Abstract
Purpose
The present study was undertaken to determine efficacy of phenethyl isothiocyanate (PEITC) for sensitization of androgen-independent human prostate cancer cells (AIPC) to Docetaxel-induced apoptosis using cellular and xenograft models.
Methods
Cell viability was determined by trypan blue dye exclusion assay. Microscopy and DNA fragmentation assay was performed to quantify apoptotic cell death in cultured cells. Protein levels were determined by immunoblotting. PC-3 prostate cancer xenograft model was utilized to determine in vivo efficacy of the PEITC and/or Docetaxel treatments.
Results
Pharmacologic concentrations of PEITC augmented Docetaxel-induced apoptosis in PC-3 and DU145 cells in association with suppression of Bcl-2 and XIAP protein levels and induction of Bax and Bak. The PEITC-Docetaxel combination was markedly more efficacious against PC-3 xenograft in vivo compared with PEITC or Docetaxel alone. Significantly higher count of apoptotic bodies were also observed in tumor sections from mice treated with the PEITC-Docetaxel combination compared with PEITC or Docetaxel alone group. The PEITC and/or Docetaxel-mediated changes in the levels of apoptosis regulating proteins in the tumor were generally consistent with the molecular alterations observed in cultured cells.
Conclusion
These results offer obligatory impetus to test PEITC-Docetaxel combination for the treatment of AIPC in a clinical setting.
Keywords: Prostate Cancer, Phenethyl Isothiocyanate, Docetaxel, Apoptosis
INTRODUCTION
Prostate cancer is one of the most commonly diagnosed visceral malignancies and a leading cause of cancer-related deaths among men in the United States (1). Molecular mechanisms underlying onset and progression of prostate cancer are not fully understood, but the factors implicated in pathogenesis of this devastating disease include age, race, diet, androgen secretion and metabolism, and activated oncogenes (2-4). Early detection techniques (e.g., screening for prostate specific antigen and digital rectal exams) have undoubtedly improved the survival of prostate cancer patients by permitting treatment of localized disease (4,5). Early stage prostate cancer is responsive to androgen ablation therapy (5,6). However, this treatment modality is palliative and often leads to disease recurrence (6,7). Furthermore, nearly all recurrent prostate cancers eventually transition to androgen-independent state (also referred to as “castration-resistant” or “hormone-refractory”) that is highly aggressive, resistant to chemotherapy, and unfortunately lethal (4,8). The mechanisms underlying transition of hormone-dependent prostate cancer to androgen independence is not fully understood, but both androgen-dependent and -independent signaling by the androgen receptor and upregulation of pro-survival pathways have been implicated in this phenomenon (8-11).
Docetaxel (Taxotere®) has become the treatment of choice for management of androgen-independent prostate cancer (AIPC) (12,13). Although implementation of Docetaxel-based regimen has significantly improved survival of AIPC patients, the response rate is not overwhelming (12,13). Moreover, Docetaxel is severely dose-limited due to adverse side effects including neutropenia, diarrhea, nausea, and fatigue (12). Clearly novel combinations to reduce dose-limiting toxicity of Docetaxel and/or to increase its efficacy are highly desirable and could have a significant impact on disease-related cost, morbidity, and mortality for a large segment of population. Natural products have received increasing attention in recent years for the discovery of novel cancer chemotherapeutics agents (14,15).
Epidemiological data continue to support the premise that dietary intake of cruciferous vegetables may reduce the risk of different malignancies including cancer of the prostate (16,17). Anticancer effect of cruciferous vegetables is attributed to organic isothiocyanates (ITCs) that are generated due to hydrolysis of corresponding glucosinolates abundant in many edible plants including watercress and broccoli (18). Phenethyl isothiocyanate (PEITC) is one such ITC compound that has attracted a great deal of research interest due to its anticancer effects. Known anticancer effects of PEITC include (a) prevention of cancer development in animal models induced by chemical carcinogens (e.g., constituents of cigarette smoke) (19-21), (b) suppression of cancer cell viability in association with cell cycle arrest, apoptosis induction, and autophagic cell death (22-30), and (c) inhibition of angiogenesis in vitro and ex vivo (31).
In the present study, we used cellular (PC-3 and DU145) and xenograft (PC-3 xenograft) models to test efficacy of PEITC for sensitization of AIPC to Docetaxel-induced apoptosis. We provide first preclinical evidence of synergy between pharmacologic concentrations of PEITC and Docetaxel for growth suppression as well as apoptosis induction in human AIPC cells in vitro as well as in vivo. These preclinical results provide obligatory impetus to test PEITC-Docetaxel combination for the treatment of AIPC in a clinical setting.
MATERIALS AND METHODS
Reagents
PEITC (purity >98%) and Docetaxel were purchased from LKT Laboratories (St. Paul, MN). Cell culture reagents and fetal bovine serum (FBS) were purchased from Life Technologies (Carlsbad, CA); 4',6-diamidino-2-phenylindole (DAPI) was from Sigma-Aldrich (St. Louis, MO); and a kit for quantification of cytoplasmic histone-associated DNA fragmentation was purchased from Roche Diagnostics- USA (Indianapolis, IN). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) kit was from Chemicon International-Millipore (Billerica, MA). Caspase-3 activation was measured by flow cytometry using a kit from Cell Signaling Technology (Danvers, MA). The antibodies against Bak and Bax were from Santa Cruz Biotechnology (Santa Cruz, CA); the antibodies against Bcl-2 and proliferating cell nuclear antigen (PCNA) were from DakoCytomation (Carpinteria, CA); and the anti-XIAP antibody was from BD Biosciences Pharmingen (San Diego, CA).
Cell Culture and Cell Viability Assay
The PC-3 and DU145 cells were procured from the American Type Culture Collection (Manassas, VA), and maintained as described by us previously (25,26,28,32). Parental HCT-116 cells and its XIAP deficient variant (HCT116-XIAP−/−) were maintained in McCoy's 5A medium supplemented with 10% (v/v) FBS and antibiotics. Stock solutions of PEITC and Docetaxel were prepared in dimethyl sulfoxide (DMSO) and diluted with complete medium. An equal volume of DMSO (final concentration <0.05%) was added to the controls. Effect of PEITC and/or Docetaxel treatments on cell viability was determined by trypan blue dye exclusion assay as described by us previously (32).
