is linear (180°), m-Abc2K creates a 120° angle, and o-Abc2K creates a 60° angle. m-Abc2K and o-Abc2K are derivatives of 3’-amino-[1,1’-biphenyl]-4-carboxylic acid and 2’-amino-[1,1’-biphenyl]-4-carboxylic acid, with two propyloxyammonium side chains for water solubility. m-Abc2K and o-Abc2K are prepared as Fmoc-protected derivatives Fmoc-m-Abc2K(Boc)-OH (1a) and Fmoc-o-Abc2K(Boc)-OH (1b). These derivatives can be used alone or in conjunction with Fmoc-Abc2K(Boc)-OH (1c) as ordinary amino acids in Fmoc-based solid-phase peptide synthesis. Building blocks 1a–c were used to synthesize macrocyclic “triangles” 9a–c, “parallelograms” 10a,b, and hexagonal “rings” 11a–d. The macrocycles range from a trimer to a dodecamer, with ring sizes from 24 to 114 atoms, and are 1–4 nm in size. Molecular modeling studies suggest that all the macrocycles except 10b should have well-defined triangle, parallelogram, and ring shapes if all of the amide linkages are trans and the ortho-alkoxy substituents are intramolecularly hydrogen bonded to the amide NH groups. The macrocycles have good water solubility and are readily characterized by standard analytical techniques, such as RP-HPLC, ESI-MS, and NMR spectroscopy. 1H and 13C NMR studies suggest that the macrocycles adopt conformations with all trans-amide linkages in CD3OD, that the “triangles” and “parallelograms” maintain these conformations in D2O, and that the “rings” collapse to form conformations with cis-amide linkages in D2O.
Introduction
This paper introduces the unnatural amino acids m-Abc2K and o-Abc2K as nanometer-sized building blocks for the creation of water-soluble macrocycles with well-defined shapes. There is intense interest in nanometer-sized macrocycles with shapes such as hexagons,1 triangles,2 and parallelograms,1c,3 because “shape-persistent” molecules are central to a bottom-up approach to functional well-defined nanometer-scale molecules and molecular assemblies (i.e., “nanotechnology”).1–5 Most of these structures have been designed for solubility in organic solvents, and many are assembled from building blocks through carbon-carbon or metal-ligand bond formation. The creation of large (e.g., ≥ 2 nm) water-soluble macrocycles with well-defined shapes remains largely undeveloped. Such structures offer the promise of bridging the gap between the aqueous world of biology and the synthetic world of nanotechnology. If the structures are sufficiently easy to assemble from building blocks, they may even allow researchers from other disciplines to create molecular architectures for their own applications. Here, we describe our approach to large, water-soluble macrocycles.
We designed m-Abc2K and o-Abc2K as homologues of the nanometer-sized amino acid Abc2K, which we recently introduced for the synthesis of water-soluble molecular rods of precise length.6 Abc2K is a derivative of the nanometer-long amino acid 4’-amino-[1,1’-biphenyl]-4-carboxylic acid, with two propyloxyammonium side chains for water solubility. Abc2K is linear (180°), m-Abc2K creates a 120° angle, and o-Abc2K creates a 60° angle.7 In this paper, we report the syntheses of Fmoc-protected derivatives Fmoc-m-Abc2K(Boc)-OH (1a) and Fmoc-o-Abc2K(Boc)-OH (1b) and the application of these derivatives to the synthesis of nanometer-scale water-soluble macrocycles.
Results and Discussion
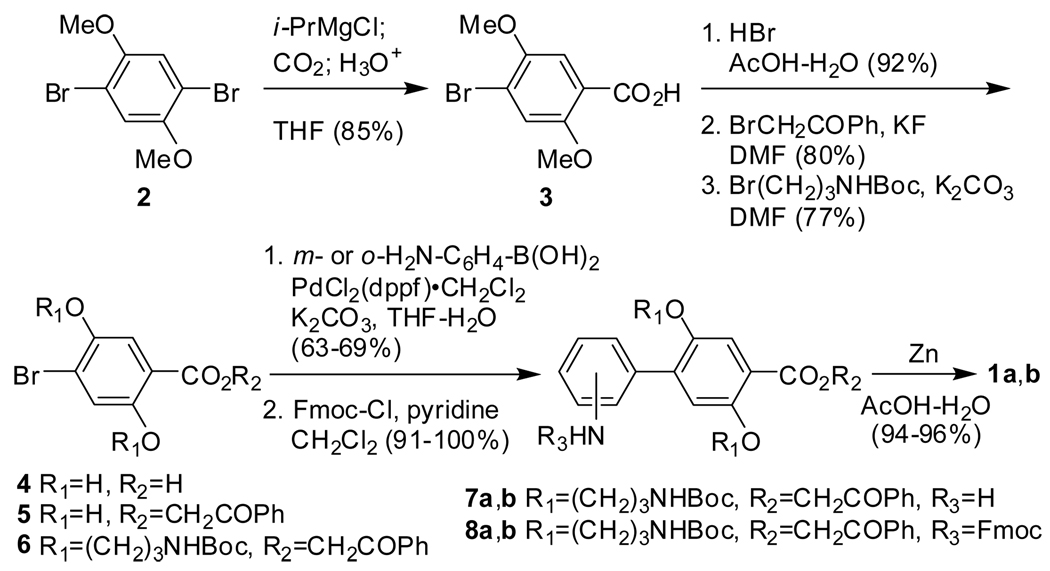
The Fmoc-m-Abc2K(Boc)-OH (1a) and Fmoc-o-Abc2K(Boc)-OH (1b) building blocks were prepared in a fashion analogous to Fmoc-Abc2K(Boc)-OH (1c), as shown in Scheme 1.6 Central to the synthesis of Abc2K building blocks is the Suzuki cross-coupling reaction of 4-bromobenzoic acid derivative 6 with 3-aminophenylboronic acid or 2-aminophenylboronic acid. All of the reactions proceed in good yield, and the synthesis permits the preparation of gram-scale quantities of 1a and 1b. One noteworthy improvement to the synthesis involves the use of an unusual Grignard-based halogen-metal exchange reaction to generate 4-bromo-2,5-dimethoxybenzoic acid (3).8 This reaction, which was reported recently by Yokozawa and coworkers in a related system, proceeds in double the yield of the conventional Grignard reaction.6,8
SCHEME 1.
