Abstract
The purpose of this study was to investigate certified diabetes educators’ (CDEs) perceptions of the adequacy of their diabetes education curricula in providing oral health information. A questionnaire was mailed to all CDEs with a mailing address in South Carolina (SC), United States (US). Of the 130 respondents, almost all (93.8%) expressed that oral health should be part of the curriculum. However, the majority of them (76.9%) reported that their curricula did not include an oral health module; the two predominant reasons were: not having enough time (61.0%), and not knowing enough about oral health and its relationship to diabetes (37.0%). Respondents who expressed that they did not know enough about oral health and its relationship to diabetes were less likely to provide adequate oral-health-related information (p = 0.008), especially information about the effect of periodontal disease on diabetes (p = 0.016). This study indicates that SC CDEs do not routinely provide oral health education to people with diabetes primarily due to lack of time and knowledge related to oral health. To better serve their patients, CDEs should integrate oral health education in the diabetes education curriculum.
Keywords: Diabetes Self-Management Training, Oral Health Education, Certified Diabetes Educators
Introduction
Diabetes mellitus is a chronic metabolic disease with serious oral health implications. People with diabetes, especially those with poorly controlled or uncontrolled diabetes, have an increased susceptibility to chronic infections and inflammation of oral tissues, including periodontal diseases (chronic gingivitis and periodontitis) [1–3], dental caries [4, 5], and oral candidiasis [5, 6], which contribute to substantial oral functional disability [7]. The likelihood of having periodontal disease among people with diabetes is about 3 times greater than for people without diabetes [2]. For people with poorly controlled or uncontrolled diabetes, periodontal disease progresses more rapidly and more severely than in their controlled or non-diabetic counterparts [2]. Studies [5, 8–12] also suggest a bidirectional relationship between periodontal disease and diabetes, with periodontal disease worsening glycemic control, and poorly controlled or uncontrolled diabetes increasing the likelihood of destructive periodontitis.
In addition, dry mouth is a common diabetic phenomenon [5]. The decrease of salivary flow may predispose people with diabetes to dental caries and oral candidiasis [13]. Frequent daily intake of refined carbohydrates may also contribute to a higher incidence of caries among people with diabetes [14]. Effective control of oral disease can be attained systemically through better glycemic control and locally through improved oral hygiene [15, 16].
Furthermore, studies [17, 18] have shown that poor perception of one’s oral health status (including dissatisfaction with teeth and mouth, and feeling of dry mouth) among people with diabetes has a strong negative impact on their health-related quality of life. Therefore, people with diabetes must be educated about the importance of removing oral plaque daily through meticulous oral hygiene, managing mouth dryness and diet, ceasing tobacco use [19–21], and obtaining regular professional dental care and cleaning [16, 22]. Studies also show that improved oral health may facilitate better glycemic control in people with poorly controlled diabetes [23–25].
Although adults with diabetes are more likely to develop periodontitis than their peers without diabetes, they are less likely to visit a dentist. Several national and statewide studies [26–29] found that the proportion of adults with diabetes who reported a dental visit during the preceding year was consistently below 70% (ranging from 60% to 69%). In those without diabetes, 73% of adults reported a dental visit within the preceding year [29]. The proportion of smokers with diabetes who reported a dental visit was even lower (59%) [28]. Subsequently, adults with diabetes required more emergency dental care service than adults without diabetes [30]. Karjalainen and colleagues also found dental health behaviors (oral hygiene and dental visit frequency) were more irregular among those individuals with poorly controlled diabetes [31]. The main reason for adults with diabetes not visiting a dentist was that they did not perceive a need [29]. Moore et al. [16] found most adults with diabetes were unaware of the oral health complications of the disease. In addition, they were less willing to spend time and money on their teeth compared to their peers without diabetes [30].
Because adults with diabetes were less likely to have seen a dentist than to have seen a health care provider for diabetes care in the preceding year (<70% vs. 86.3%) [29], diabetes educators can be a potential source for diabetes-related oral health information. Patients with diabetes who identified a regular primary provider for their diabetes care were more likely to have received more recommended elements of diabetes care (though not dental checkups) and to have better glycemic control than patients without such a provider [27]. Lack of significant differences related to dental care between the two groups (regular primary health care provider versus no regular primary health care provider) may be due to the fact that the provider did not educate the patients about the importance of dental checkups.