Determination of Apoptosis and Membrane Potential
Apoptosis induction by PEITC and/or Docetaxel treatments was assessed by fluorescence microscopy, and cytoplasmic histone-associated DNA fragmentation and caspase-3 activation assays as described by us previously (32,33). The effects of PEITC and/or Docetaxel treatments on mitochondrial membrane potential was determined by flow cytometry using 5,5',6,6'- tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Molecular Probes, Eugene, OR) as described by us previously (28).
Immunoblotting
Cell lysates and tumor supernatants were prepared as described by us previously (34,35). Immunoblotting was performed as described by us previously (34,35). Change in protein level was determined by densitometric scanning of the immunoreactive bands followed by correction for actin loading control.
Xenograft Assay
Male athymic mice (6-week old) were purchased from Harlan Sprague Dawley (Indianapolis, IN). Exponentially growing PC-3 cells with stable expression of luciferase (Caliper Life Sciences, Mountain View, CA) were mixed in a 1:1 ratio with Matrigel and a 0.1 mL suspension containing 3×106 cells was injected subcutaneously on flank of each mouse. Mice were randomized into four groups of five mice per group and treatment was started on the day of tumor cell injection. Group 1 (control) mice received 0.1 mL PBS by gavage five times per week (Monday-Friday) and 0.1 mL PBS by intraperitoneal injections three times at weekly intervals. Group 2 mice were gavaged with 9 μmol PEITC in 0.1 mL PBS five times per week (Monday-Friday). Group 3 mice were intraperitoneally injected with 10 mg Docetaxel/kg body weight in 0.1 mL PBS three times at weekly intervals. Group 4 mice were treated with PEITC and Docetaxel as described above for mice of groups 2 and 3. The experiment was terminated 38 days after tumor cell injection. Tumor size was measured using a caliper as described by us previously (36). Body weights of the control and treated mice were recorded periodically. Mice of each group were also monitored every other day for symptoms of side effects including food and water withdrawal, ruffled fur, and impaired posture or movement.
The in vivo xenograft experiment was repeated with some modifications. In the second experiment, 6-week old male athymic mice were subcutaneously implanted with PC-3 cells as described above in the first experiment. Fifteen days after tumor cell injection when average tumor volume approached nearly 50 mm3, the mice were randomized into four groups. Mice of control group (group 1) were gavaged with 0.1 mL PBS five times per week (Monday-Friday) and 0.1 mL PBS on day 15, 22 and 29 after tumor cell implantation (n= 6). The group 2 mice were gavaged with 6 μmol PEITC in 0.1 mL PBS by gavage five times per week (Monday-Friday; n= 7). Mice of group 3 were treated with 5 mg Docetaxel/kg body weight by intraperitoneal injection on day 15, 22 and 29 after tumor cell implantation (n= 6). The mice of group 4 were treated with PEITC and Docetaxel as described above (n= 7). The experiment was terminated on day 38 after tumor cell implantation.
TUNEL Assay and Immunohistochemical Analysis of PCNA Expression
Apoptotic bodies in the tumor sections were visualized by TUNEL assay as described by us previously (37). Immunohistochemical analysis of PCNA expression was performed as previously described by us (37).
Statistical Analysis
The t-test or ANOVA was used to test the significance of difference in measured variables between control and treated groups. Difference was considered significant at P= 0.05.
RESULTS
PEITC Sensitized PC-3 and DU145 Cells to Growth Suppression by Docetaxel
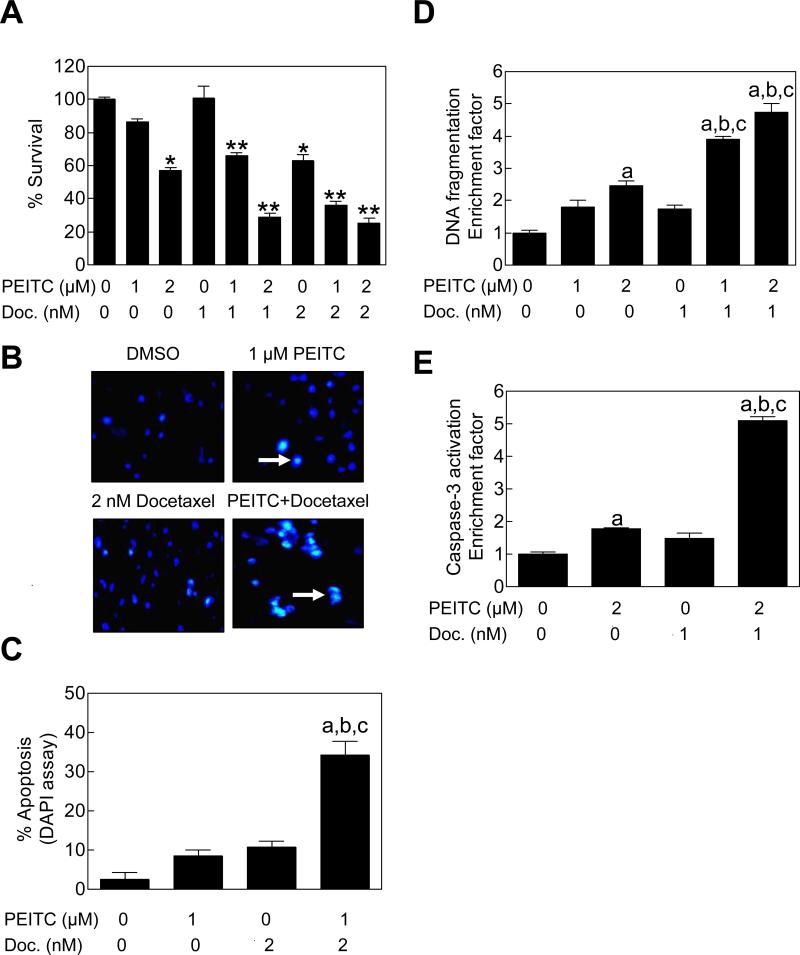
We have shown previously that PEITC treatment inhibits growth of PC-3 cells by causing apoptotic cell death (25,28). In the present study, we used the same cell line to test if growth inhibition by Docetaxel is increased in the presence of PEITC. Fig. 1A shows viability of PC-3 cells following 24 h exposure to PEITC and/or Docetaxel as judged by trypan blue dye exclusion assay. The PEITC and Docetaxel combination was significantly more efficacious against viability of PC-3 cells compared with PEITC or Docetaxel treatment alone. Further analysis of the results using the method described by Lee et al (38) revealed that the PC-3 cell growth suppression by Docetaxel was synergistically augmented in the presence of PEITC (Table 1). For example, viability of PC-3 cells was marginally affected in the presence of 1 nM Docetaxel alone, while exposure to 2 μM PEITC resulted in about 43% inhibition of cell survival compared with DMSO-treated control (Table 1). The viability of PC-3 cells was reduced by about 71% by a 24 h co-treatment with 2 μM PEITC and 1 nM Docetaxel in comparison with vehicle-treated control cells with observed combination index of 1.97 indicating synergy between PEITC and Docetaxel (Table 1).