Synthesis of Building Blocks 1a and 1b
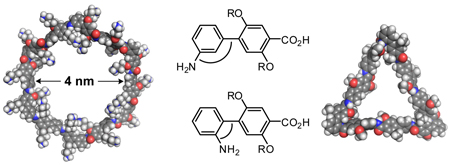
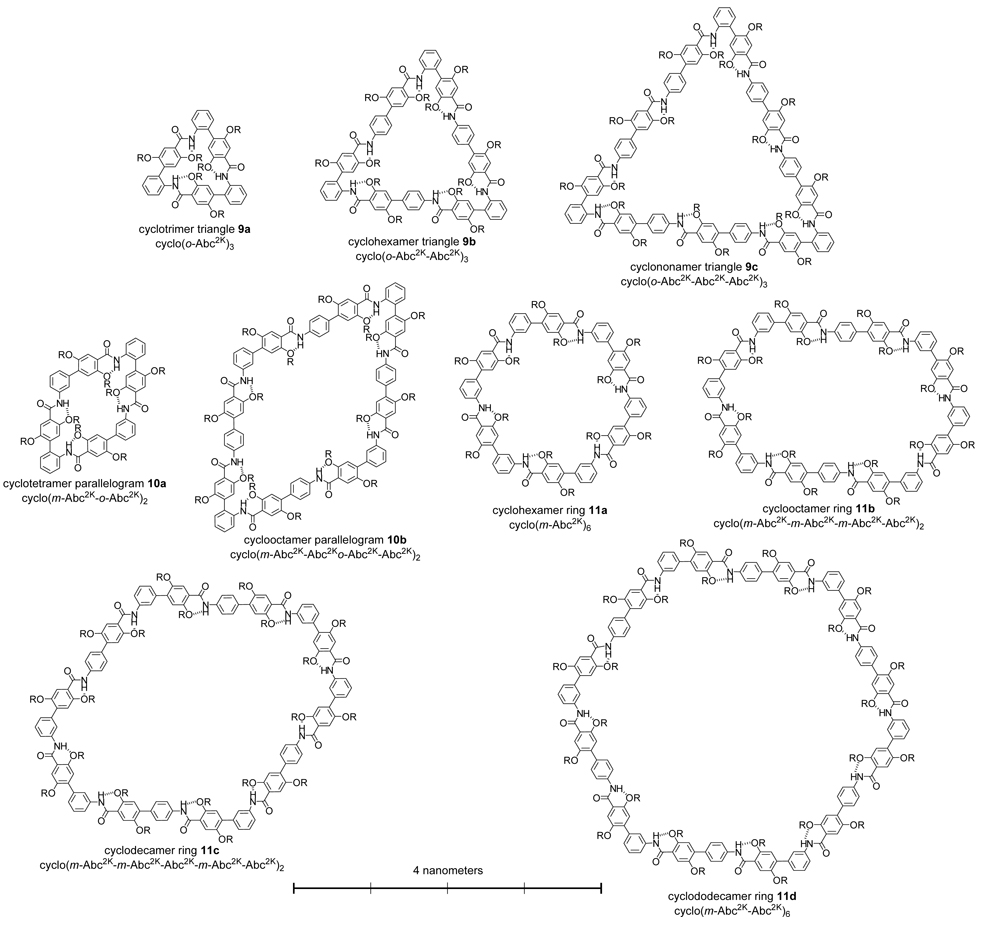
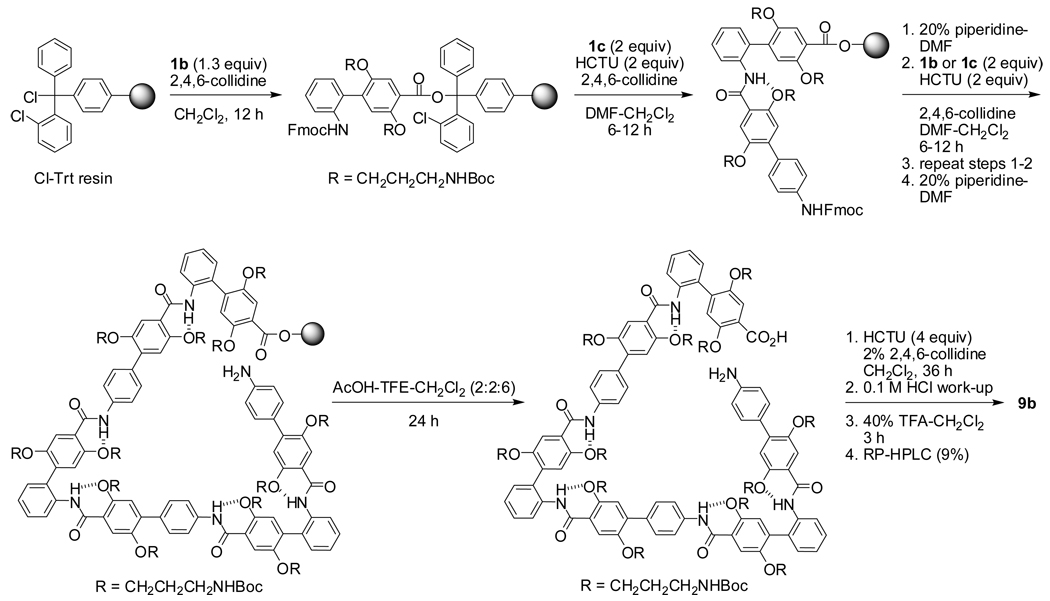
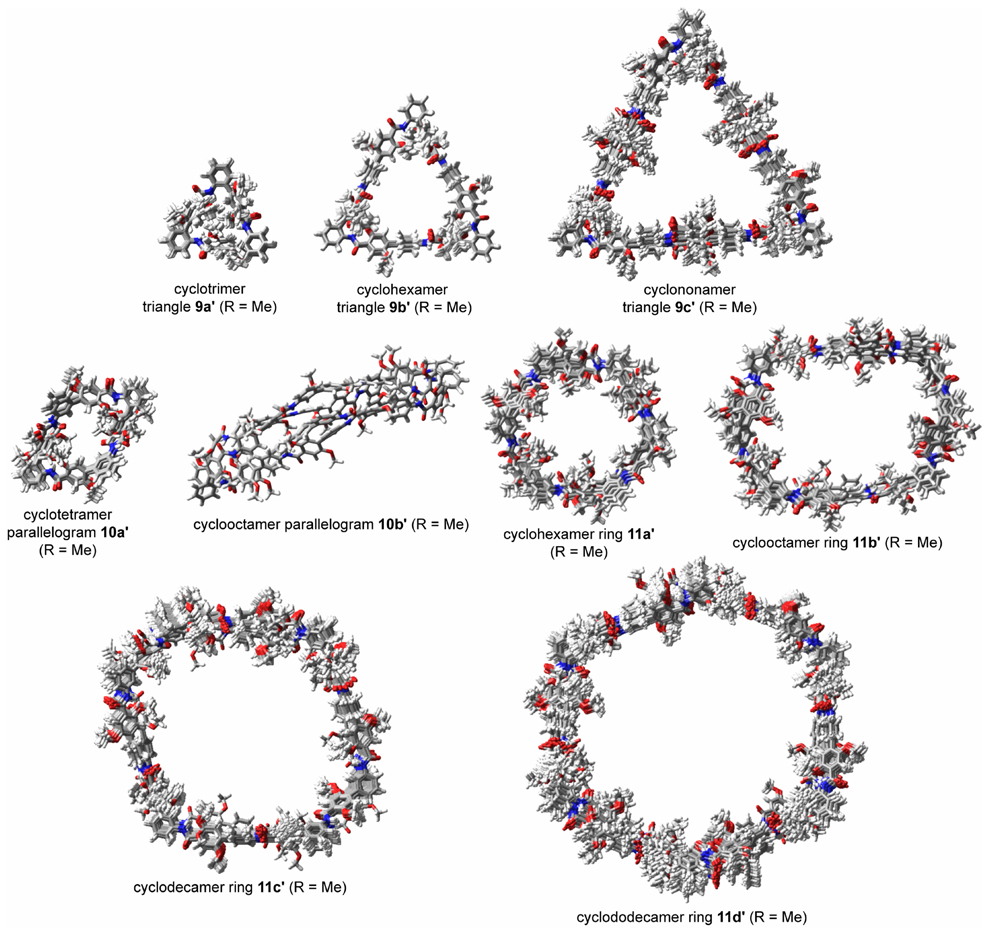
To evaluate the utility of building blocks 1a–c for the preparation of macrocycles, we synthesized “triangles” 9a–c, “parallelograms” 10a,b, and hexagonal “rings” 11a–d (Figure 1). The triangles are composed of 3 o-Abc2K units and 0, 3, or 6 Abc2K units; the parallelograms are composed of 2 o-Abc2K units, 2 m-Abc2K units, and 0 or 4 Abc2K units; and the rings are composed of 6 m-Abc2K units and 0, 2, 4, or 6 Abc2K units. The macrocycles range from a trimer to a dodecamer, with ring sizes from 24 to 114 atoms. The triangles are 1–3 nm on an edge, the parallelograms are 1–2 nm on an edge, and the rings are 2–4 nm across.
FIGURE 1.
Nanometer-scale macrocyclic peptides prepared from m-Abc2K, o-Abc2K, and Abc2K (R = CH2CH2CH2NH3+ CF3CO2−).
The macrocycles were synthesized by Fmoc-based solid-phase synthesis of protected linear oligomers, followed by macrocyclization, deprotection, and RP-HPLC purification (Scheme 2). The linear oligomers were prepared on chlorotrityl resin with ca. 2 equiv of protected amino acid, HCTU coupling reagent, 2,4,6-collidine base, and 8–12 h coupling times. After final Fmoc-deprotection, the protected linear oligomers were cleaved from the resin and cyclized at tenth-millimolar concentrations with HCTU, 2,4,6-collidine, and 24 h reaction times. Analytical HPLC studies established that the purities of the unpurified cyclized peptides were generally high, with the exception of 11d, which proved difficult to cyclize. The cyclic peptides were generally easy to purify by RP-HPLC. Lyophilization afforded the trifluoroacetate salts as fluffy white solids. A typical synthesis starting with 0.05 mmol of chlorotrityl resin gave an initial resin loading of ca. 0.03 mmol and afforded 0.002–0.008 mmol of analytically pure macrocycle after a single purification.
SCHEME 2.