Given the importance of good oral hygiene among people with diabetes and the relationship of poor glycemic control to the severity of periodontal disease, adequate oral hygiene instruction and healthy lifestyle information related to oral health are essential for this population. Thus, the main purposes of this study were to 1) determine diabetes educators’ perceptions of the adequacy of their diabetes education curricula in providing diabetes-related oral health information to people with diabetes; 2) identify potential barriers related to the inclusion of oral health education in their curricula; and 3) explore the association between the inclusion of an oral health module in the diabetes education curriculum and the diabetes educator respondents’ practice characteristics, as well as adequacy in the coverage of various diabetes-related oral health topics. Results obtained from this study may provide information for diabetes educators to improve oral health education for people with diabetes, and over time, may lead to improvements in oral health as well as diabetes control in people with diabetes.
Methods
Questionnaire
A 12-question survey instrument was developed based on questions drawn from a review of related literature [32–36], and input from practicing diabetes educators and dental hygienists. The questionnaire was reviewed for content validity by a panel of four practicing dental hygienists and two diabetes educators.
The questionnaire asked about the practice characteristics of certified diabetes educators (CDEs) including number of years in practice, type of work setting, number of hours worked per week, number of patients with diabetes they educated in a typical week, time spent in direct patient contact per week, average length of time spent in a diabetes education session, and format of the education session (individual and/or group).
Respondents were asked to rate the coverage adequacy of their diabetes education curricula with respect to educating patients about general and oral health issues. The coverage adequacy questions comprised 24 topics, including 10 on controlling diabetes, preventing complications of diabetes, and healthy life-style topics, and 14 on oral hygiene and oral health. Respondents rated curriculum coverage of these topics on a 3-point scale as “Adequate,” “Not adequate,” or “Not covered.” Specific wordings of the 24 content areas pertaining to curriculum coverage of general and oral health topics are presented in Table 1. In addition, respondents were asked to respond ‘Yes’ or ‘No’ to the question, “Does your patient diabetes education curriculum include a module on oral health?” For those responding ‘No’, a follow-up question asked them to identify the main reasons for not including an oral health module.
Table 1.
Percent of respondents (n = 130) reporting coverage of general and oral health topics in the diabetes education curriculum as ‘Adequate’, ‘Not adequate’ or ‘Not covered’. Questions are arranged in descending order of ‘Adequate’ coverage.
| General health topic | Adequate | Not adequate | Not covered |
|---|---|---|---|
| Q02a: Control blood sugar level | 99.2 | 0.8 | 0 |
| Q02b: Nutrition and dietary counseling | 96.9 | 3.1 | 0 |
| Q02g: Monitoring blood pressure, cholesterol level, and blood sugar level | 96.9 | 2.3 | 0.8 |
| Q02h: Long-term complications (eyes, kidneys and nerves) | 93.9 | 4.6 | 1.5 |
| Q02c: Physical exercise | 92.3 | 7.7 | 0 |
| Q02f: Foot problems | 90.0 | 6.2 | 3.8 |
| Q02e: Avoiding alcohol | 70.0 | 26.2 | 3.8 |
| Q02j: Flu and pneumonia vaccines | 70.0 | 21.5 | 8.5 |
| Q02i: Stress management | 68.5 | 27.7 | 3.8 |
| Q02d: Tobacco / smoking cessation | 60.8 | 33.8 | 5.4 |
| Oral health topic | |||
| Q02r: Frequent prophylactic dental visits | 59.2 | 28.5 | 12.3 |
| Q02s: Daily brushing and flossing | 56.9 | 26.9 | 16.2 |
| Q02k: Importance of good oral hygiene (plaque control) | 51.5 | 34.6 | 13.9 |
| Q02p: Effect of uncontrolled diabetes on periodontal disease | 45.4 | 36.2 | 18.5 |
| Q02o: Effect of periodontal disease on diabetes | 36.2 | 42.3 | 21.5 |
| Q02l: Causes and results of plaque, calculus, gingivitis and periodontal disease | 19.2 | 46.9 | 33.9 |
| Q02n: Use bleeding to monitor the health of gums | 18.5 | 40.8 | 40.8 |
| Q02q: Managing dry mouth | 13.8 | 46.2 | 40.0 |
| Q02m: Benefits of fluoride | 6.2 | 44.6 | 49.2 |
| Q02x: Care of removable prosthetic appliances (i.e., denture) | 4.6 | 23.1 | 72.3 |
| Q02u: Shown proper flossing techniques | 3.8 | 26.2 | 70.0 |
| Q02t: Shown proper tooth brushing techniques | 3.1 | 28.5 | 68.5 |
| Q02v: Have patients demonstrate recommended brushing techniques | 0.8 | 25.4 | 73.9 |
| Q02w: Have patients demonstrate recommended flossing techniques | 0.8 | 24.6 | 74.6 |
Survey Process
A cover letter, a one-page questionnaire printed on both sides, an acknowledgement slip to indicate completion of the questionnaire, and two preaddressed, return postage-paid envelopes were mailed to all (n = 250) certified diabetes educators with a mailing address of South Carolina, US. Address labels were purchased from the National Certification Board for Diabetes Educators. To maintain anonymity, respondents returned the completed questionnaire and the acknowledgment slip separately in two different envelopes. Upon receipt of the return acknowledgement slip, a US$5 store gift card was sent to the person’s address. A complete follow-up mailing about 2 months afterward was sent to nonrespondents. Approval for the study was obtained from the Institutional Review Board of the Medical University of South Carolina.