Figure 1.
PEITC sensitized PC-3 cells to growth inhibition and apoptosis induction by Docetaxel. A, Survival (trypan blue dye exclusion assay) of PC-3 cells following 24 h treatment with DMSO (control) or the indicated concentrations of PEITC and/or Docetaxel. Results shown are mean ± SE (n= 3). Significantly different (P<0.05) compared with *DMSO-treated control; and **corresponding PEITC alone as well as Docetaxel alone group. B, Visualization of apoptotic cells (identified by arrows) by DAPI staining (40× magnification) in PC-3 cells treated for 24 h with DMSO (control) or the indicated concentrations of PEITC and/or Docetaxel. C, Quantitation of apoptotic cells (DAPI assay) following 24 h treatment with the indicated concentrations of PEITC and/or Docetaxel. D, Analysis of cytoplasmic histone-associated DNA fragmentation, and E, analysis of caspase-3 activation in PC-3 cells treated for 24 h with the indicated concentrations of PEITC and/or Docetaxel. In panels C-E, results shown are mean ± SE (n= 3-4). Significantly different (P<0.05) compared with aDMSO-treated control, bPEITC alone, and cDocetaxel alone by one-way ANOVA followed by Bonferroni's or Tukey's test.
Table 1.
Analysis of synergy between phenethyl isothiocyanate (PEITC) and Docetaxel combination against PC-3 and DU145 cell proliferation.
| PC-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Docetaxel |
PEITC |
Combination treatment |
Indexe | ||||||
| Dose | MSRa | pb | Dose | MSR | p | expectedc | observedd | p | |
| 1 nM | 1.00 | >0.05 | 1 μM | 0.86 | >0.05 | 0.86 | 0.66 | <0.01 | 1.30 |
| 1 nM | 1.00 | >0.05 | 2 μM | 0.57 | <0.001 | 0.57 | 0.29 | <0.001 | 1.97 |
| 2 nM | 0.63 | <0.001 | 1 μM | 0.86 | >0.05 | 0.54 | 0.37 | <0.001 | 1.46 |
| 2 nM | 0.63 | <0.001 | 2 μM | 0.57 | <0.001 | 0.36 | 0.29 | <0.001 | 1.24 |
| DU145 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Docetaxel |
PEITC |
Combination treatment |
Indexe | ||||||
| Dose | MSRa | pb | Dose | MSR | p | expectedc | observedd | p | |
| 1 nM | 0.99 | >0.05 | 1 μM | 0.97 | >0.05 | 0.96 | 0.94 | >0.05 | 1.02 |
| 1 nM | 0.99 | >0.05 | 2 μM | 0.89 | >0.05 | 0.90 | 0.78 | >0.05 | 1.12 |
| 2 nM | 0.80 | >0.05 | 1 μM | 0.97 | >0.05 | 0.78 | 0.71 | <0.05 | 1.10 |
| 2 nM | 0.80 | >0.05 | 2 μM | 0.89 | >0.05 | 0.73 | 0.56 | <0.001 | 1.30 |
MSR: mean survival rate compared with DMSO-treated control group.
p value was determined by one-way ANOVA followed by Tukey's test.
survival rate of Docetaxel-treated group multiplied by that of the PEITC-treated group.
survival rate of combination treatment group.
Index was calculated by dividing the expected survival rate by the observed survival rate. An index of >1 indicates synergistic effect and an index <1 indicates less than additive effect.
Next, we raised the question of whether the PEITC-mediated sensitization to growth suppression by Docetaxel was a cell line-specific response unique to the PC-3 cell line. We addressed this question using DU145 cell line, which is another well-accepted cellular model of human AIPC. The PEITC-mediated synergistic sensitization to growth inhibition by Docetaxel was also evident in the DU145 cell line (Table 1). Collectively, these observations indicated that the PEITC and Docetaxel combination was synergistic against proliferation of cultured PC-3 and DU145 cells.
PEITC Sensitized Cultured PC-3 and DU145 Cells to Docetaxel-induced Apoptosis
We proceeded to test whether the PEITC-mediated sensitization of human AIPC cells to growth inhibition by Docetaxel was due to increased apoptosis. This possibility was likely considering apoptosis induction is the main mechanism by which both PEITC and Docetaxel kill cancer cells (15,25). We explored this possibility by fluorescence microscopy to visualize apoptotic cells with condensed and fragmented DNA (DAPI assay). Fig. 1B depicts representative fluorescence microscopic images of PC-3 cells treated for 24 h with DMSO (control), 1 μM PEITC, 2 nM Docetaxel, and PEITC+Docetaxel combination (apoptotic cells are marked with arrows). A 24 h exposure of PC-3 cells to 1 and 2 μM PEITC alone or 2 nM Docetaxel alone was marginally proapoptotic compared with DMSO-treated control. The apoptotic fraction with condensed and fragmented DNA was significantly higher in PC-3 cells exposed to the PEITC-Docetaxel combination when compared with either DMSO-treated control or PEITC and Docetaxel treatment alone (Fig. 1C). The results of DAPI assay were verified by analysis of cytoplasmic histone-associated DNA fragmentation (Fig. 1D) and caspase-3 activation (Fig. 1E), which are well-accepted and reliable techniques for quantitation of apoptotic cell death. In both these assays, the PEITC-Docetaxel combination was significantly more efficacious in eliciting apoptotic response compared with control as well as PEITC or Docetaxel alone.