Synthesis of Cyclohexamer Triangle 9b from Building Blocks Fmoc-o-Abc2K(Boc)-OH (1b) and Fmoc-Abc2K(Boc)-OH (1c)
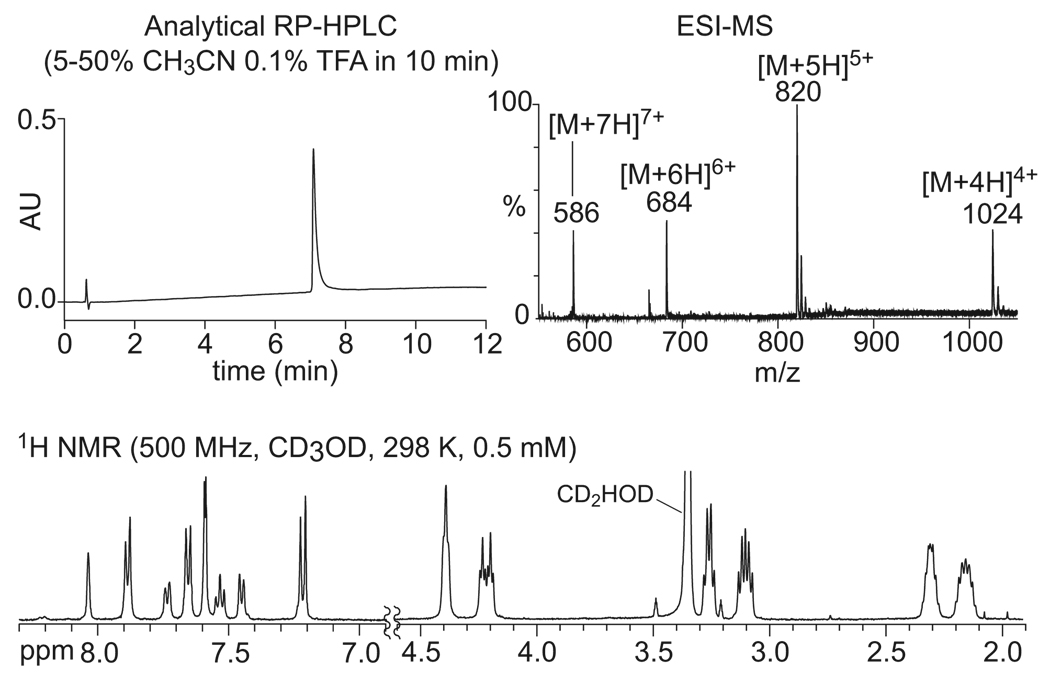
The macrocycles have good water solubility and are readily characterized by standard analytical techniques, such as RP-HPLC, ESI-MS, and NMR spectroscopy in D2O or CD3OD solution. Figure 2 shows characterization data for cyclododecamer 11d. The HPLC trace shows a single peak; the mass spectrum shows a series of multiply-charged molecular ions consistent with the molecular weight of the dodecamer, and the 1H NMR spectrum is sharp and well resolved.
FIGURE 2.
Characterization data for cyclododecamer ring 11d.
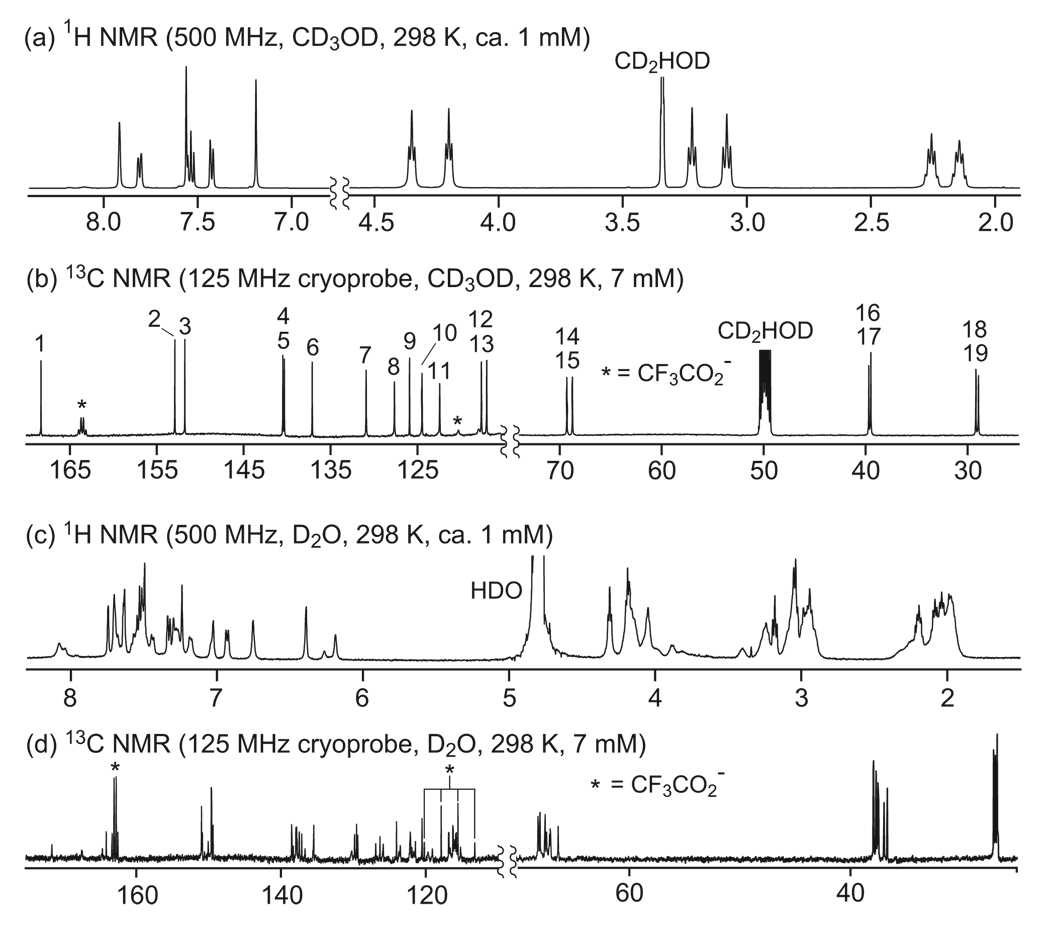
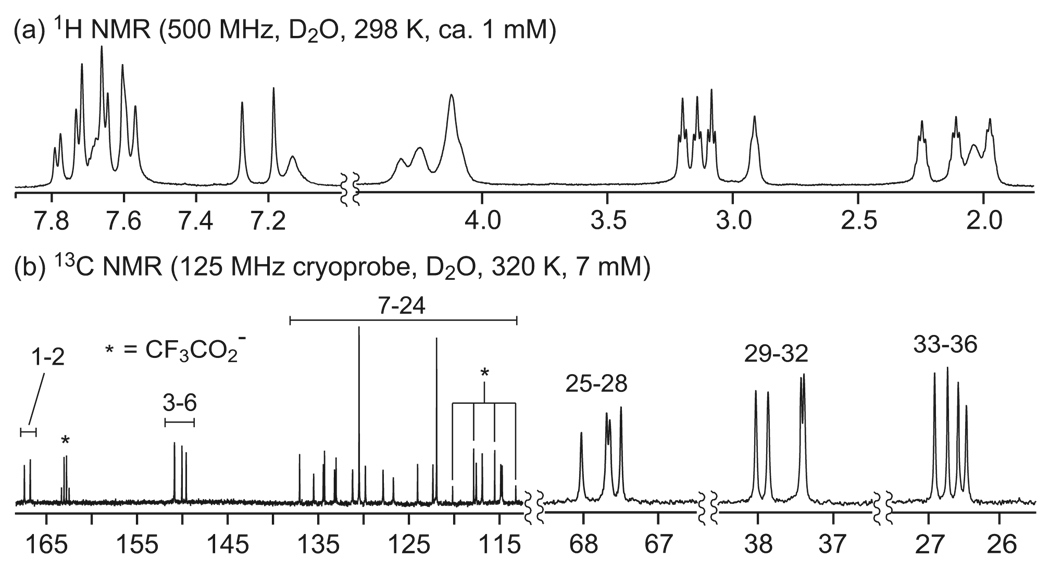
1H and 13C NMR studies suggest that the macrocycles adopt conformations with all trans-amide linkages in CD3OD, that the “triangles” and “parallelograms” maintain these conformations in D2O, and that the “rings” collapse to form conformations with cis-amide linkages in D2O. The 1H and 13C NMR spectra of ring 11a in CD3OD are sharp and well resolved and clearly show sixfold symmetry (Figure 3a,b). The 1H NMR spectrum shows six aromatic resonances and two sets of aliphatic resonances for each of the three methylene groups, consistent with a single type of m-Abc2K unit. The 13C NMR spectrum shows one carbonyl resonance, twelve aromatic resonances, and six aliphatic resonances, also consistent with one type of m-Abc2K unit and sixfold symmetry of ring 11a. All of the aromatic 1H resonances are downfield (7–8 ppm), suggesting that all of the amide linkages are trans and that the ring adopts an open structure without significant interactions between the aromatic rings.