Statistical Analysis
For survey questions measured on a continuous scale, we summarized measures of central tendency and dispersion using median and interquartile range (IQR) due to positively skewed data distributions. Bivariable associations between respondents’ practice characteristics and inclusion of an oral health module (yes or no) in the patient diabetes education curriculum were assessed using Fisher’s exact test and Wilcoxon’s rank-sum test for categorical and continuous variables, respectively. Among respondents with no oral health module in their diabetes education curricula, a second binary outcome was measured taking on the value of one if the respondent reported not knowing enough about oral health and its relationship to diabetes as a main reason for not including an oral health module. Within this subgroup, we assessed bivariable associations between respondents’ practice characteristics and this second outcome variable using both Fisher’s exact test and Wilcoxon’s rank-sum test as appropriate.
Because of the clinical importance of the relationship between diabetes and periodontal disease, we assessed the associations between adequate coverage in the diabetes education curriculum of the ‘Effect of periodontal disease on diabetes’ and the ‘Effect of uncontrolled diabetes on periodontal disease’ with the second binary outcome defined above. Specifically, within the subgroup of respondents with no oral health module, we used Fisher’s exact test to compare the percent of respondents reporting ‘Adequate’ coverage of these two topics among those who did not and those who did report not knowing enough about oral health and its relationship to diabetes as a main reason for not including an oral health module.
We conducted an exploratory factor analysis (EFA) of topics assessing respondents’ diabetes education curriculum on general and oral health content coverage to identify the number of latent variables and to uncover the underlying structure of the factors. Execution of the EFA was based on the matrix of polychoric correlations, a correlation measuring the strength of linear association between ordinal variables. Factors were identified based on the scree plot and the Kaiser-Guttman criterion that specifies retaining factors with eigenvalues greater than one. A variable was considered important in explaining the variance of a factor if its loading on that factor exceeded 0.50. Orthogonal biquartimax rotation was used to enhance interpretability.
We used the results of the EFA to establish an initial model for a confirmatory factor analysis (CFA). We performed the CFA using the underlying variable approach for ordinal data described in Bartholomew et al [37]. We assessed goodness-of-fit using the adjusted goodness-of-fit index (AGFI), a fit measure that is robust against normality violations and that is insensitive to small sample sizes [38, 39]. AGFI takes on values between zero and one, measures the improvement in fit relative to no model at all, and reflects good model fit when its value exceeds 0.9. We used this measure, together with the estimated error variances, to compare competing models and to guide the final model selection.
To assess associations between the factors identified by the CFA and the previously defined binary outcome variables (inclusion of an oral health module; and identification of not knowing enough about oral health and its relationship to diabetes as a main reason for not including an oral health module), we computed latent variable scores for each of the factors [40] and performed univariable logistic regressions with factor-specific latent variable scores as independent variables in each model. In addition, for each factor, we summarized separately for respondents who did and did not include an oral health module in their diabetes education curricula, the percent of respondents who indicated adequate coverage of those general and oral health topics associated with the specified factor.
Data analysis was conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina). The CFA was performed using LISREL, version 8.8 (Scientific Software International Inc.). All hypothesis tests were based on a two-sided alpha of 0.05.
Results
Of the 250 listed diabetes educators, 10 had moved out of state, 2 had no forwarding address, and 4 were temporarily away from the current address. Of the 234 delivered letters, 150 were returned, for a response rate of 64.1%. Of the respondents, 20 stated that they had retired or no longer practiced as a diabetes educator. These 20 respondents were excluded. Thus, the analytic sample consisted of 130 respondents with usable data.
The diabetes educators responding to the survey had been practicing for a median of 8 years (IQR = 5 to 14 years; range = 1 to 32 years). About 76.7% of the respondents reported working at least 25 hours per week and seeing a median of 15 patients with diabetes per week (IQR = 10 to 25 patients; range = 2 to 80 patients). The median length of an educational session was 1 hour 20 minutes (IQR = 1 to 2 hours; range = 15 minutes to 7 hours), with 56.8% of the respondents reporting that typical treatment sessions were between 1 hour and 1.5 hours. The respondents worked in a variety of settings, including hospitals (28.5%), community (23.8%), outpatient facilities (23.8%), and private offices (18.5%).