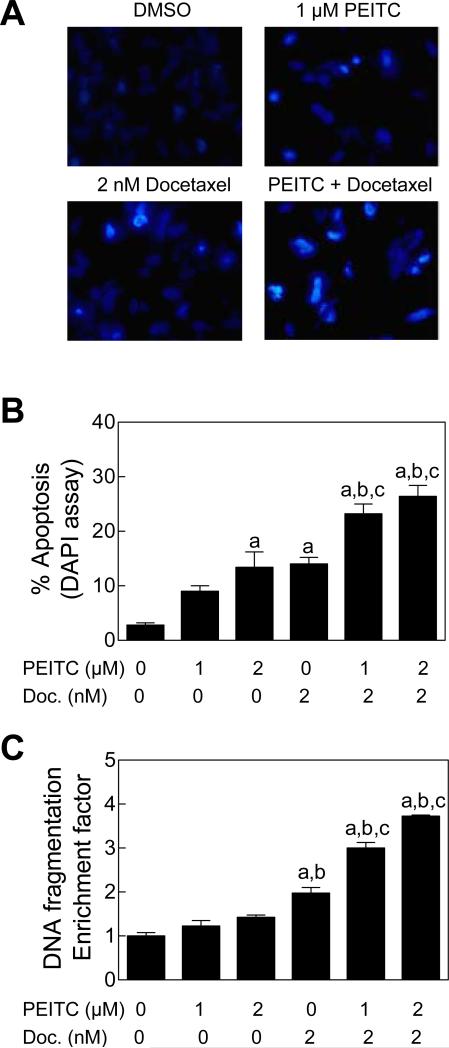
We designed experiments using DU145 cell line to test generality of these observations. Similar to the PC-3 cell line (Fig. 1), a 24 h exposure of DU145 cells to the PEITC (1 or 2 μM) and Docetaxel (2 nM) combination resulted in statistically significantly higher apoptotic cell death compared with DMSO-treated control, PEITC alone, and Docetaxel alone as judged by DAPI assay (Fig. 2A and 2B) and analysis of cytoplasmic histone-associated DNA fragmentation (Fig. 2C). Collectively, these results indicated that the PEITC-mediated sensitization to growth inhibition by Docetaxel was indeed due to increased apoptosis.
Figure 2.
PEITC sensitized DU145 cells to apoptosis induction by Docetaxel. A, Visualization of apoptotic cells by DAPI assay (40× magnification) in DU145 cells treated for 24 h with DMSO, 1 μM PEITC, 2 nM Docetaxel, or the PEITC-Docetaxel combination. B, Quantitation of apoptotic cells (DAPI assay) in DU145 cultures treated for 24 h with PEITC and/or Docetaxel (mean ± SE, n= 6). C, Analysis of cytoplasmic histone-associated DNA fragmentation in DU145 cells following 24 h treatment with PEITC and/or Docetaxel (mean ± SE, n= 3). Significantly different (P<0.05) compared with aDMSO-treated control, bPEITC, and cDocetaxel by one-way ANOVA followed by Tukey's test.
Effects of PEITC and/or Docetaxel Treatments on Expression of Bcl-2 Family Proteins
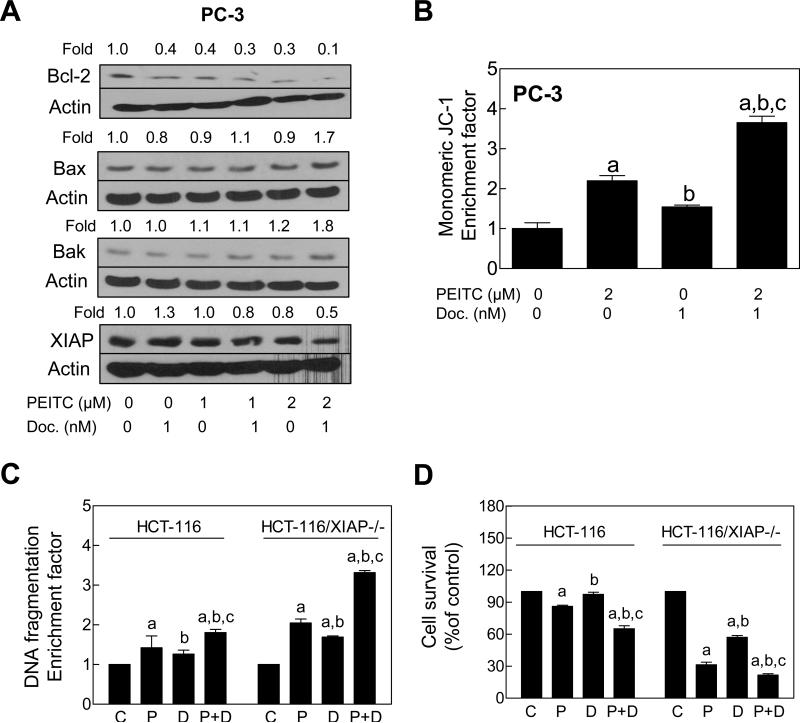
The Bcl-2 family proteins have emerged as critical regulators of apoptosis by functioning as either promoters (e.g., Bax and Bak) or inhibitors (e.g., Bcl-2) of the cell death process. We have shown previously that the PEITC-induced cell death in prostate cancer cells is associated with changes in protein levels of Bcl-2 family of anti-apoptotic and/or pro-apoptotic proteins favoring apoptosis (27,28). To gain insight into the mechanism behind increased apoptosis by PEITC-Docetaxel combination, we performed immunoblotting for Bcl-2 family proteins using lysates from PC-3 cells treated for 24 with 1 nM Docetaxel in the absence or presence of 1 or 2 μM PEITC. Both Docetaxel and PEITC treatments resulted in down-regulation of Bcl-2 protein, which was exacerbated by co-treatment with these agents (Fig. 3A). The levels of multidomain proapoptotic Bcl-2 family members Bax and Bak were not altered appreciably by treatment with Docetaxel alone or PEITC alone. On the other hand, the levels of these proteins were increased by about 1.7 to 1.8-fold by co-treatment with PEITC and Docetaxel in comparison with DMSO-treated control (Fig. 3A). Finally, Docetaxel alone resulted in a modest increase in the protein level of XIAP, which was abrogated in the presence of PEITC especially at the 2 μM dose.
Figure 3.