FIGURE 3.
NMR spectra of cyclohexamer ring 11a in CD3OD and D2O.
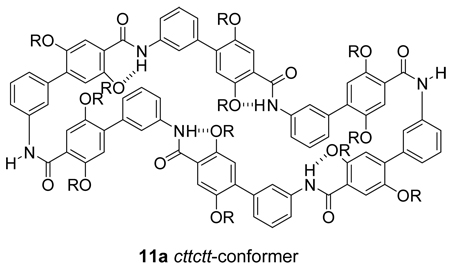
In D2O, the 1H and 13C NMR spectra of 11a are more complex (Figure 3c,d). Some of the aromatic 1H resonances are shifted upfield (6–7 ppm), suggesting the presence of cis-amide linkages.9 The 13C NMR spectrum shows ca. four times as many resonances; while the 13C NMR spectrum in CD3OD clearly shows two sets of propyloxyammonium groups, the 13C NMR spectrum in D2O appears to show eight. Collectively, these data suggest the presence of two conformers in D2O—a collapsed twofold-symmetrical cttctt-conformer, with two cis-amide linkages, in addition to the open ringlike tttttt-conformer, with all trans-amide linkages. We have previously documented the formation of both open (tttt) and collapsed (ctct) conformers in a related fourfold-symmetrical macrocycle and have prepared and studied control compounds that adopt cis-amide conformers.9 These related systems have also exhibited upfield shifting of the 1H aromatic resonances, reflecting the proximity of the aromatic rings that is bought on by the cis-amide geometry.
Rings 11b–d show similar behavior. The 1H NMR spectrum of 11d in CD3OD shows one set of resonances associated with sixfold symmetry, and the aromatic resonances are downfield (7–8 ppm). The 1H NMR spectrum in D2O is more complex, and some of the aromatic resonances are shifted upfield (6–7 ppm), suggesting a collapsed conformer with cis-amide linkages. The 1H NMR spectra of 11b and 11c in CD3OD show downfield aromatic resonances and appear to be consistent with all trans-amide conformers with twofold symmetry. The spectra of 11b and 11c in D2O are more complex, and some of the aromatic resonances are shifted upfield, suggesting cis-amide conformers.
In contrast to cyclohexamer ring 11a, cyclohexamer triangle 9b appears to adopt an open conformation with all trans-amide linkages in both D2O and CD3OD. The 13C NMR spectrum clearly shows threefold symmetry; the 1H NMR spectrum, although slightly broadened, suggests threefold symmetry and shows downfield aromatic resonances (Figure 4). The cyclononamer triangle 9c also shows threefold symmetry and appears to adopt an open conformation with all trans-amide linkages in D2O.
FIGURE 4.
NMR spectra of cyclohexamer triangle 9b in D2O.
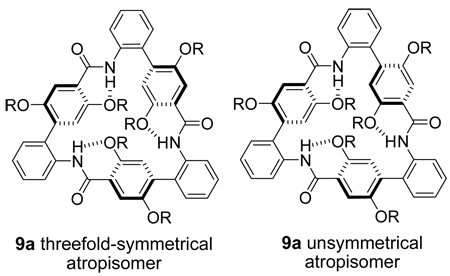
The cyclotrimer triangle 9a exhibits behavior atypical of the other macrocycles. It elutes from RP-HPLC as two peaks in a 2:1 ratio that re-equilibrate upon standing. The 1H and 13C NMR spectra of 9a in D2O and in CD3OD show two conformers in a 2:1 ratio—a threefold-symmetrical conformer and an unsymmetrical conformer. The symmetrical conformer likely has all trans-amide linkages and must have threefold symmetry. The unsymmetrical conformer could either contain a cis-amide linkage or be an unsymmetrical atropisomer associated with slow rotation about the ortho-disubstituted biphenyl groups of the o-Abc2K units (shown below). The small size of the macrocycle likely raises the energy barrier to conformational interconversion and slows equilibration of the two conformers.
Parallelogram 10b appears to adopt conformations with all trans-amide linkages in both D2O and CD3OD. The 13C NMR spectrum in D2O shows a single twofold-symmetrical conformer. The 1H NMR spectra show no significant upfield shifting of the aromatic resonances in either D2O or CD3OD, suggesting that the twofold-symmetrical conformer has all trans-amide linkages. Parallelogram 10a shows similar behavior at slightly elevated temperatures (e.g., 330 K). Some decoalescence of the 1H NMR spectra in either D2O or CD3OD occurs at lower temperatures (e.g., 298 K), suggesting the occurrence of atropisomers or, less likely, cis-amide linkages.
Molecular modeling studies suggest that all the macrocycles except 10b should have well-defined triangle, parallelogram, and ring shapes if all the amide linkages are trans and the ortho-alkoxy substituents are intramolecularly hydrogen bonded to the amide NH groups. The macrocycles were modeled as simplified homologues in which the propyloxyammonium side chains (R = CH2CH2CH2NH3+) were replaced with methoxy groups (R = Me, Figure 5). Each molecule was modeled using Maestro/MacroModel v8.5 with the MMFFs implementation of the MMFF force field and MCMM conformational searching. The Ar–Ar and Ar–N bonds were rotated during the search procedure. Rotations about the Ar–CO and Ar–O bonds were not performed. Amide linkages were assumed to adopt trans conformations and were not allowed to adopt cis conformations (except as noted below). 1000 Monte-Carlo search steps were performed for each structure, and no effort was made to assure that all of the lowest-energy conformers or the global minimum were identified.10,11
FIGURE 5.
Molecular models of low-energy conformers of macrocycles 9a–c, 10a,b, and 11a–d. The macrocycles were modeled as simplified homologues 9a'–c', 10a',b', and 11a'–d' in which the propyloxyammonium side chains (R = CH2CH2CH2NH3+) were replaced with methoxy groups (R = Me). Overlays represent conformers identified within the lowest 5.00 kJ/mol from 1000 Monte-Carlo conformational search steps.
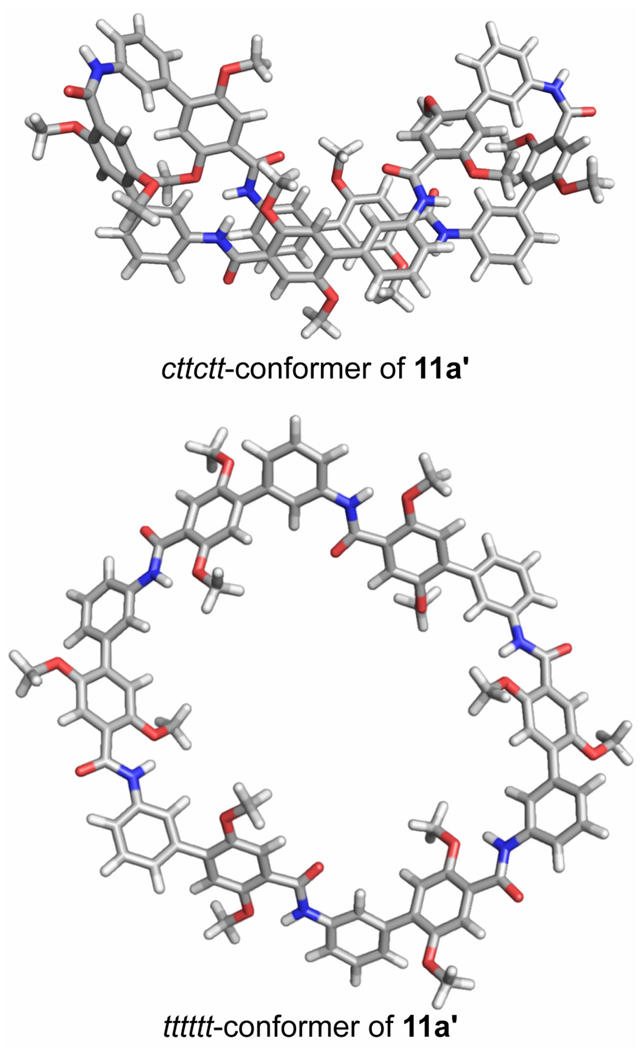
Molecular modeling studies also suggest that introduction of cis-amide linkages permits the formation of collapsed structures. A simplified model of the cttctt-conformer of 11a (11a', R = Me) was generated and is shown with the tttttt-conformer in Figure 6. The conformer represents the global minimum among the cttctt-conformers and was identified from 1000 Monte-Carlo search steps starting with a cttctt-conformer.12,13 In this conformer, the cis-amide linkages and adjacent aromatic rings create tight turn structures, and the other aromatic rings of the m-Abc2K units stack.