Table 1 summarizes the percent of respondents reporting coverage of general and oral health topics in their diabetes education curricula as ‘Adequate,’ ‘Not adequate’ or ‘Not covered.’ Among the 10 general health topics, the percentage of responses for the adequate categories ranged from a low of 60.8% on tobacco/smoking cessation to a high of 99.2% on controlling blood sugar level. Among the 14 oral health topics, the percentages of responses for the adequate category ranged from a low of 0.8% on having patient demonstrate recommended oral hygiene (brushing and flossing) techniques to a high of 59.2% on recommending frequent prophylactic dental visits.
Almost all respondents (93.8%, n = 122) expressed that oral health should be part of the patient diabetes education curriculum. However, 76.9% (n = 100) of respondents reported their curricula did not include a module on oral health. Even though eight respondents did not include an oral health module in their curricula, they briefly covered some basic oral health issues (such as regular dental visit and brushing regularly).
Among respondents with no oral health module in their curricula, the two predominant reasons for not including an oral health module were: not having enough time (61.0%), and not knowing enough about oral health and its relationship to diabetes (37.0%). However, patients’ lack of interest in the topic of oral health was not an issue, as only 7% of respondents in this subgroup reported patient lack of interest as a main reason for not including an oral health module. Four respondents reported that they did not have sufficient oral health educational materials and patient handouts for their patients. Finally, five respondents were not aware or had not thought about including information on oral health prior to completing the survey.
Respondents who reported not knowing enough about oral health and its relationship to diabetes as a main reason for not including an oral health module were less likely to adequately address both the effect of periodontal disease on diabetes as well as the effect of uncontrolled diabetes on periodontal disease, compared to those who did not report not knowing enough about oral health (10.8% versus 33.3%, p = 0.016; and 24.3 % versus 42.9%, p = 0.084, respectively),
Table 2 summarizes respondents’ practice characteristics as they relate to the inclusion of an oral health module (included or not included) in their curricula. Among respondents with no oral health module, those who reported not knowing enough about oral health and its relationship to diabetes as one of the main reasons for not including an oral health module have been practicing as diabetes educators a significantly shorter length of time relative to those who did not report this reason (median = 7 years, IQR = 4 to 10 years and median = 10 years, IQR = 6 to 15 years, respectively; p = 0.006). No other practice characteristics were significantly associated with the inclusion of an oral health module.
Table 2.
Summary of respondents practice characteristics by inclusion of an oral health module (not included versus included) in the patient diabetes education curriculum.
| Oral health module† | ||
|---|---|---|
| Practice characteristic* | Not included (n = 100) | Included (n = 30) |
| Work environment | ||
| Hospital | 29 (29.0) | 8 (27.6) |
| Community | 23 (23.0) | 6 (20.7) |
| Outpatient clinic | 22 (22.0) | 9 (31.0) |
| Private office | 19 (19.0) | 5 (17.2) |
| Other | 7 (7.0) | 1 (3.5) |
| Hours worked/week | ||
| 8 or less | 9 (9.0) | 2 (6.9) |
| 9–16 | 6 (6.0) | 2 (6.9) |
| 17–24 | 7 (7.0) | 4 (13.8) |
| 25–32 | 9 (9.0) | 6 (20.7) |
| 33 or more | 69 (69.0) | 15 (51.7) |
| Hours/week with direct patient contact | ||
| 8 or less | 16 (16.3) | 3 (10.3) |
| 9–16 | 14 (14.3) | 7 (24.2) |
| 17–24 | 19 (19.4) | 8 (27.6) |
| 25–32 | 26 (26.5) | 6 (20.7) |
| 33 or more | 23 (23.5) | 5 (17.2) |
| Primary education session type | ||
| One-on-one | 33 (33.0) | 7 (24.1) |
| Group | 12 (12.0) | 1 (3.5) |
| One-on-one and group | 55 (55.0) | 21 (72.4) |
| No. years practicing as a diabetes educator‡ | 8 (5–14) | 8 (6–13) |
| No. patients receiving services in a typical week‡ | 15 (10–26) | 14 (10–18) |
| Session duration (hours)‡ | 1.3 (1–2) | 1.5 (1–2) |
No practice characteristic was significantly associated with inclusion of an oral health module, p-values range = 0.20 to 0.90.
