Abstract
Myocardial [ATP] falls in the failing heart. One potential compensatory mechanism for maintaining a near normal free energy of ATP hydrolysis (ΔG∼ATP), despite a fall in [ATP], may be the reduction of myocardial creatine (Cr). To test this, we conducted a longitudinal study using transgenic mice overexpressing cardiac Gsα, which slowly developed cardiomyopathy. Myocardial energetics measured using 31P NMR spectroscopy and isovolumic contractile performance were determined in perfused hearts isolated from 5-, 10-, 17-month-old Gsα and age-matched littermate wild type (WT) mice. In young Gsα hearts, contractile performance was enhanced with near normal cardiac energetics. With age, as contractile performance progressively decreased in Gsα hearts, [ATP] and [PCr] progressively decreased while [Pi] increased only modestly; no changes were observed in WT hearts. Myocardial (but not skeletal) [Cr] in Gsα mice decreased, beginning at an early age (1.5-months). Consequently, cytosolic [ADP] and the free energy available from ATP hydrolysis were maintained at normal levels in Gsα hearts, despite decreased [ATP]. During increased cardiac work caused by supplying isoproterenol, the relationship between the rate pressure product (RPP) and ΔG∼ATP in Gsα mouse hearts demonstrated an increased cost of contraction in failing hearts. Thus, our results suggest that the decrease of myocardial [Cr] and net Pi efflux play compensatory roles by maintaining a nearly normal free energy of ATP hydrolysis in the dysfunctional heart; however, it also increased the cost of contraction, which may contribute to the lower contractile reserve in the failing heart.
Keywords: Adenosine triphosphate, ΔG∼ATP, Creatine, Cardiomyopathy, Gsα transgenic mice
Introduction
Adenosine triphosphate (ATP) is the high energy phosphate-containing compound directly used for excitation and contraction in the heart. Phosphocreatine (PCr), present in high concentrations in striated muscle, is the high energy phosphate-containing compound used to support temporal and spatial utilization of ATP during high work load. Transfer of the phosphoryl group between ADP and Cr is catalyzed by creatine kinase. Results from studies of both failing human myocardium and animal models of heart failure show that myocardial [ATP] and [PCr] progressively fall during the development of heart failure. [ATP] in the failing myocardium is as much as ∼25% lower than in normal myocardium while the fall in [PCr] is even greater, falling by ∼ 50% (1,2,3,4). The observation that [ATP] is reduced in the failing heart raises important questions central to our understanding of bioenergetics: Is the free energy released from ATP hydrolysis (ΔG∼ATP) also decreased, or are there compensatory mechanisms maintaining ΔG∼ATP to near normal values? If ΔG∼ATP is maintained near normal, is it sufficient to support high cardiac workload? These questions are important because a fall in ΔG∼ATP can limit the ability of the heart to increase its contractile performance (3,4,5,6).
In a longitudinal study of the failing canine heart, we found that the tissue content of creatine ([Cr]) was progressively depleted and that the loss of myocardial [Cr] preceeded the fall in [ATP](2). We hypothesized that the loss of [Cr] maintained a near normal ratio of PCr/Cr which in turn blunted any increase in [ADP] caused by higher rates of ATP utilization needed to support high wall stress characteristic of the failing heart (2). If this is the case, loss of creatine in the failing heart would be compensatory. However, since [PCr] was not measured in that study, this hypothesis could not be directly tested. If this hypothesis is correct, it has two important consequences for the energetics of the failing heart: 1) by maintaining low [ADP], [AMP] would also remain near normal and the purine nucleotide pool would be better preserved (7) and 2) by maintaining low [ADP], ΔG∼ATP would remain near normal, despite decreased [ATP]. Maintaining a near normal level of ΔG∼ATP, required to support cellular ATPase reactions, is essential for cell survival (5,6). Here, we test this hypothesis.