FIGURE 6.
Molecular models of cttctt-and tttttt-conformers of macrocycle 11a. The macrocycle was modeled as simplified homologue 11a' in which the propyloxyammonium side chains (R = CH2CH2CH2NH3+) were replaced with methoxy groups (R = Me). Structures represent the lowest energy cttctt-and tttttt-conformers identified from 1000 Monte-Carlo conformational search steps.
Conclusion
In summary, we have now developed a family of nanometer-sized amino acids—Abc2K, m-Abc2K, and o-Abc2K—that can easily be combined to create water-soluble nanometer-scale macrocycles with well-defined shapes. The macrocycles are easy to synthesize, purify, and characterize, because they are water-soluble cationic peptides. The macrocycles can adopt conformations with all trans-amide linkages and cavities as large as 4 nm or undergo hydrophobic collapse depending on their composition and on the solvent. The size, water solubility, and ease of synthesis of these compounds should help bridge the gap between biology and nanotechnology.
Experimental Section
General Procedures
Commercial solvents and reagents were used without further purification, unless otherwise stated. All solution-phase reactions were carried out under an atmosphere of nitrogen and were monitored by thin-layer chromatography (TLC) and carried out on 250 µm silica gel polyester plates on fluorescent silica gel with UV visualization. Column chromatography was performed on 40–63 µm silica gel (EMD Science) using flash chromatography. Solvents were removed by rotary evaporation, followed by further drying of residual solvents under vacuum (< 0.01 mmHg) or by air suction through a filter funnel. High resolution mass spectra were obtained by electrospray ionization (ESI) on a Waters Micromass LCT Premier (instrument variation σ < 5 ppm). NMR spectra were recorded using a 500 MHz Bruker AVANCE™ spectrometer. Chemical shifts are reported in parts per million (ppm) on the δ scale. 1H NMR spectra in CD3SOCD3 were referenced with TMS (δ = 0.00 ppm); 1H NMR spectra in D2O were referenced to HDO (δ = 4.80 ppm); 1H NMR spectra in CD3OD were referenced to CHD2OD (δ = 3.34). IR spectra were obtained using a Galaxy Series FTIR 5000. HPLC analysis was performed on an analytical RP-HPLC instrument, using a C18 column (Alltech, Platinum Rocket, 3µm packing, 7 mm × 53 mm). Preparative RP-HPLC was performed using an Agilent C18 column (1 inch diameter).
4-Bromo-2,5-dimethoxybenzoic acid (3).8
A flame dried 3-necked 100-mL round bottomed flask equipped with a magnetic stirring bar, rubber septum, ground-glass stopper, and a nitrogen inlet adapter, was charged with 1,4-dibromo-2,5-dimethoxybenzene (2) (5.0 g, 16.9 mmol). THF (ca. 25 mL) was added by syringe until a clear solution was formed. A 2.0 M solution of i-PrMgCl in THF (8.7 mL) was then added by syringe in a single portion at 25 °C to a stirring solution of 2, and was then stirred under an atmosphere of nitrogen for 20 h. Dry ice (ca. 20 g) was added in small portions resulting in a color change of the reaction mixture from light yellow to bluish-green. The solution returned to its original light yellow color after being allowed to mix for an additional 90 min. The reaction mixture was acidified with concd HCl (aq) (ca. 5 mL) and then extracted into ether (3 × 100 mL) with water (75 mL). The organic layer was washed with water (ca. 100 mL) and then extracted into aq 1 M KOH (3 × ca. 30 mL). The alkaline solution was acidified with concd HCl (aq), and the resultant white precipitate was washed with water to provide 4-bromo-2,5-dimethoxybenzoic acid (3) as a white powder (3.7 g, 85%).15
H-m-Abc2K(Boc)-OCH2COPh (7a)
Diether 66 (5.63 g, 8.86 mmol) was dissolved in THF (150 mL) by heating to ca. 60 °C in an oil bath. To the warmed solution was added 3-aminophenylboronic acid (1.16 g, 8.86 mmol), PdCl2(dppf)•CH2Cl2 (0.345 g, 0.423 mmol), K2CO3 (5.86 g, 42.3 mmol), water (30 mL), and the mixture was stirred at 60 °C for 24 h under an atmosphere of nitrogen. The solution was then concentrated by rotary evaporation and the resultant oil was dissolved in CH2Cl2 (ca. 100 mL). The organic layer was washed with water (ca. 100 mL), dried (MgSO4), and filtered. The filtrate was concentrated by rotary evaporation to afford a brown oil. Purification using column chromatography (silica gel, ethyl acetate–hexanes, 2/1, v/v) provided H-m-Abc2K(Boc)-OCH2COPh (7a) as an off-white solid (3.60 g, 63%): mp 118–120 °C; IR (KBr) 3430, 3361, 1729, 1681 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 298 K) δ 8.03 (d, J = 7.2 Hz, 2 H), 7.72 (t, J = 7.4 Hz, 1 H), 7.59 (t, J = 7.8 Hz, 2 H), 7.45 (s, 1 H), 7.08 (t, J = 7.8 Hz, 1 H), 7.03 (s, 1 H), 6.90 (t, J = 5.4 Hz, 1 H), 6.85 (t, J = 5.5 Hz, 1 H), 6.80 (s, 1 H), 6.72 (d, J = 7.7 Hz, 1 H), 6.59 (d, J = 7.9 Hz, 1 H), 5.72 (s, 2 H), 5.15 (br s, 2 H), 4.05 (t, J = 6.1 Hz, 2 H), 3.93 (t, J = 6.2 Hz, 2 H), 3.12 (q, J = 6.5 Hz, 2 H), 3.06 (q, J = 6.4 Hz, 2 H), 1.81 (quintet, J = 6.4 Hz, 2 H), 1.77 (quintet, J = 6.5 Hz, 2 H), 1.37 (s, 9 H), 1.32 (s, 9 H); 13C NMR (125 MHz, CD3SOCD3, 298 K) δ 192.8, 164.3, 155.6, 155.5, 152.6, 148.8, 148.16, 137.5, 136.7, 133.9, 133.9, 128.8, 128.4, 127.7, 117.7, 116.9, 116.6, 115.2, 114.8, 113.3, 77.4, 77.3, 67.2, 66.8, 66.2, 37.0, 36.7, 29.3, 29.0, 28.14, 28.09; HRMS (ESIMS) m/z for C37H47N3O9Na [M+Na]+ calcd 700.3210, found 700.3194.
Fmoc-m-Abc2K(Boc)-OCH2COPh (8a)
A solution of H-m-Abc2K(Boc)-OCH2COPh (7a) (3.60 g, 5.31 mmol), pyridine (0.52 mL, 6.4 mmol), and CH2Cl2 (100 mL) was cooled to 0 °C in an ice-bath. Fmoc-Cl (1.51 g, 5.84 mmol) in CH2Cl2 (50 mL) was added in drops over 5 min. After 30 min, the solution was allowed to warm to 25 °C and stirred for an additional 30 min. The mixture was then washed with water (ca. 100 mL), dried (MgSO4), filtered, and the filtrate was concentrated by rotary evaporation to give a yellow oil. The yellow oil was dissolved in CH2Cl2 (ca. 15 mL), precipitated using ethyl acetate (ca. 50 mL) and hexanes (ca. 100 mL), and filtered to provide Fmoc-m-Abc2K(Boc)-OCH2COPh (8a) as a white solid (4.36 g, 91%): mp 118–120 °C; IR (KBr) 3369, 1733, 1697 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 298 K) δ 9.63 (s, 1 H), 8.01 (d, J = 8.5 Hz, 2 H), 7.89 (d, J = 7.5 Hz, 2 H), 7.75 (d, J = 7.5 Hz, 2 H), 7.70 (t, J = 7.4 Hz, 1 H), 7.65 (br s, 1 H), 7.58 (t, J = 7.7 Hz, 2 H), 7.51-7.44 (m, 2 H), 7.42 (t, J = 7.5 Hz, 2 H), 7.37-7.30 (m, 3 H), 7.21 (d, J = 7.7 Hz, 1 H), 7.05 (s, 1 H), 6.66 (br s, 2 H), 5.68 (s, 2 H), 4.49 (d, J = 6.7 Hz, 2 H), 4.31 (t, J = 6.7 Hz, 2 H), 4.05 (t, J = 6.1 Hz, 2 H), 3.94 (t, J = 6.3 Hz, 2 H), 3.12 (q, J = 6.6 Hz, 2 H), 3.02 (q, J = 6.2 Hz, 2 H), 1.82 (quintet, J = 6.5 Hz, 2 H), 1.76 (quintet, J = 6.5 Hz, 2 H), 1.34 (s, 9 H), 1.32 (s, 9 H); 13C NMR (125 MHz, CD3SOCD3, 298 K) δ 192.7, 164.3, 155.54, 155.50, 153.4, 152.5, 148.8, 143.7, 140.7, 138.8, 137.4, 135.6, 133.9, 128.8, 128.3, 127.7, 127.6, 127.0, 125.0, 123.5, 120.1, 119.2, 118.3, 117.6, 116.8, 115.2, 77.4, 77.3, 67.3, 66.8, 66.3, 65.5, 46.5, 37.0, 36.6, 29.3, 29.1, 28.10, 28.08; HRMS (ESIMS) m/z for C52H57N3O11Na [M+Na]+ calcd 922.3891, found 922.3863.