Categorical and continuous variables are reported as frequency (column percent) and median (IQR), respectively. Frequencies do not sum to column totals due to missing data. One respondent did not answer the questions on the back side of the questionnaire which consists of questions related to practice characteristics and inclusion of oral health module in the diabetes education curriculum.
Median (IQR) for ‘No. years practicing’, ‘No. patients receiving services’ and ‘Session duration’ are based on frequencies of: 99 not included (NI), 29 included (I); 98 NI, 29 I; and 97 NI, 28 I, respectively.
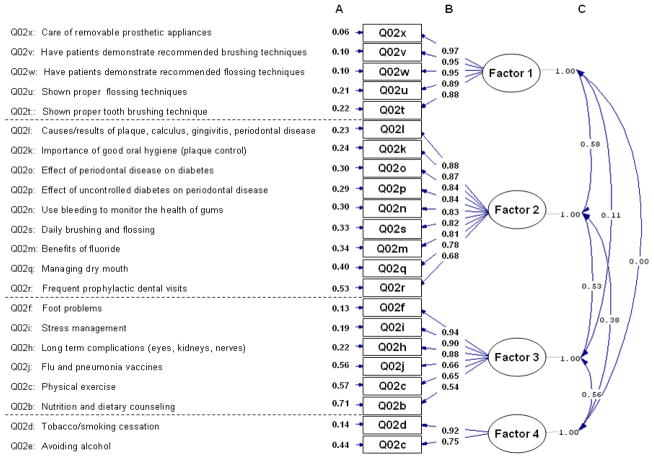
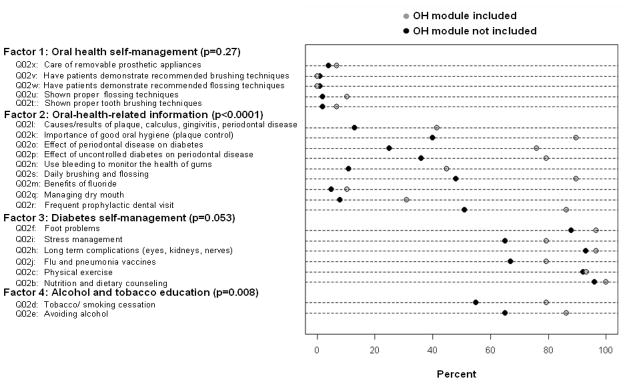
Figure 1 shows the path diagram summarizing the final CFA model. We identified four factors: oral health self-management; oral-health-related information; diabetes self-management; and alcohol and tobacco education. The AGFI was 0.94, indicating excellent model fit. In both the CFA and EFA, we excluded data from the question assessing curriculum coverage of blood sugar level control (Q02a) due to uniformity in diabetes educators’ responses (99% of respondents reported adequate coverage of blood sugar level control). In the CFA, we also excluded the question on monitoring of blood pressure, cholesterol level, and blood sugar levels (Q02g) in order to improve the goodness-of-fit of the model. Figure 2 shows the percent of respondents reporting adequate coverage for survey questions related to general and oral health content among diabetes educators that do and do not include an oral health module in their curricula. Questions are grouped according to their associations with the factors based on the CFA. Reported p-values are based on the simple logistic regression of the binary outcome measuring the inclusion of an oral health module regressed on the latent variable scores for each factor.
Figure 1.
Path diagram for the four-factor model based on the confirmatory factor analysis (AGFI = 0.94). Survey question groups are arranged in the order of decreasing factor loadings, and correspond to collections of manifest variables associated with the following factors: factor 1 = oral health self-management; factor 2 = oral-health-related information; factor 3 = diabetes self-management; and factor 4 = alcohol and tobacco education. Measures: column A is error variances; column B is factor loadings; and column C is factor correlations
Figure 2.
Percent of respondents reporting adequate coverage for survey questions related to general and oral health content among diabetes educators that do and do not include an oral health (OH) module in their patient education curriculum. Questions are grouped according to their associations with the factors based on the confirmatory factor analysis, and arranged in order of decreasing factor loadings. Reported p-values are based on the simple logistic regression of the binary outcome measuring the inclusion of an oral health module regressed on the latent variable scores for each factor.
The factors ‘oral-health-related information’ and ‘alcohol and tobacco education’ were significantly associated with the inclusion of an oral health module in the patient curriculum (p < 0.0001 and p = 0.008, respectively). The association between the factor ‘diabetes self-management’ and inclusion of an oral health module bordered on significance (p = 0.053). The factor ‘oral health self-management’ was not significantly associated with the inclusion of an oral health module (p = 0.27).