The animal model chosen for this study was the well-characterized mouse with over expressed cardiac Gsα transgene (Gsα mice) (8). Hearts of these transgenic mice exhibit enhanced inotropic and chronotropic responses to the chronic increase in β-adrenergic signaling. However, with age, these animals slowly develop cardiomyopathy characterized by myocyte hypertrophy, fibrosis, apoptosis, left ventricular (LV) dilation, depression of LV mechanical function, and an increase in sudden death (8-11). Thus, the Gsα transgenic mouse model provides an opportunity to conduct a longitudinal study of cardiac energetics during the development of cardiomyopathy and heart failure.
Using this model, our first goal was to determine the sequence of changes in cardiac ATP, PCr and Cr levels as cardiomyopathy and heart failure developed. The second goal was to determine whether the free energy of ATP hydrolysis, ΔG∼ATP, in failing hearts was maintained at a near normal level. The third goal was to determine the energetic cost of increasing work in failing hearts by measuring the change in ΔG∼ATP as a consequence of increasing work load. Simultaneous 31P NMR spectroscopy and isovolumic contractile performance measurements were made using hearts isolated from 5-, 10-, 17-month-old Gsα mice and age-matched wild type (WT) mice. The primary hypothesis tested is that the decrease in myocardial Cr content is a compensatory mechanism blunting an increase in cytosolic [ADP] (despite decreased [ATP] and [PCr]) in response to increased wall stress, thereby maintaining a near normal ΔG∼ATP during the development of cardiomyopathy and heart failure.
Methods
Animals
The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experimental protocol was approved by the Standing Committee on Animals of Harvard Medical Area and followed the recommendations of current NIH and APS guidelines. Three age groups of mice, 5-, 10- and 17-month-old littermate transgenic mice selectively overexpressing cardiac Gsα (Gsα, n=4, 4, and 7 in each age group) and wildtype (WT, n=5, 4, and 7 in each age group), were used for this study.
Isolated Perfused Heart Preparation and Protocols
Hearts were isolated from experimental mice and perfused in the Langendorff isovolumic mode (balloon-in-LV) as described (12,13). The coronary perfusate consisted of phosphate-free Krebs-Henseleit buffer containing (mM) NaCl (118), KCl (5.3), CaCl2 (2.0), NaHCO3 (25), MgSO4 (1.2), EDTA (0.5), glucose (10) and pyruvate (0.5) equilibrated with 95% O2 + 5% CO2 to maintain a pH of 7.4.
Measurements of contractile performance and 31P NMR spectroscopy were made simultaneously at baseline and two levels of higher contractile performance induced by supplying isoproterenol (ISO). ISO was administered (1% coronary flow) via a fine cannula into the aortic perfusion catheter to final concentrations of 5×10-10 M and 1×10-8 M. Hearts were perfused at each ISO concentration for 20 minutes to allow for the collection of two 31P NMR spectra (see below). At the end of the protocol, subsets of 10- and 17-month-old Gsα and WT hearts were freeze-clamped for high pressure liquid chromatography analysis (HPLC) of ATP (2); other hearts were weighed. In addition, samples of cardiac and skeletal (from the upper leg) muscles were collected from 0.5, 1.5, 5, 10 and 17 month-old Gsα mice and age-matched WT mice for analysis of Cr content.
Measurement of Isovolumic Contractile Performance
A custom-made water-filled balloon inserted into the LV was connected to a pressure transducer (Statham P23Db, Gould, Oxnard, CA) for continuous recording of LV developed pressure (LVdevP) and heart rate (HR). The balloon volume was adjusted to set LV end-diastolic pressure at ∼8 mmHg, and then held constant. Contractile performance data were collected on-line at a sampling rate of 200Hz using data acquisition system (MacLab, AD Instruments). Isovolumic contractile performance was expressed as the product of HR and LVdevP, the rate-pressure product (RPP).