Fmoc-m-Abc2K(Boc)-OH (1a)
Zn dust (8.0 g, 122 mmol) was added to a solution of Fmoc-m-Abc2K(Boc)-OCH2COPh (8a) (4.36 g, 4.84 mmol), AcOH (90 mL), and H2O (10 mL) and was stirred at 25 °C for ca. 18 h. The suspension was then diluted with CH2Cl2 (75 mL) and stirred for an additional ca. 1 h, concentrated to approximately one-half of its original volume, and partitioned between CH2Cl2 (75 mL) and aq 0.2 N HCl (ca. 75 mL). The aqueous layer was extracted with CH2Cl2 (2 × 75 mL), and the combined organic layers were washed with H2O (ca. 100 mL), saturated aq sodium chloride (ca. 100 mL), and dried (MgSO4). Filtration and concentration by rotary evaporation afforded a yellow oil. The oil was dissolved in ether (ca. 20 mL) and CH2Cl2 (ca. 10 mL) and the resulting solution was added in drops to hexanes (ca. 150 mL) over ca. 2 min. The resulting white suspension was filtered to afford Fmoc-m-Abc2K(Boc)-OH (1a) as a white solid (3.78 g, 94%): mp 98–100 °C; IR (KBr) 3361, 1702 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 298 K) δ 12.58 (br s, 1 H), 9.77 (br s, 1 H), 7.91 (d, J = 7.5 Hz, 2 H), 7.76 (d, J = 7.4 Hz, 2 H), 7.64 (br s, 1 H), 7.49 (br s, 1 H), 7.43 (t, J = 7.4 Hz, 2 H), 7.39-7.26 (m, 4 H), 7.18 (d, J = 7.6 Hz, 1 H), 6.99 (s, 1 H), 6.87 (t, J = 5.6 Hz, 1 H), 6.84 (t, J = 5.7 Hz, 1 H), 4.49 (d, J = 6.5 Hz, 2 H), 4.31 (t, J = 6.7 Hz, 1 H), 4.01 (t, J = 6.0 Hz, 2 H), 3.92 (t, J = 6.2 Hz, 2 H), 3.10 (q, J = 6.5 Hz, 2 H), 3.01 (q, J = 6.2 Hz, 2 H), 1.81 (quintet, J = 6.4 Hz, 2 H), 1.74 (quintet, J = 6.4 Hz, 2 H), 1.34 (s, 18 H); 13C NMR (125 MHz, CD3SOCD3, 298 K) δ 166.8, 155.5, 153.3, 151.7, 148.8, 143.7, 140.7, 138.7, 137.6, 134.3, 128.3, 127.6, 127.0, 125.0, 123.5, 120.9, 120.1, 119.1, 117.4, 116.7, 115.0, 77.38, 77.34, 67.2, 66.2, 65.5, 46.5, 36.9, 36.6, 29.2, 29.1, 28.1; HRMS (ESIMS) m/z for C44H51N3O10 [M+Na]+ calcd 804.3472, found 804.3470.
H-o-Abc2K(Boc)-OCH2COPh (7b)
Diether 66 (2.31 g, 3.47 mmol) was dissolved in THF (80 mL) by heating to ca. 60 °C in an oil bath. To the warmed solution was added 2-aminophenylboronic acid (0.874 g, 3.99 mmol), K2CO3 (2.40 g, 17.4 mmol), water (20 mL), PdCl2(dppf)•CH2Cl2 (0.142 g, 0.174 mmol), and the mixture was stirred at 60 °C for 24 h under an atmosphere of nitrogen. The solution was then concentrated by rotary evaporation to an oily slurry and was partitioned between water (150 mL) and CH2Cl2 (75 mL). The aqueous layer was extracted with CH2Cl2 (2 × 75 mL) and the combined organic layers were washed with saturated aqueous sodium chloride (ca. 100 mL) and dried (MgSO4). The suspension was then filtered and concentrated by rotary evaporation to afford a brown oil. The oil was purified using column chromatography (silica gel, ethyl acetate–hexanes, 2/1, v/v) to provide H2N-o-Abc2K(Boc)-OCH2COPh (7a) as an off-white solid (1.61 g, 69%): mp 54–56 °C; IR (KBr) 3367, 1701 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 298 K) δ 8.03 (d, J = 7.0 Hz, 2 H), 7.72 (t, J = 7.5 Hz, 1 H), 7.59 (t, J = 7.7 Hz, 2 H), 7.46 (s, 1 H), 7.06 (t, J = 7.0 Hz, 1 H), 6.98 (J = 7.5 Hz, 1 H), 6.95 (s, 1 H), 6.82 (t, J = 5.5 Hz, 2 H), 6.61 (t, J = 7.3 Hz, 1 H), 5.72 (s, 2 H), 4.72 (br s, 2 H), 4.02 (t, J = 6.0 Hz, 2 H), 3.93 (t, J = 6.5 Hz, 2 H), 3.11 (q, J = 6.5 Hz, 2 H), 3.00 (q, J = 6.0 Hz, 2 H), 1.80 (quintet, J = 6.5 Hz, 2 H), 1.68 (quintet, J = 6.25 Hz, 2 H), 1.37 (s, 9 H), 1.32 (s, 9 H); 13C NMR (125 M, Hz, CD3SOCD3, 298 K) δ 192.8, 164.5, 155.50, 155.48, 152.5, 149.2, 145.6, 134.6, 133.89, 133.85, 130.5, 128.8, 128.43, 127.7, 122.1, 118.1, 117.6, 115.9, 114.9, 114.8, 77.34, 77.29, 67.1, 66.8, 65.8, 37.0, 36.3, 29.3, 29.2, 28.13, 28.09;. HRMS (ESIMS) m/z for C37H48N3O9 [M+H]+ calcd 678.3391, found 678.3391.
Fmoc-o-Abc2K(Boc)-OCH2COPh (8b)
A solution of H-o-Abc2K(Boc)-OCH2COPh (7b) (1.51 g, 2.23 mmol), pyridine (0.220 mL, 2.68 mmol), and CH2Cl2 (75 mL) was cooled to 0 °C in an ice-bath. Fmoc-Cl (1.51 g, 3.70 mmol) in CH2Cl2 (20 mL) was added in drops over 10 min. After 20 min, the solution was allowed to warm to 25 °C and stirred for an additional 30 min. The mixture was then washed with water (150 mL), dried (MgSO4), filtered, and the filtrate was concentrated by rotary evaporation to afford a yellow oil. Purification using column chromatography (silica gel, ethyl acetate–hexanes, 2/1, 5% CH2Cl2, v/v) provided Fmoc-o-Abc2K(Boc)-OCH2COPh (8b) as a white solid (2.0 g, 100%): mp 68–70 °C; IR (KBr) 3373, 1732, 1695 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 298 K) δ 8.51 (br s, 1 H), 8.05 (d, J = 8.1 Hz, 2 H), 7.88 (d, J = 7.9 Hz, 2 H), 7.72 (t, J = 7.7 Hz, 1 H), 7.65-7.55 (m, 4 H), 7.48 (s, 2 H), 7.44-7.34 (m, 3 H), 7.34-7.27 (m, 3 H), 7.24 (t, J = 7.7 Hz, 1 H), 6.96 (s, 1 H), 6.80 (t, J = 5.5 Hz, 1 H), 6.73 (t, J = 5.5 Hz, 1 H), 5.75 (s, 2 H), 4.30 (d, J = 7.0 Hz, 2 H), 4.22 (t, J = 7.0 Hz, 1 H), 4.01-3.92 (m, 2 H), 3.85 (t, J = 6.3 Hz, 2 H), 3.08 (q, J = 6.4 Hz, 2 H), 2.86 (q, J = 6.2 Hz, 2 H), 1.78 (quintet, J = 6.5 Hz, 2 H), 1.60 (quintet, J = 6.5 Hz, 2 H), 1.32 (s, 9 H), 1.31 (s, 9 H); 13C NMR (125 MHz, CD3SOCD3, 298 K) δ 192.7, 164.5, 155.5, 155.4, 154.1, 152.3, 149.0, 143.6, 140.6, 135.5, 133.89, 133.87, 133.5, 131.6, 130.6, 128.8, 128.2, 127.8, 127.6, 127.0, 125.1, 124.6, 120.0, 118.7, 117.7, 114.9, 77.4, 77.3, 67.1, 66.9, 66.3, 65.9, 46.4, 37.1, 36.3, 29.2, 29.0, 28.1; HRMS (ESIMS) m/z for C52H57N3O11Na [M+Na]+ calcd 922.3891, found 922.3895.