No factor was significantly associated with the outcome variable for reporting not knowing enough about oral health and its relationship to diabetes as one of the main reasons for not including an oral health module (results not shown).
Discussion
This study indicates that SC CDEs do not routinely engage in the provision of diabetes-related oral and periodontal patient education primarily due to lack of time and knowledge related to oral health. Consistent with a pilot qualitative study by Koerber et al.[41], respondents appreciated the importance of including oral health education in their patient diabetes education curriculum. However, time and resources devoted to oral or periodontal health education are limited. These results suggest a need for improving training of SC CDEs by integrating oral health education into the curriculum in a more efficient and effective manner. The integration of oral health informaiton is an important component of quality diabetes care and has the potential for improving outcomes for the person with diabetes.
In addition, not knowing enough about oral health and its relationship to diabetes is a significant limiting factor to providing adequate oral health education to respondents’ patients. Among the diabetes education curricula without oral health content, respondents who report not knowing enough about oral health and its relationship to diabetes were less likely to provide adequate ‘oral-health-related information,’ especially information about the relationship between diabetes and periodontal disease. The significant negative association between diabetes educators’ years of practice and lack of an oral health module because of not knowing enough about oral health and its relationship to diabetes indicates that diabetes educators’ practice experience (as an indirect indicator of receiving more continuing education in various issues related to diabetes) may allow them to learn more about the relationship between oral health and diabetes.
Thus, continuing education on various diabetes-related oral and periodontal health topics is critical for SC CDEs to better equip them with sufficient knowledge, skills, and confidence to educate their patients. Additionally, information on oral health complications and prevention of the complications should be included in diabetes educators’ clinical training programs [29], as well as in the education of other health professionals who provide care to people with diabetes. The American Diabetes Association recommends that persons with diabetes learn how gum problems start, and look for early signs of gum disease, in addition to brushing teeth twice daily, flossing once daily, and visiting the dentist at least twice each year (www.diabetes.org/type-2-diabetes/mouth-care.jsp). Thus, diabetes educators need ongoing education and training that integrates an evidence-based approach for educating the person with diabetes about dental health. Patients with diabetes, especially those with poor control may need more frequent visits to their dental health provider as well as more frequent dental hygiene.
According to the American Diabetes Association’s Clinical Practice Recommendations [42], referral for a dental examination is one component of the comprehensive diabetes evaluation. Diabetes educators should encourage their patients with poorly controlled or uncontrolled diabetes, particularly smokers and minorities (African-Americans and Hispanics) to have a regular dental visit at least every 6 months [26, 33]. A written referral from the diabetes educator or the provider of diabetes care may serve as a reminder for the patient.
Integrating evidence-based dental health education and written referrals for comprehensive dental exams and regular dental care require more time on the part of the educator whose schedule is already crowded with activities. The key to successfully behavior change involves integrating the community context with the evidence-based approach to diabetes education and oral health care, whether the location is in a community health center or in a physician’s exam room or other places where care is delivered.
The factor analysis results suggest that the inclusion of an oral health module in the diabetes curriculum will lead to a higher coverage of diabetes related topics such as oral-health-related information and alcohol and tobacco education. One of the implications of the findings is that partnership of diabetes educators with dental health professionals (or professional associations) to educate patients on diabetes and oral health has the potential to expand current diabetes self-management training and education curriculum for improving diabetes and oral health outcomes.
In some rural areas in the US and in many areas outside the US, access to certified diabetes educators may be limited; thus, the health professionals who provide care for the patient with diabetes may need to assume the role of diabetes educator and integrate dental health education into episodic and ongoing care. Oral health education is an important component of diabetes education and should be integrated into ongoing care of persons with diabetes. By screening and providing oral health education, diabetes educators and others providing diabetes care may make a significant difference in oral health, as well as diabetes control, with their patients. Furthermore, mouth cavity examination and oral health education should be listed as a component of National Standards for Diabetes Self-Management Education, through working with the American Diabetes Association and the Association of Diabetes Educators to include an oral health module as a component of the diabetes education curriculum in prevention of chronic complications.