31P NMR Spectroscopy and Data Analysis
31P NMR free induction decays were collected at 161.94 MHz using a GE-400 wide-bore Omega spectrometer (Freemont, CA) (12,13). Spectra were obtained without proton decoupling over 8 minutes by signal-averaging 208 scans (pulse width 15 μsec, pulse angle 60°, recycle time 2.14 sec and sweep width 6000 Hz).
31P NMR spectra were analyzed as previously described (12,13). To determine the cytosolic concentrations of PCr, Pi, and ATP, the mean of the resonance areas for [γ−P]ATP and [β-P]ATP was normalized by heart weight. We made the assumption that the fractional volume of intracellular water in the myocytes of Gsα and WT hearts were similar and equal to values typical of well perfused rodent hearts (0.48 μl/mg wet weight, adjusted to take into account any changes in protein content). In this case, resonance areas/mg wet weight were directly proportional to intracellular metabolite concentrations. The values for [ATP] obtained from HPLC for 10- and 17-month-old Gsα and WT hearts were used to calibrate the 31P NMR spectra. [Pi] and [PCr] were calculated by multiplying the ratio of their resonance areas to ATP resonance areas. Intracellular pH was determined by comparing the chemical shift difference between the Pi and PCr resonances to standard values.
Cytosolic [ADP] was calculated using the creatine kinase equilibrium expression: ADP = ([ATP][free creatine]) / ([PCr][H+]Keq), using measured values for [ATP], [PCr] and [Cr] (14) and Keq of 1.66×109 (mol/L)-1 for a [Mg2+] of 1.0 mmol/L (15,16). [AMP] was calculated using the adenylate kinase equilibrium expression: AMP = 1.05[ADP]2 / [ATP], measured values of [ATP] and calculated values for [ADP]. The free energy of ATP hydrolysis (ΔG∼ATP) was calculated from: ΔG∼ATP (kJ/mol) = ΔG° - RT ln ([ATP]/[ADP][Pi]), where ΔG° (-30.5 kJ/mol) is the value of ΔG∼ATP under standard conditions of molarity, temperature, pH, and [Mg2+], R is the gas constant (8.3 J/mol K), and T is temperature (Kelvin) (16), measured values of [ATP] and [Pi] and calculated values for [ADP].
Statistical Analysis
Data were expressed as mean ±SE. ANOVA followed by Fisher's PLSD test were applied to compare the differences among the age groups using of Statview (Brainpower, Calabasas); significance was defined at P<0.05. The relationships between RPP and ΔG∼ATP during ISO challenge were constructed using linear regressions.
Results
Cardiac Hypertrophy in Old Gsα Mice
Body weight, heart weight, heart weight to body weight ratio, and myocyte protein content were measured in 5-, 10- and 17-month-old Gsα and WT mice (Table I). Compared with age-matched WT mice, the heart weight and the heart weight to body weight ratio for young Gsα mice were comparable to young WT mice, but were 19% and 23% higher for 17-month-old Gsα mice. Myocyte protein content was unchanged in young Gsα mice, but was slightly lower in old Gsα mouse heart. As observed for previous studies (9,11), hearts of 17-month-old Gsα mice were hypertrophied.
Table I. Body Weight, Heart Weight and Myocardial Protein Content in Gsα and WT Mice at Different Ages.
| WT (n=5) | Gsα (n=4) | WT (n=4) | Gsα (n=4) | WT (n=7) | Gsα (n=7) | |
|---|---|---|---|---|---|---|
|
Age (months) |
5.2±0.2 | 5.3±0.1 | 10.2±0.2 | 10.2±0.2 | 17±0.2 | 17±0.2 |
|
Body Weight (g) |
33±1.0 | 33±1.9 | 33±1.4 | 33±1.3 | 34.6±1.9 | 33.0±0.8 |
|
Heart Weight (mg) |
147±5 | 142±10 | ---- | ---- | 147±12 | 175±11*§ |
|
Heart wt/Body wt (mg/g) |
4.5±0.2 | 4.3±0.1 | ---- | ---- | 4.3±0.2 | 5.3±0.3*§ |
|
Protein Content (mg/g wet wt) |
15.1±0.56 | 14.8±0.12 | ---- | ---- | 14.7±0.17 | 13.8±0.21*§ |
Mean±SE,
P<0.05 vs. age matched WT,
P<0.05, vs. 5-month-old mouse hearts.