Fmoc-o-Abc2K(Boc)-OH (1b)
Zn dust (3.2 g, 49 mmol) was added to a solution of Fmoc-o-Abc2K(Boc)-OCH2COPh (8b) (1.85 g, 2.06 mmol), AcOH (90 mL), and H2O (10 mL) and was stirred at 25 °C for 18 h. The suspension was then diluted with CH2Cl2 (100 mL) and stirred for an additional 3 h, washed with 0.2 N HCl (100 mL), H2O (100 mL), and saturated aqueous sodium chloride (100 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation to give a yellow oil. The oil was dissolved in ether (ca. 20 mL) and the resultant solution was added in drops to hexanes (ca. 80 mL) over ca. 2 min. The resultant white suspension was filtered to provide Fmoc-o-Abc2K(Boc)-OH (1b) as a white solid (1.54 g, 96%): mp 98–100 °C. An analytical sample of Fmoc-o-Abc2K(Boc)-OH (1b) was prepared by purification of 50 mg by RP-HPLC (water–CH3CN buffers with 0.1 % TFA), concentrating, dissolving the resultant oil in CH2Cl2, and passing the solution through a plug of silica gel (eluted with ethyl acetate): mp 98–100 °C; IR (KBr) 3500-3100, 1706 cm−1; 1H NMR, (500 MHz, CD3SOCD3, 320 K) δ 12.50 (br s, 1 H), 8.11 (br s, 1H), 7.85 (d, J = 7.6 Hz, 2 H), 7.56 (d, J = 7.4 Hz, 2 H), 7.49 (br d, J = 7.6 Hz, 1 H), 7.39 (t, J = 7.4 Hz, 2 H), 7.33 (td, J = 7.7, 1.2 Hz, 1 H), 7.31-7.23 (m, 4 H), 7.20 (td, J = 7.3, 0.9 Hz, 1 H), 6.84 (s, 1 H), 6.77 (br s, 1 H), 6.55 (br s, 1 H), 4.29 (d, J = 7.1 Hz, 2 H), 4.21 (t, J = 7.0 Hz, 1 H), 3.95 (t, J = 6.1 Hz, 2 H), 3.84 (t, J = 6.4 Hz, 2 H), 3.09 (q, J = 6.4 Hz, 2 H), 2.86 (q, J = 6.3 Hz, 2 H), 1.77 (quintet, J = 6.4 Hz, 2 H), 1.61 (quintet, J = 6.6 Hz, 2 H), 1.34 (s, 9 H), 1.32 (s, 9 H); 13C NMR (125 MHz, CD3SOCD3, 298 K) δ 166.9, 155.5, 155.4, 154.0, 151.5, 149.0, 143.6, 140.6, 135.4, 132.2, 131.8, 130.6, 128.0, 127.5, 126.9, 125.1, 124.9, 124.6, 121.2, 120.0, 117.6, 114.7, 67.1, 66.3, 65.9, 46.4, 37.0, 36.4, 29.1, 29.0, 28.11, 28.08; HRMS (ESIMS) m/z for C44H51N3O10Na [M+Na]+ calcd 804.3472, found 804.3463.
Representative Procedure for Macrocycle Synthesis: Synthesis of Cyclohexamer Triangle 9b
A Bio-Rad Poly-Prep column® was charged with 2-chlorotrityl resin (73 mg, nominally 1.4 mmol/g, 0.10 mmol, Novabiochem). The resin was derivatized by gently agitating with a solution of Fmoc-o-Abc2K(Boc)-OH (1b) (102 mg, 0.13 mmol) in 20% 2,4,6-collidine-CH2Cl2 (ca. 1 mL) for ca. 12 h. The solution was then drained using nitrogen pressure, and the resin was washed with CH2Cl2 (5 × ca. 5 mL, 1 min each). After the loading step, unreacted sites on the resin were capped using a solution of CH2Cl2-MeOH-DIPEA (17/2/1) for ca. 30 min. (Measurement of the weight gain in related experiments demonstrated the efficiency of the resin loading step to be ca. 50%.) The column was then drained, and the resin was washed with DMF (6 × ca. 5 mL, 1 min each) and then CH2Cl2 (6 × ca. 5 mL, 1 min each). The Fmoc group was removed by adding a solution of 20% piperidine–DMF (1 × ca. 5 mL for 1 min, 1 × ca. 5 mL for 20 min) to the resin followed by gentle agitation of the resultant suspension. The piperidine solution was drained, and the resin was washed with DMF (6 × ca. 5 mL, 1 min each) followed by CH2Cl2 (6 × ca. 5 mL, 1 min each).
Elongation of the protected linear Abc2K oligomer was accomplished by pre-activating the appropriate Fmoc-protected Abc2K building block (Abc2K or o-Abc2K for 9b; 78 mg, 0.10 mmol) with HCTU (0.10 mmol, 41 mg) in a ca. 1 mL solution of 20% 2,4,6-collidine–DMF. (These amounts correspond to ca. 2 equiv, based on 50% loading of the resin.) The coupling solution was then added to the resin and gently agitated for ca. 12 h. To determine if Abc2K couplings were complete, a small amount of resin was treated in a new Bio-Rad column with a solution of CF3COOH/water (9/1, v/v) and the column was vigorously agitated for ca. 2 h. The solution was then drained, concentrated by rotary evaporation, and the residue was dissolved in a mixture of water and CH3CN and then injected into an analytical RP-HPLC instrument to determine if any unreacted amine was present. In all cases, the amount of uncoupled amine was not significant enough to warrant a second coupling. The coupling procedure was repeated until the desired length of the linear oligomer was obtained [H-(o-Abc2K(Boc)-Abc2K(Boc))3-OH].
The protected linear Abc2K(Boc) oligomer was cleaved from the resin using a solution of AcOH-TFE-CH2Cl2 (1/1/4) for ca. 12 h. The peptide solution was drained from the resin and concentrated to form a gel. From the gel, remaining acetic acid was azeotropically removed by rotary evaporating with hexanes (3×) and CH2Cl2 (3×) to provide a white a solid. (This step is particularly important to avoid acetylation of the aniline group by trace amounts of acetic acid during cyclization.) The protected linear oligomer was split into two equal portions, and the cyclization step was carried out using half the material. (The remaining half of the material was reserved for studies of the uncyclized linear peptide.)