Acknowledgments
The authors thank the four dental hygienists: Sharon Crossley, MPH, RDH; Linda Morrison, RDH; Pemra L. Hudson, RDH, Lisa M. Summerlin, MA, RDH; Elizabeth Slate, PhD (biostatistician), Carlos F. Salinas, DMD (dentist), Kathryn M. Magruder, MPH, PhD (epidemiologist) and several diabetes educators for their valuable suggestions on the content of the questionnaire. This study was completed with support from the South Carolina Centers of Biomedical Research Excellence (COBRE) for Oral Health with funding provided by the National Institutes of Health (NIH) and the National Center for Research Resources (NCRR) with a P20 RR-017696.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hon K. Yuen, Email: yuen@musc.edu, Division of Occupational Therapy, Department of Health Professions, College of Health Professions, Medical University of South Carolina, CHP Complex Bldg B, 151 Rutledge Ave., Charleston, SC 29425, United States Tel: 843-792-3788; Fax: 843-792-0710
Georgiana Onicescu, Email: onicescu@musc.edu, Division of Biostatistics and Epidemiology, Department of Medicine, Hollings Cancer Center, MUSC, Charleston, SC 29425, United States.
Elizabeth G. Hill, Email: hille@musc.edu, Division of Biostatistics and Epidemiology, Department of Medicine, Hollings Cancer Center, MUSC, Charleston, SC 29425, United States
Carolyn Jenkins, Email: Jenkinc@musc.edu, College of Nursing, MUSC, Charleston, SC 29425, United States.
References
- 1.Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc. 2006;137 (Suppl):26S–31S. doi: 10.14219/jada.archive.2006.0404. [DOI] [PubMed] [Google Scholar]
- 2.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodont 2000. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 3.Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 4.Mattson JS, Cerutis DR. Diabetes mellitus: a review of the literature and dental implications. Compend Contin Educ Dent. 2001;22:757–760. 762, 764. [PubMed] [Google Scholar]
- 5.Soell M, Hassan M, Miliauskaite A, Haikel Y, Selimovic D. The oral cavity of elderly patients in diabetes. Diabetes Metab. 2007;33 (Suppl 1):S10–18. doi: 10.1016/s1262-3636(07)80053-x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend ContinEduc Dent. 2004;25:179–184. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Dental visits among dentate adults with diabetes--United States, 1999 and 2004. MMWR Morbid Mortal Wkly Rep. 2005;54:1181–1183. [PubMed] [Google Scholar]
- 8.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodont. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 9.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodont. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 10.Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–141. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura F, Iwamoto Y, Mineshiba J, Shimizu A, Soga Y, Murayama Y. Periodontal disease and diabetes mellitus: the role of tumor necrosis factor-alpha in a 2-way relationship. J Periodontol. 2003;74:97–102. doi: 10.1902/jop.2003.74.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Perrino MA. Diabetes and periodontal disease: an example of an oral/systemic relationship. N Y State Dent J. 2007;73:38–41. [PubMed] [Google Scholar]
- 13.Daniels TE, Wu AJ. Xerostomia--clinical evaluation and treatment in general practice. CDA J. 2000;28:933–941. [PubMed] [Google Scholar]
- 14.Ciglar L, Skaljac G, Sutalo J, Keros J, Jankovic B, Knezevic A. Influence of diet on dental caries in diabetics. Coll Antropol. 2002;26:311–317. [PubMed] [Google Scholar]
- 15.Hallmon WW, Mealey BL. Implications of diabetes mellitus and periodontal disease. Diabetes Educ. 1992;18:310–315. doi: 10.1177/014572179201800409. [DOI] [PubMed] [Google Scholar]
- 16.Moore PA, Orchard T, Guggenheimer J, Weyant RJ. Diabetes and oral health promotion: a survey of disease prevention behaviors. J Am Dent Assoc. 2000;131:1333–1341. doi: 10.14219/jada.archive.2000.0388. [DOI] [PubMed] [Google Scholar]
- 17.Lee IC, Shieh TY, Yang YH, Tsai CC, Wang KH. Individuals’ perception of oral health and its impact on the health-related quality of life. J Oral Rehabil. 2007;34:79–87. doi: 10.1111/j.1365-2842.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg GE, Wikblad KF. Oral health and health-related quality of life in type 2 diabetic patients and non-diabetic controls. Acta Odontol Scand. 2003;61:141–148. doi: 10.1080/00016350310002559. [DOI] [PubMed] [Google Scholar]
- 19.Grossi S. Smoking and stress: common denominators for periodontal disease, heart disease, and diabetes mellitus. Compend Contin Educ Dent Suppl. 2000;30:31–39. [PubMed] [Google Scholar]