Progressive Decrease in Contractile Performance in Gsα Hearts with Age
To determine changes in cardiac function in Gsα mouse hearts with increasing duration of chronic β-AR activation, we measured contractile performance in age-matched 5-, 10- and 17-month-old Gsα and WT hearts using isolated isovolumic heart preparations perfused at the same constant perfusion pressure (Table II).
Table II. Contractile and Energetic Responses to ISO Challenge in Gsα and WT Mouse Hearts at Different Ages.
| 5-month-old | 10-month-old | 17-month-old | |||||
|---|---|---|---|---|---|---|---|
| WT (n=5) | Gsα (n=4) | WT (n=4) | Gsα (n=4) | WT (n=7) | Gsα (n=7) | ||
|
HR (beat/min) |
Baseline | 279±7 | 499±18‡ | 336±16 | 487±12‡ | 339±13 | 446±38‡ |
| Iso 5×10-10 M | 382±40* | 547±34‡ | 406±15* | 539±18‡ | 425±21* | 514±35‡ | |
| Iso 10-8 M | 535±13* | 598±29*‡ | 565±17* | 599±8* | 577±30* | 613±16* | |
|
LVdevP (mmHg) |
Baseline | 108±4 | 86±4‡ | 96±3 | 74±5‡ | 87±6§ | 49±7‡§ |
| Iso 5×10-10 M | 113±6 | 103±6* | 117±5* | 87±3*‡ | 94±7 | 57±7‡§ | |
| Iso 10-8 M | 127±7* | 118±4* | 116±5* | 91±6*‡§ | 95±9§ | 60±6‡§ | |
|
CF/HW (ml/min/g) |
Baseline | 13±1 | 15±1 | ---- | ---- | 16±1§ | 14±1 |
| Iso 5×10-10 M | 18±1* | 19±2* | ---- | ---- | 20±1* | 16±2‡ | |
| Iso 10-8 M | 22±1* | 21±2* | ---- | ---- | 24±1* | 19±1*‡ | |
ISO, isoproterenol; HR, heart rate; LVdevP, left ventricular developed pressure; RPP, rate pressure product; CF, coronary flow; HW, heart wet weight. Mean ± SE,
P<0.05, vs. Baseline,
P<0.05, vs. age matched WT,
P<0.05, vs. 5-month-old mouse hearts.
Baseline (Table II, Figure 1)
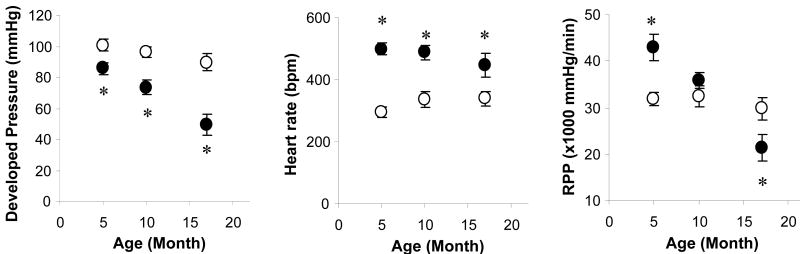
Figure 1.
Changes in LV developed pressure, heart rate (HR) and rate-pressure product (RPP) with age in Gsα and WT mouse hearts. There were progressive decreases in LV developed pressure (a) and RPP (c) and with age in Gsα hearts. Heart rate is higher in all age groups of Gsα hearts than in WT hearts (b). Wild type (○), Gsα (●), * P<0.05, Gsα vs. age-matched WT.