Cyclization was accomplished by charging a 250-mL round bottomed flask with H-(o-Abc2K(Boc)-Abc2K(Boc))3-OH (half of the material from above step) and 100 mL of 2% collidine-CH2Cl2. HCTU (0.1 mmol, dissolved in 1 mL of DMF) was added in drops over 2 min and the mixture was stirred for ca. 24 h. The reaction solution was then washed with ca. 100 mL of 0.2 N HCl (aq) to remove excess base, and the organic layer was concentrated to an oil. Global deprotection of the Boc groups was carried out in a small round bottomed flask equipped with a magnetic stirring bar, and mixed with 10–20 mL of CF3COOH/CH2Cl2 (1/1, v/v) for ca. 4 h. The solution was then concentrated by rotary evaporation, and the resultant oil was purified by preparative RP-HPLC (water–CH3CN with 0.1 % TFA). Pure fractions of the product (ca. 98% purity) were concentrated by rotary evaporation to remove most of the CH3CN, frozen, and then lyophilized to afford a white powder (16 mg, 0.005 mmol, 9 % based on the nominal 1.4 mmol/g loading of the resin and use of half of the linear oligomer in the cyclization step). The remaining less pure HPLC fractions (< 98% purity) were also lyophilized (10 mg, 0.003 mmol, 6 %) and stored for future use. Macrocycles 9a,c, 10a,b, and 11a–d were prepared in a similar fashion.14
It should be noted that the percentage yields based on resin loading are significantly higher than the above percentages would indicate, because the actual resin loading was found to be roughly half (0.7–1.0 mmol/g) of the nominal resin loading (1.4 mmol/g). Treatment of 36 mg of 2-chlorotrityl resin (nominally 1.4 mmol/g, 0.050 mmol, Novabiochem,) with Fmoc-o-Abc2K(Boc)-OH (1b) (51 mg, 0.065 mmol) in 20% 2,4,6-collidine-CH2Cl2 resulted in a weight gain of 19 mg, thus indicating 0.7 mmol/g actual loading. Treatment of 36 mg of 2-chlorotrityl resin (nominally 1.4 mmol/g, 0.050 mmol, Novabiochem,) with Fmoc-m-Abc2K(Boc)-OH (1a) (51 mg, 0.065 mmol) in 20% 2,4,6-collidine-CH2Cl2 resulted in a weight gain of 27 mg, thus indicating 1.0 mmol/g actual loading. A control study in which 36 mg of 2-chlorotrityl resin (nominally 1.4 mmol/g, 0.050 mmol, Novabiochem) was treated with MeOH in 20% 2,4,6-collidine-CH2Cl2 resulted in no weight gain, thereby validating the use of gravimetric analysis to assess resin loading. Gravimetric analysis after elongation to the full-length linear peptides indicated a final resin loading of 0.3–0.6 mmol/g, suggesting that some loss of peptide from the labile 2-chlorotrityl resin occurs during the synthesis.
Supplementary Material
Acknowledgement
We thank the ACS-PRF (38986-AC1) and NSF (CHE-0750523) for grant support for grant support. C. M. G thanks the UCI Institute for Brain Aging and Dementia for training grant support (NIA-5T32AG00096).
Footnotes
Supporting Information Available: NMR, ESI-MS, HPLC data, and additional details of molecular modeling studies. This material is available free of charge via the Internet at http://pubs.acs.org/.
References and Notes
- 1.(a) Mayor M, Lehn J-M. J. Am. Chem. Soc. 1999;121:11231–11232. [Google Scholar]; (b) Höger S, Bonrad K, Mourran A, Beginn U, Möller M. J. Am. Chem. Soc. 2001;123:5651–5659. doi: 10.1021/ja003990x. [DOI] [PubMed] [Google Scholar]; (c) Tobe Y, Utsumi N, Kawabata K, Nagano A, Adachi K, Araki S, Sonoda M, Hirose K, Naemura K. J. Am. Chem. Soc. 2002;124:5350–5364. doi: 10.1021/ja012458m. [DOI] [PubMed] [Google Scholar]; (d) Höger S, Morrison DL, Enkelmann V. J. Am. Chem. Soc. 2002;124:6734–6736. doi: 10.1021/ja017628+. [DOI] [PubMed] [Google Scholar]; (e) Grave C, Lentz D, Schäfer A, Samori P, Rabe JP, Franke P, Schlüter AD. J. Am. Chem. Soc. 2003;125:6907–6918. doi: 10.1021/ja034029p. [DOI] [PubMed] [Google Scholar]; (f) Huang X-C, Zhang J-P, Chen X-M. J. Am. Chem. Soc. 2004;126:13218–13219. doi: 10.1021/ja045249l. [DOI] [PubMed] [Google Scholar]; (g) Yang H-B, Das N, Huang F, Hawkridge AM, Diaz DD, Arif AM, Finn MG, Muddiman DC, Stang PJ. J. Org. Chem. 2006;71:6644–6647. doi: 10.1021/jo0608117. [DOI] [PubMed] [Google Scholar]; (h) Pan G-B, Cheng X-H, Höger S, Freyland W. J. Am. Chem. Soc. 2006;128:4218–4219. doi: 10.1021/ja060469f. [DOI] [PubMed] [Google Scholar]
- 2.(a) Youngs WJ, Tessier CA, Bradshaw JD. Chem. Rev. 1999;99:3153–3180. doi: 10.1021/cr9902446. [DOI] [PubMed] [Google Scholar]; (b) Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193–5198. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]; (c) Martin-Redondo MP, Scoles L, Sterenberg BT, Udachin KA, Carty AJ. J. Am. Chem. Soc. 2005;127:5038–5039. doi: 10.1021/ja050155c. [DOI] [PubMed] [Google Scholar]; (d) Heo J, Jeon Y-M, Mirkin CA. J. Am. Chem. Soc. 2007;129:7712–7713. doi: 10.1021/ja0716812. [DOI] [PubMed] [Google Scholar]; (e) Ferguson JS, Yamato K, Lui R, He L, Zeng XC, Gong B. Angew. Chem. Int. Ed. 2009;48:3150–3154. doi: 10.1002/anie.200900584. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]; (b) Li F, Gan Q, Xue L, Wang Z-m, Jiang H. Tetrahedron Lett. 2009;50:2367–2369. [Google Scholar]; (c) Zhu Y-Y, Wang G-T, Li Z-T. Org. Biomol. Chem. 2009;7:3243–3250. doi: 10.1039/b907457k. [DOI] [PubMed] [Google Scholar]
- 4.Nakao K, Nishimura M, Tamachi T, Kuwatani Y, Miyasaka H, Nishinaga T, Iyoda M. J. Am. Chem. Soc. 2006;128:16740–16747. doi: 10.1021/ja067077t. [DOI] [PubMed] [Google Scholar]
- 5.(a) Höger S. Chem.—Eur. J. 2004;10:1320–1329. doi: 10.1002/chem.200305496. [DOI] [PubMed] [Google Scholar]; (b) Zhang W, Moore JS. Angew. Chem., Int. Ed. 2006;45:4416–4439. doi: 10.1002/anie.200503988. [DOI] [PubMed] [Google Scholar]
- 6.Gothard CM, Rao NA, Nowick JS. J. Am. Chem. Soc. 2007;129:7272–7273. doi: 10.1021/ja072648i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neustadt BR, Smith EM, Lindo N, Nechuta T, Bronnenkant A, Wu A, Armstrong L, Kumar C. Bioorg. Med. Chem. Lett. 1998;8:2395–2398. doi: 10.1016/s0960-894x(98)00433-8. [DOI] [PubMed] [Google Scholar]
- 8.Miyakoshi R, Shimono K, Yokoyama A, Yokozawa T. J. Am. Chem. Soc. 2006;128:16012–16013. doi: 10.1021/ja067107s. [DOI] [PubMed] [Google Scholar]
- 9.Kang SW, Gothard CM, Maitra S, Atia-tul-Wahab, Nowick JS. J. Am. Chem. Soc. 2007;129:1486–1487. doi: 10.1021/ja0677970. [DOI] [PubMed] [Google Scholar]
- 10.Thorough identification of all low-energy conformers is not practical for the larger structures, and is only marginally practical for the smaller structures.
- 11.The modeling should be interpreted with several caveats: (1) The MMFF and MMFFs force fields lack good parameters for some of the stretches, bends, and torsions associated with the structures. (2) The MMFFs force field is designed to enforce planarity of the amide nitrogen atoms and may therefore overemphasize the conformational regularity of the structures. (3) The absence of H2O solvation in the modeling should decrease the effect of hydrophobic interactions within the structures. (4) The conformational search procedure excluded conformers with cis-amide linkages and did not explicitly seek those lacking intramolecular hydrogen bonds between the ortho-methoxy group and the amide NH group or with alternative rotations about the Ar–OMe bonds.
- 12.Other low-energy cttctt-conformers found in the search are virtually identical in structure to the global-minimum structure.
- 13.A meaningful comparison of the relative energies of the cttctt- and tttttt-conformers is not possible, because of the absence of charges and solvation in the modeling studies and because of the limitations of the force field.
- 14.Using similar procedures, yields of 4–16% of pure (ca. 98% purity) product were recorded for 9a (11%), 9c (13%), 10a (16%), 10b (10%), 11a (5%), 11c (4%), and 11d (1%) based on the nominal 1.4 mmol/g loading of the resin. Only 11d proved difficult to prepare, requiring three separate attempts on the synthesis and multiple HPLC purifications and giving a low yield of product.
- 15.We had previously6 generated intermediate 3 by way of a conventional Grignard reaction, in which the Grignard reagent was formed by treatment of 1,4-dibromo-2,5-dimethoxybenzene with magnesium. Generating the Grignard reagent through halogenmetal exchange8 of 1,4-dibromo-2,5-dimethoxybenzene with isopropylmagnesium chloride doubles the yield of 3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.