XIAP protected against PEITC-mediated sensitization to Docetaxel. A,Immunoblotting for Bcl-2, Bax, Bak, and XIAP proteins using lysates from PC-3 cells treated for 24 h with PEITC and/or Docetaxel. Numbers on top of the bands represent change in protein level relative to DMSO-treated control (first lane). B, Flow cytometric analysis of mitochondrial membrane potential (monomeric JC-1 associated fluorescence) in PC-3 cells treated for 6 h with 2 μM PEITC and/or 1 nM Docetaxel (mean ± SE, n= 3). C, Cytoplasmic histone-associated DNA fragmentation, and D, cell viability in parental HCT-116 cells and its XIAP−/− variant (HCT-116/XIAP−/−) following 24 h treatment with DMSO, 2 μM PEITC (P), 1 nM Docetaxel (D), or the combination of PEITC and Docetaxel (P+D) Results shown are mean ± SE (n= 3). Significantly different (P<0.05) compared with aDMSO-treated control, bPEITC, and cDocetaxel by one-way ANOVA followed by Bonferroni's test.
Because co-treatment with PEITC and Docetaxel resulted in up-regulation of Bax and Bak (Fig. 3A), we designed experiments to test whether PEITC-mediated sensitization to Docetaxel-induced apoptosis was associated with disruption of mitochondrial membranepotential. Disruption of the mitochondrial membrane potential, judged by flow cytometric analysis of monomeric JC-1 associated fluorescence, was much more pronounced in PC-3 cells treated for 6 h with the PEITC-Docetaxel combination compared with PEITC or Docetaxel alone treatment group (Fig. 3B).
We used wild-type HCT-116 cells and its isogenic XIAP-knockout variant (HCT-116/XIAP−/−) to further test role of XIAP in PEITC-mediated sensitization to Docetaxel-induced apoptosis. The PEITC-mediated sensitization to Docetaxel-induced apoptosis (Fig. 3C) as well as growth suppression (Fig. 3D) was much more pronounced in the HCT-116/XIAP−/− cell line compared with the wild-type HCT-116 cells. Collectively, these results indicated excellent correlation between increased apoptosis induction (Figs. 1 and 2) and changes in levels of Bcl-2 family proteins and XIAP (Fig. 3) by the PEITC-Docetaxel combination.
Effects of Docetaxel and/or PEITC Treatments on Growth of PC-3 Xenografts
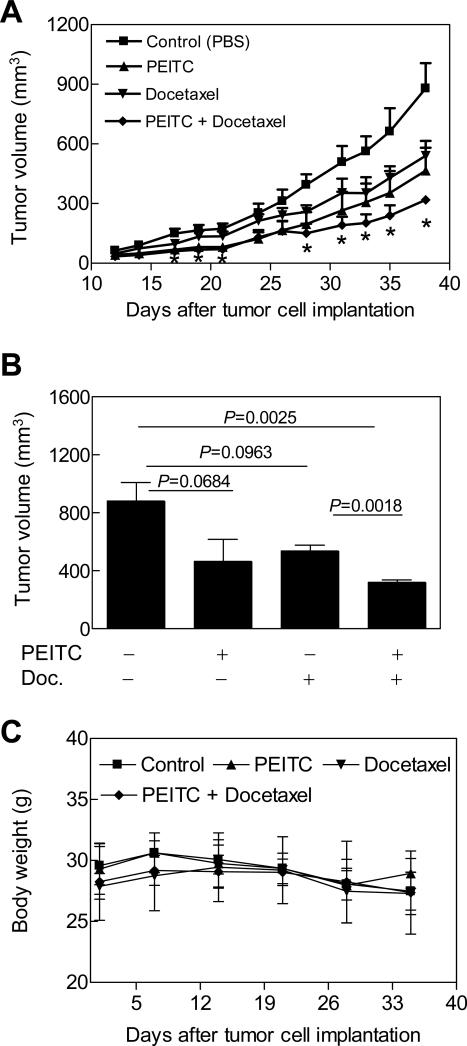
In vivo validation of the cellular observations is essential for logical design of promising combination regimens for future clinical investigations. We therefore designed a xenograft study to determine the in vivo anticancer effects of PEITC and/or Docetaxel treatments using the PC-3 cell line. The doses of the PEITC and Docetaxel were selected from published literature (39,40). Tumor did not grow in one mouse of the Docetaxel alone treatment group. One mouse from the Docetaxel alone treatment group died on day 31 after tumor cell implantation possibly due to toxicity. Even though the average tumor volume in mice treated with PEITC alone or Docetaxel alone was generally lower compared with vehicle-treated control mice, the differences were statistically insignificant on most days of tumor measurement (Fig. 4A). On the other hand, the average tumor volume in mice treated with the combination of PEITC and Docetaxel was statistically significantly lower in comparison with control mice on several days of tumor measurement (Fig. 4A). For example the average tumor volume in mice of control group on day 38 (day of sacrifice) was 879 ± 127 mm3, which was approximately 2.8-fold higher compared with that of the PEITC-Docetaxel combination group (Fig. 4B). Consistent with these results, average wet weight of the tumors excised from control mice was also about 2.6-fold higher compared with that of the PEITC-Docetaxel combination group (results not shown). The body weights of the control and PEITC and/or Docetaxel-treated mice did not differ significantly throughout the experimental period (Fig. 4C). Moreover, none of the mice of any group exhibited any other signs of overt toxicity.
Figure 4.
Effects of PEITC and/or Docetaxel treatments on growth of PC-3 xenografts in male athymic mice. A, Average tumor volume over time, B, average tumor volume on the day of sacrifice, and C, average body weight over time in mice treated with PBS (control), 9 μmol PEITC five times per week by gavage (Monday-Friday), 10 mg Docetaxel/kg body weight by i.p. injections, once per week for three weeks, or the PEITC and Docetaxel combination. Results shown are mean ± SE (n= 5 except for the Docetaxel alone group where n= 3). *Significantly different (P<0.05) compared with control by t-test.
In a different in vivo experiment involving a slightly modified treatment protocol (6 μmol PEITC by gavage 5 times per week and 5 mg Docetaxel/kg body weight by intraperitoneal injection on days 15, 22 and 29 after tumor cell implantation with treatments starting when average tumor volume was about 50 mm3), average tumor volume on the last day of measurement (38 days after tumor cell injection) in mice treated with the PEITC-Docetaxel combination (564 ± 114 mm3) was significantly lower compared with control mice (1071 ± 148 mm3; P= 0.019 by t-test), PEITC alone treated mice (1043 ± 71 mm3; P= 0.004 by t-test), and Docetaxel alone treated mice (931 ± 126 mm3; P= 0.053 by t-test) (results not shown). There were no deaths in any group in the second experiment. These results indicated that the PEITC-Docetaxel combination was markedly more efficacious against PC-3 xenograft compared with control as well as PEITC or Docetaxel alone.