Compared with age-matched WT hearts, RPP was higher in young Gsα hearts (+43%, P<0.05), similar in 10-month-old Gsα hearts and lower in old Gsα hearts (-30%, P<0.05). The slope of the relationship for RPP and age was very steep for Gsα hearts (-1685 mm Hg/min/month), but close to zero for WT hearts. All Gsα hearts had higher heart rates (>400 beats/min) than for WT hearts (<350 beats/min) that was independent of age. All Gsα hearts exhibited a lower LVdevP (<100 mmHg) than WT hearts, and the difference became greater with age. The progressive decrease in LVdevP was the major factor responsible for the reduction of RPP for Gsα hearts.
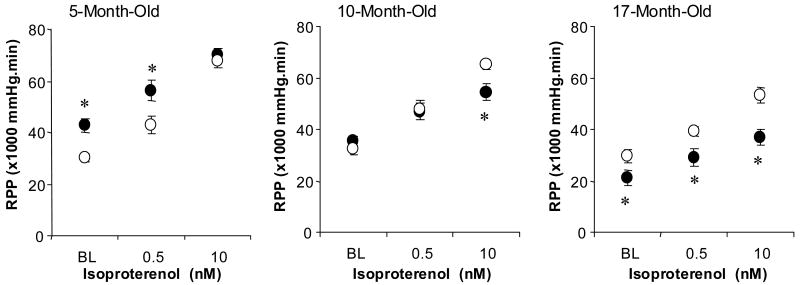
Contractile reserve was determined with acute ISO challenge. RPP increased with ISO in the dose dependent manner for all age groups for both Gsα and WT mouse hearts (Figure 2). The maximum RPP observed for Gsα hearts at high dose ISO (10 nM) was the same as for 5-month-old WT hearts (70,400±2600 vs 67,800±2600 mmHg/min), 16% lower for 10-month-old hearts (54,500±3300 vs 64,700±2100 mmHg/min) and 33% lower for 17-month-old hearts (36,800±2900 vs 55,100±2400 mmHg/min, p<0.05). Coronary flow during the ISO challenge was increased, which was not different in young Gsα and WT hearts, but was lower in the old Gsα hearts, compared to the age matched old WT hearts (Table II). Thus, chronically enhanced sympathetic activity in the mouse led to large and progressive decreases in cardiac contractile performance and contractile reserve encompassing a hyper-contractile state, a transition state and finally cardiomyopathy.
Figure 2.
Left ventricular rate-pressure product (RPP) in response to isoproterenol challenge among different age groups of WT and Gsα mouse hearts. The RPP response to isoproterenol was not different among three age groups of WT mouse hearts, but progressively decreased with age in Gsα mouse hearts. Wild type (○), Gsα (●), * P<0.05, vs. age-matched WT.
Changes in Energetics in Gsα Hearts with Age
To determine the sequence of changes in energetics associated with these three different contractile states, we obtained 31P NMR spectra for 5-, 10- and 17-month-old Gsα and WT mouse hearts. Figure 3 shows representative 31P NMR spectra obtained from 17-month-old Gsα and WT hearts. The mean values for [ATP], [ADP], [AMP], [PCr], [Pi], [Cr], [PCr]/[ATP], [PCr]/[Cr], [ATP]/[ADP] and ΔG∼ATP are shown in Figures 4-6 and Table III.
Figure 3.