- 20.Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol. 2006;33:408–414. doi: 10.1111/j.1600-051X.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 21.Orbak R, Tezel A, Canakci V, Demir T. The influence of smoking and non-insulin-dependent diabetes mellitus on periodontal disease. J Int Med Res. 2002;30:116–125. doi: 10.1177/147323000203000203. [DOI] [PubMed] [Google Scholar]
- 22.Rees TD. Periodontal management of the patient with diabetes mellitus. Periodontol 2000. 2000;23:63–72. doi: 10.1034/j.1600-0757.2000.2230105.x. [DOI] [PubMed] [Google Scholar]
- 23.Almas K, Al-Lazzam S, Al-Quadairi A. The effect of oral hygiene instructions on diabetic type 2 male patients with periodontal diseases. J Contemp Dent Pract. 2003;4:24–35. [PubMed] [Google Scholar]
- 24.Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res. 2005;84:1154–1159. doi: 10.1177/154405910508401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M, Lee JY, Rozier RG. Oral health literacy among adult patients seeking dental care. J Am Dent Assoc. 2007;138:1199–1208. doi: 10.14219/jada.archive.2007.0344. [DOI] [PubMed] [Google Scholar]
- 26.Gregg EW, Geiss LS, Saaddine J, Fagot-Campagna A, Beckles G, Parker C, et al. Use of diabetes preventive care and complications risk in two African-American communities. Am J Prev Med. 2001;21:197–202. doi: 10.1016/s0749-3797(01)00351-8. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor PJ, Desai J, Rush WA, Cherney LM, Solberg LI, Bishop DB. Is having a regular provider of diabetes care related to intensity of care and glycemic control? J Fam Prac. 1998;47:290–297. [PubMed] [Google Scholar]
- 28.Solberg LI, Desai JR, O’Connor PJ, Bishop DB, Devlin HM. Diabetic patients who smoke: are they different? Ann Fam Med. 2004;2:26–32. doi: 10.1370/afm.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomar SL, Lester A. Dental and other health care visits among U.S. adults with diabetes. Diabetes Care. 2000;23:1505–1510. doi: 10.2337/diacare.23.10.1505. [DOI] [PubMed] [Google Scholar]
- 30.Thorstensson H, Falk H, Hugoson A, Kuylenstierna J. Dental care habits and knowledge of oral health in insulin-dependent diabetics. Scand J Dental Res. 1989;97:207–215. doi: 10.1111/j.1600-0722.1989.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 31.Karjalainen KM, Knuuttila ML, von Dickhoff KJ. Association of the severity of periodontal disease with organ complications in type 1 diabetic patients. J Periodont 2000. 1994;65:1067–1072. doi: 10.1902/jop.1994.65.11.1067. [DOI] [PubMed] [Google Scholar]
- 32.Basson WJ. Oral health education provided by oral hygienists in private practice. SADJ. 1999;54:53–57. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Take Charge of Your Diabetes. 3. Atlanta: U.S. Department of Health and Human Services; 2003. [Google Scholar]
- 34.McConaughy FL, Lukken KM, Toevs SE. Health promotion behaviors of private practice dental hygienists. J Dent Hyg. 1991;65:222–230. [PubMed] [Google Scholar]
- 35.McConaughy FL, Toevs SE, Lukken KM. Adult clients’ recall of oral health education services received in private practice. J Dent Hyg. 1995;69:202–211. [PubMed] [Google Scholar]
- 36.Milgrom P, Weinstein P, Chapko M, Grembowski D, Spadafora A. Dentists’ attitudes and behaviors in counseling patients about oral self care. J Am Coll of Dent. 1988;55:48–53. [PubMed] [Google Scholar]
- 37.Bartholomew DJ, Steele F, Moustaki I, Galbraith JI. The Analysis and Interpretation of multivariate data for social scientist. Boca Raton, FL: Chapman & Hall/CRC; 2002. pp. 207–234. [Google Scholar]
- 38.Joreskog KG, Sorbom D. LISREL 8: User’s Reference Guide. Lincolnwood, IL.: Scientific Software International; 1996. pp. 29–30. [Google Scholar]
- 39.Joreskog KG, Sorbom D. LISREL 8: Structural Equation Modeling with the Simplis Command Language. Chicago: Scientific Software International; 1998. pp. 122–123. [Google Scholar]
- 40.Joreskog KG. Latent Variable Scores and Their Uses. 2000 [Google Scholar]
- 41.Koerber A, Peters KE, Kaste LM, Lopez E, Noorullah K, Torres I, et al. The views of dentists, nurses and nutritionists on the association between diabetes and periodontal disease: a qualitative study in a Latino community. J Pub Health Dent. 2006;66:212–215. doi: 10.1111/j.1752-7325.2006.tb02583.x. [DOI] [PubMed] [Google Scholar]
- 42.American Diabetes Association. Summary of revisions for the 2009 Clinical Practice Recommendations. Diabetes Care. 2009;32 (Suppl 1):S3–5. doi: 10.2337/dc09-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]