Increased Tumor Apoptosis In Vivo by the PEITC-Docetaxel Combination Treatment
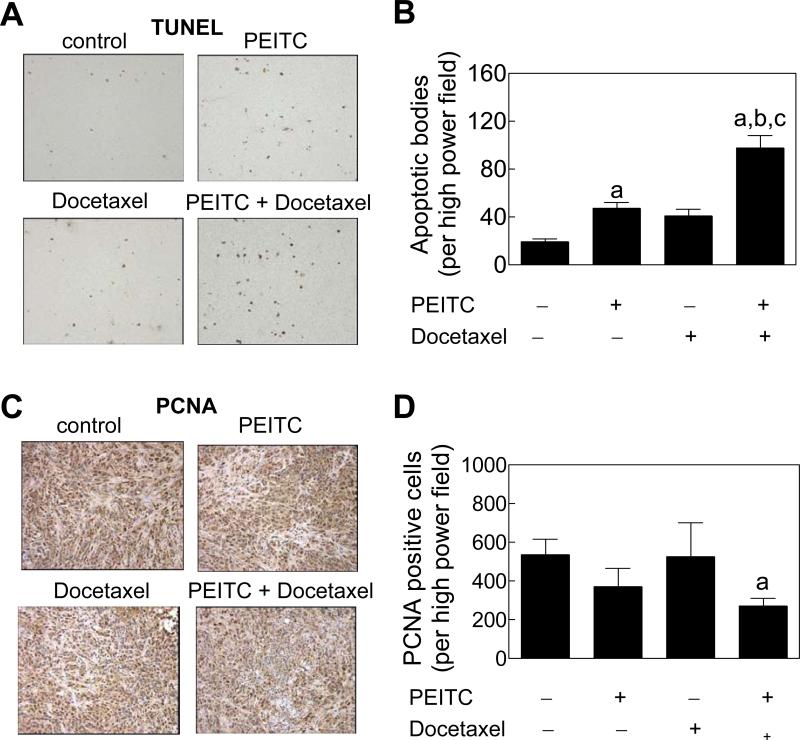
Fig. 5A depicts TUNEL-positive apoptotic bodies in representative tumor sections from control and PEITC and/or Docetaxel treatment groups. Number of TUNEL-positive apoptotic bodies in tumor sections from mice treated with the PEITC-Docetaxel combination was significantly higher in comparison with control as well as PEITC or Docetaxel alone treatment groups (Fig. 5B). For example, average number of TUNEL-positive apoptotic bodies in tumor sections from the PEITC-Docetaxel combination group was higher by about 5.0-, 2.1- and 2.4-fold, respectively, compared with control, PEITC alone, and Docetaxel alone groups (P<0.001).
Figure 5.
Apoptosis induction and proliferating cell nuclear antigen (PCNA) expression in the tumor sections. Microscopic images depicting A, TUNEL-positive apoptotic bodies, and C, PCNA expression in representative tumor section of the indicated group. Quantitation of B,TUNEL-positive apoptotic bodies/high power field, and D, PCNA expression/high power field in tumor sections from control, and PEITC and/or Docetaxel-treated mice. Results shown are mean ± SE (n= 3). Significantly different (P<0.001 for panel B and P<0.05 for panel D) compared with acontrol, bPEITC alone, and cDocetaxel alone group. Tumor sections from 3 individual mice of each group were examined.
Fig. 5C shows immunohistochemical analysis for PCNA expression in representative tumor sections of control and PEITC and/or Docetaxel-treated mice. The PEITC-Docetaxel combination treatment caused ~45% decrease (P= 0.045 compared with control by t-test) in tumor PCNA expression compared with control (Fig. 5D). Collectively, these results indicated that the PEITC-Docetaxel combination was significantly more proapoptotic compared with other treatments as revealed by the TUNEL assay.
Effects of PEITC and/or Docetaxel Treatments on Tumor Levels of Bcl-2 Family Proteins
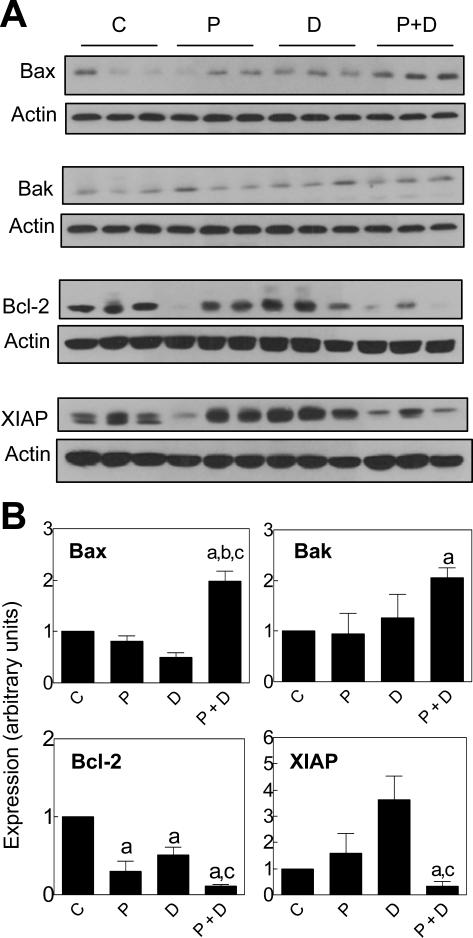
Tumor tissues harvested from three mice of each group (first xenograft study) were examined for expression of Bax, Bak, Bcl-2, and XIAP by immunoblotting to gain insight into the mechanism of increased apoptosis by the combination treatment. As can be seen in Fig. 6A, the PEITC and/or Docetaxel-mediated changes in levels of apoptosis regulating proteins in the tumor tissue were generally in good agreement with the molecular alterations observed in cultured PC-3 cells. For example, the PEITC-Docetaxel combination treatment caused a statistically significant increase in protein level of Bax compared with control and PEITC or Docetaxel alone treatment groups. The level of Bak protein was significantly higher in tumor of mice treated with the PEITC-Docetaxel combination compared with control mice. Likewise, down-regulation of Bcl-2 was relatively more pronounced in the tumors from PEITC-Docetaxel combination group compared with other groups (Fig. 6B). Finally, the level of XIAP protein was reduced significantly in tumors from PEITC-Docetaxel treated mice compared with control and Docetaxel alone groups (Fig. 6B). These results clearly indicated that Bcl-2 family proteins and XIAP are targets of PEITC-mediated sensitization of PC-3 xenografts to apoptosis induction by Docetaxel in vivo.