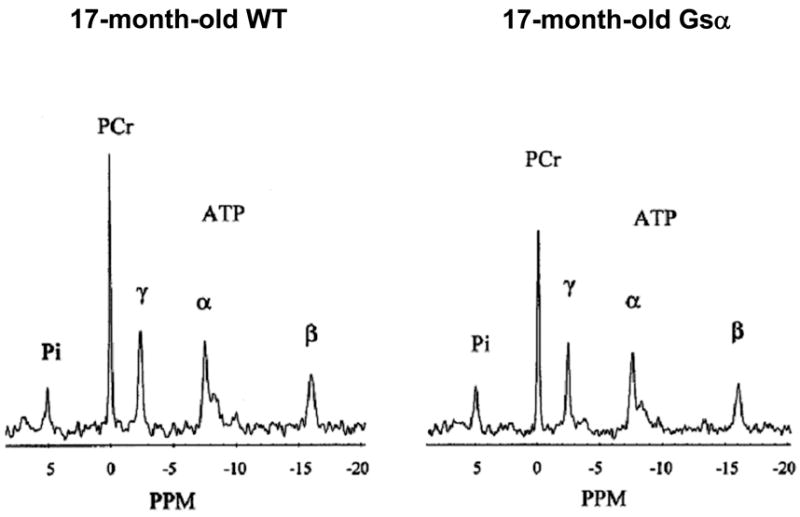
Representative 31P NMR spectra from hearts isolated from a 17-month-old Gsα mouse (right) and an age-matched wild-type mouse (left). Each spectrum is the average of 416 consecutive scans collected over 16 minutes. The major resonances are assigned (from left to right) as Pi, PCr, and γ-, α-, and β-phosphates of ATP. The area under each peak is proportional to the number of molecules of that substance in the heart. The 17-month-old Gsα mouse heart had lower PCr and ATP than did age-matched wild-type mouse heart.
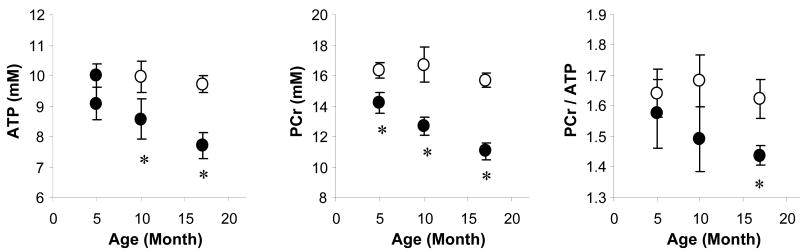
Figure 4.
Changes in myocardial [ATP], [PCr] and PCr/ATP with age in Gsα and WT mouse hearts. [ATP] and [PCr] progressively decreased with age in Gsα mice (a and b). The ratio of PCr/ATP was not different in 5- and 10-month-old Gsα and WT hearts, and fell only in the 17-month-old Gsα mice. Wild type (○), Gsα (●), * P<0.05, Gsα vs. age-matched WT.
Figure 6.
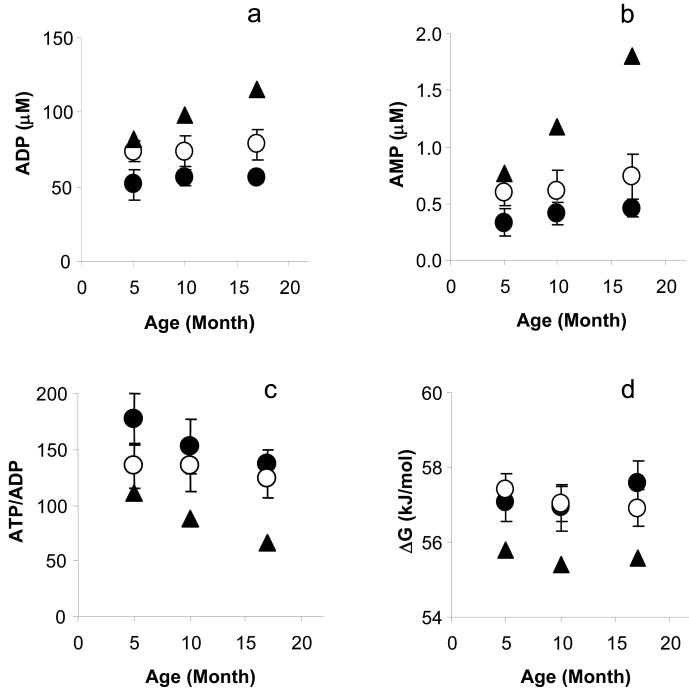
The cytosolic [ADP], [AMP], ATP/ADP ratio and free energy from ATP hydrolysis (|ΔG∼ATP|) in 5-, 10-, 17-month-old Gsα and WT mouse hearts. Using measured values for ATP, PCr, Cr, and H+ determined by 31P NMR spectroscopy and chemical assays, and using the creatine kinase and adenylate kinase equilibrium expression, we calculated cytosolic concentrations of ADP, AMP, ATP/ADP ratio and |ΔG∼ATP|. The cytosolic [ADP], [AMP], ATP/ADP ratio and |ΔG∼ATP| in all age groups of Gsα mouse hearts (●) were not different, and they were also not different from the age matched WT mouse hearts (○). In addition, we also calculated the values (▲) for cytosolic [ADP], [AMP], ATP/ADP ratio and |ΔG∼ATP| if the [Cr] had been maintained at a normal level, the cytosolic [ADP] and [AMP] would have increased with age (a,b), and ATP/ADP ratio and |ΔG∼ATP| would have been significantly lower at all ages in Gsα hearts (c, d).
Table III. Pi and |ΔG∼ATP| for Gsα and WT Mouse Hearts during Isoproterenol Challenge.
| 5 months | 10 months | 17 months | |||||
|---|---|---|---|---|---|---|---|
| WT (n=5) | Gsα (n=4) | WT (n=4) | Gsα (n=4) | WT (n=7) | Gsα (n=7) | ||
|
Pi (mM) |
Baseline | 4.1±0.6 | 6.0±0.1‡ | 4.6±0.3 | 5.5±0.9 | 4.6±0.5 | 3.9±0.6§ |
| Iso 5×10-10 M | 5.5±1.0 | 9.1±0.3*‡ | 5.4±0.3 | 8.8±0.6*‡ | 5.9±0.3 | 6.8±0.4*§ | |
| Iso 10-8 M | 10.2±0.8* | 12.9±1.1*‡ | 10.6±1.0* | 12.6±0.3*‡ | 10.3±0.8* | 11.1±0.5* | |
| |ΔG∼ATP| (kJ/mol) |
Baseline | 57.4±0.4 | 57.1±0.5 | 57.0±0.5 | 56.9±0.6 | 56.9±0.5 | 57.6±0.6 |
| Iso 5×10-10 M | 56.1±0.4* | 54.7±0.5*‡ | 55.9±0.3 | 54.7±0.5*‡ | 55.8±0.5 | 55.4±0.3* | |
| Iso 10-8 M | 53.3±0.3* | 52.9±0.6* | 53.3±0.2* | 52.7±0.4* | 53.2±0.3* | 53.0±0.3* | |
Pi, inorganic phosphate; |ΔG∼ATP|, free energy from ATP hydrolysis. Mean ± SE,
P<0.05, vs. Baseline,
P<0.05, vs. age matched WT,
P<0.05, vs. 5-month-old mouse hearts.