Figure 6.
Expression of Bax, Bak, Bcl-2, and XIAP proteins in tumor supernatants. A, Immunoblotting for Bax, Bak, Bcl-2, and XIAP using tumor supernatants from control mice and those treated with PEITC and/or Docetaxel. B, Quantitation of Bax, Bak, Bcl-2, and XIAP protein levels by densitometric scanning of the immunoreactive bands from control group (c); PEITC alone group (P); Docetaxel alone group (D); and the PEITC-Docetaxel combination group (P+D). Tumor tissues from 3 mice of each group were used for immunoblotting. Results shown are mean ± SE (n= 3). Significantly different (P<0.05) compared with acontrol, bPEITC alone, and cDocetaxel alone group.
DISCUSSION
Docetaxel remains the treatment of choice for AIPC with both symptomatic and survival benefits in men with metastatic cancer (12,13,41). Clinical benefit of Docetaxel is limited by adverse side effects (12). Clearly novel combinations to reduce dose-limiting toxicity of Docetaxel and/or to increase its efficacy are highly attractive. Such a strategy could especially benefit elderly population by allowing longer and/or frequent dosing regimens to more effectively treat AIPC. Results presented herein demonstrate that a constituent of cruciferous vegetables (PEITC) synergizes with Docetaxel against cultured human AIPC cells. We also found that the PEITC-mediated sensitization of AIPC cells to growth suppression by Docetaxel is intimately linked to increased apoptotic cell death both in cultured PC-3 and DU145 cells in vitro as revealed by DAPI assay and analysis of cytoplasmic histone-associated DNA fragmentation and in PC-3 tumor xenografts in vivo as judged by TUNEL assay. Moreover, the PEITC-Docetaxel combination is well-tolerated by the mice without weight loss or any other signs of overt toxicity. To the best of our knowledge, the present study is the first published report to document synergistic anticancer effect of PEITC and Docetaxel against AIPC in vivo. It is important to point out that the PEITC-mediated sensitization to Docetaxel-induced apoptosis occurs at pharmacologic concentrations in both PC-3 and DU145 cells (42-44).
The PEITC-mediated sensitization to growth suppression by Docetaxel was relatively stronger in the PC-3 cell line than in the DU145 cells (Table 1). Precise mechanism underlying this divergence remains to be elucidated, but most likely attributable to differential expression of pro-survival factors. One such possibility relates to constitutive activation of signal transducer and activator of transcription 3 (STAT3), which is an oncogenic transcription factor implicated in development and progression of various cancers including prostate cancer (45). The STAT3 is constitutively active in the DU145 cell line, but not in the PC-3 cells (46). It is possible that the constitutively active STAT3 hinders PEITC-mediated sensitization to growth suppression by Docetaxel in the DU145 cells. Likewise, the PC-3 cell line lacks expression of tumor suppressor PTEN, which results in constitutive activation of Akt in this cell line (47). On the other hand, serine-threonine kinase Akt is not constitutively active in DU145 cells due to robust expression of PTEN (47). Thus difference in constitutive activation of Akt between PC-3 and DU145 cells may also contribute to their differential sensitivity to the PEITC-Docetaxel combination. However, further studies are needed to systematically explore these mechanistically intriguing possibilities.
Docetaxel directly binds to the β-subunit of tubulin and alter microtubule dynamics, which ultimately leads to mitotic arrest and apoptosis (48). However, chemosensitivity to Docetaxel is also influenced by other prosurvival molecules including Bcl-2 , Stat1, and PIM1 kinase (49,50). Apoptotic response to PEITC in prostate cancer cells is accompanied by a change in the ratio of proapoptotic-anti-apoptotic Bcl-2 family members (27,28). The present study reveals that the PEITC-mediated sensitization of prostate cancer cells to Docetaxel-induced apoptosis shows excellent correlation with suppression of Bcl-2 and induction of Bax and Bak not only in cultured cells but also in the PC-3 tumor in vivo. For example, the Docetaxel-mediated suppression of Bcl-2 in cultured PC-3 cells is exacerbated in the presence of PEITC. However, the molecular mechanism underlying changes in levels of Bcl-2 family proteins upon treatment with PEITC-Docetaxel combination is yet to be determined. Nonetheless, these results suggest that Bcl-2, Bax and Bak represent valid targets to assess response to PEITC-Docetaxel combination in future clinical trials.
The XIAP protein may be another biomarker to assess response to PEITC-Docetaxel combination treatment. The XIAP is a direct inhibitor of executioner caspase-3 and initiator caspase-7 (51). The XIAP is considered as a valid therapeutic target (52). For example, XIAP inhibition has been shown to increase chemotherapy sensitivity in cancer cells (53). Increased expression of XIAP has been shown in prostate cancer biopsy specimens from all stages of the disease (54). In addition, it has been suggested that inhibition of XIAP expression may reinforce the apoptotic effect of neo-adjuvant hormonal therapy in prostate cancer patients (55). We found that Docetaxel alone treatment causes modest yet marked induction of XIAP protein level in both cultured PC-3 cells and xenograft. Interestingly, the Docetaxel-mediated induction of XIAP is not only nullified in the presence of PEITC but the combination treatment results in robust down-regulation of this protein. Expression of XIAP is regulated by the transcription factor nuclear factor-κB (NF-κB; 56). Because Docetaxel treatment can activate NF-κB in cancer cells (15,57), the possibility that the XIAP induction by Docetaxel administration in the tumor in vivo (Fig. 6A) is mediated by NF-κB can not be excluded. It is equally plausible that the suppression of XIAP expression by the PEITC-Docetaxel combination is also related to inhibition of NF-κB because PEITC inhibits NF-κB-regulated gene expression in cultured PC-3 cells (30). Further studies are needed to experimentally verify these possibilities.
In conclusion, the present study demonstrates that combination of PEITC with Docetaxel results in greater than additive apoptotic cell killing of cultured PC-3 and DU145 cells and superior antitumor activity against PC-3 xenograft in vivo compared with single agent alone. We are hopeful that our preclinical results will spark interest to test this combination regimen in a clinical setting.
ACKNOWLEDGMENTS
The authors thank Julie A. Arlotti and Yan Zeng for technical assistance, and Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD) for generous gift of HCT-116 cells and its XIAP−/− variant.
Grant support: This investigation was supported by the USPHS grant CA101753 (to S.V.S.), awarded by the National Cancer Institute.
REFERENCES
- 1.Jemal A, Siegal R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ross RK, Henderson BE. Do diet and androgens alter prostate cancer risk via a common etiologic pathway. J Natl Cancer Inst. 1994;86:252–4. doi: 10.1093/jnci/86.4.252. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–61. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 4.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff P. Recent progress in management of advanced prostate cancer. Oncology. 2005;19:631–6. [PubMed] [Google Scholar]
- 6.Labrie F, Dupont A, Belanger A, et al. New approaches in the treatment of prostate cancer: complete instead of partial withdrawal of androgens. Prostate. 1983;4:579–94. doi: 10.1002/pros.2990040605. [DOI] [PubMed] [Google Scholar]
- 7.Laufer M, Denmeade SR, Sinibaldi VJ, Carducci MA, Eisenberger MA. Complete androgen blockade for prostate cancer. What went wrong? J Urol. 2000;164:3–9. [PubMed] [Google Scholar]
- 8.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 9.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Furihata M, Tsunoda T, et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67:5117–25. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 11.Jin RJ, Lho Y, Connelly L, et al. The nuclear factor-κB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762–9. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Eng J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 13.Petrylak DP. New paradigms for advanced prostate cancer. Rev Urol. 2007;9(suppl 2):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- 14.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor κB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–42. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 16.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 17.Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 18.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochem. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 19.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–8. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 20.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989;49:2894–7. [PubMed] [Google Scholar]
- 21.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, Wagner SA. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51:2063–8. [PubMed] [Google Scholar]
- 22.Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem. 2002;277:39334–42. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62:3615–9. [PubMed] [Google Scholar]
- 24.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–52. [PubMed] [Google Scholar]
- 25.Xiao D, Johnson CS, Trump DL, Singh SV. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Caner Ther. 2004;3:567–75. [PubMed] [Google Scholar]
- 26.Xiao D, Choi S, Lee YJ, Singh SV. Role of mitogen-activated protein kinases in phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Mol Carcinogenesis. 2005;43:130–40. doi: 10.1002/mc.20099. [DOI] [PubMed] [Google Scholar]
- 27.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Lew KL, Zeng Y, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 29.Bommareddy A, Hahm ER, Xiao D, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69:3704–12. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kB and NF-kB-regulated gene expression by sulforaphane and PEITC through IkBa, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 31.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vivo and ex vivo. Cancer Res. 2007;67:2239–46. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 32.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 33.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger ROS-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–63. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 35.Xiao D, Lew KL, Kim Y, et al. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;15:6836–43. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 36.Singh SV, Mohan RR, Agarwal R, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun. 1996;225:660–5. doi: 10.1006/bbrc.1996.1226. [DOI] [PubMed] [Google Scholar]
- 37.Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly-differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–11. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HY, Oh SH, Suh Ya, et al. Response of non-small cell lung cancer cells to the inhibitors of phosphatidylinositol 3-kinase/Akt- and MAPK kinase 4/c-Jun NH2-terminal kinase pathways: an effective therapeutic strategy for lung cancer. Clin Cancer Res. 2005;11:6065–74. doi: 10.1158/1078-0432.CCR-05-0009. [DOI] [PubMed] [Google Scholar]
- 39.Howard EW, Lee DT, Chiu YT, Chua CW, Wang X, Wong YC. Evidence of a novel Docetaxel sensitizer, garlic-derived S-allylmercaptocysteine, as a treatment option for hormone refractory prostate cancer. Int J Cancer. 2008;122:1941–8. doi: 10.1002/ijc.23355. [DOI] [PubMed] [Google Scholar]
- 40.Hecht SS, Kenney PMJ, Wang M, Trushin N, Upadhyaya P. Effects of Phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 41.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 42.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Liebes L, Conaway CC, Hochster H, et al. High-performance liquid chromatography-based determination of total isothiocyanate levels in human plasma: application to studies with 2-phenethyl isothiocyanate. Anal Biochem. 2001;291:279–89. doi: 10.1006/abio.2001.5030. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm Res. 2005;22:1658–66. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–95. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.van Duijn PW, Trapman J. PI3K/Akt signaling regulates p27kip1 expression via Skp2 in PC3 and DU145 prostate cancer cells, but is not a major factor in p27kip1 regulation in LNCaP and PC346 cells. Prostate. 2006;66:749–60. doi: 10.1002/pros.20398. [DOI] [PubMed] [Google Scholar]
- 48.Bergstralh DT, Ting JP. Microtubule stabilizing agents: their molecular signaling consequences and the potential for enhancement by drug combination. Cancer Treat Rev. 2006;32:166–79. doi: 10.1016/j.ctrv.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Patterson SG, Wei S, Chen X, et al. Novel role of Stat1 in the development of Docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–22. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- 50.Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by Docetaxel and promotes survival of Docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283:20635–44. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckelman BP, Salvessen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–94. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dean EJ, Ranson M, Blackhall F, Dive C. X-linked inhibitor of apoptosis protein as a therapeutic target. Expert Opin Ther Targets. 2007;11:1459–71. doi: 10.1517/14728222.11.11.1459. [DOI] [PubMed] [Google Scholar]
- 53.Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- 54.Krajewska M, Krajewski S, Banares S, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–25. [PubMed] [Google Scholar]
- 55.Watanabe SI, Miyata Y, Kanda S, et al. Expression of X-linked inhibitor of apoptosis protein in human prostate cancer specimens with and without neo-adjuvant hormonal therapy. J Cancer Res Clin Oncol. 2009 doi: 10.1007/s00432-009-0718-x. In press. [DOI] [PubMed] [Google Scholar]
- 56.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–6. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domingo-Domenech J, Oliva C, Rovira A, et al. Interleukin 6, a nuclear factor-kB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kB inhibition by PS-1145 enhances docetaxel antitumor activity. Cancer Res. 2006;12:5578–86. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]