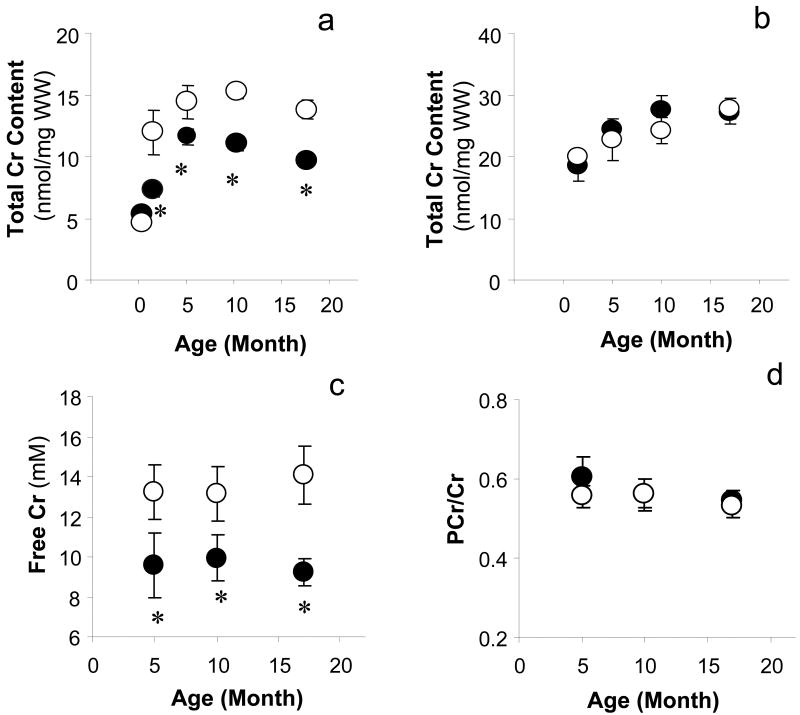
Progressive Decrease in Myocardial [ATP] and [PCr] in Gsα Hearts
[ATP] for WT hearts did not change with age. In contrast, [ATP] for Gsα hearts progressively fell with age compared to age-matched WT hearts. [ATP] in the hyper-contractile Gsα hearts was unchanged, but [ATP] was 14% lower in 10-month-old Gsα hearts and 23% lower in 17-month-old Gsα hearts (Figure 4). The 23% fall in [ATP] in Gsα hearts with cardiomyopathy is comparable to changes in [ATP] observed in other animal models of heart failure and in failing human hearts (1,2,3).
[PCr] and [Pi] also did not change with age for WT hearts. In contrast, for hyper-contractile Gsα hearts, [PCr] was 13% lower and [Pi] was 46% higher (both P<0.05) than for WT hearts. This profile is consistent with chronically hyper-contractile hearts. [PCr] progressively fell with age (-23% in 10-month-old and -32% in 17-month-old Gsα hearts (P<0.05 vs. WT littermates). Thus, for Gsα hearts, [ATP] and [PCr] progressively decreased as isovolumic contractile performance progressed from a hyper-contractile state to cardiomyopathy.
The total amount of NMR visible phosphate-containing metabolites was not different among three age groups of WT hearts (50±1, 51±2, 50±1 mM). In contrast, the total amount of NMR visible phosphate-containing metabolites progressively fell with age for the Gsα hearts: 47±2 mM (-5.9% compared to age-matched WT hearts), 44±2 mM (-14%, P<0.05) and 37±2 mM (-25%, P<0.05) for 5-, 10- and 17-month-old Gsα hearts respectively. The loss of total phosphate was due to the decreases in [ATP] and [PCr] without a concomitant increase in [Pi]. Intracellular pH was similar for all groups (pH 7.16).
Decrease in [Cr] in Gsα Mouse Hearts
To determine whether the progressive decrease in [PCr] in Gsα mouse hearts observed during the evolution of cardiomyopathy was due to a decrease in [Cr] or to increased chronic demand for ATP with unchanged [Cr], and whether this change was cardiac specific, myocardial and skeletal muscle [Cr] were measured for 5 age groups in both Gsα and WT mice (Figure 5).
Figure 5.
Changes in total creatine (Cr) pool in myocardium and skeletal muscle with age in Gsα and WT mice. Although the cardiac [Cr] in 2-week-old Gsα was the same as in WT mouse hearts, the total cardiac Cr pools and free Cr in Gsα hearts were significantly lower than age-matched WT hearts (a, c). The total Cr pool in upper leg skeletal muscle was no difference between Gsα and age-matched WT mice in any age group (b). PCr/Cr ratio was not different among Gsα and WT hearts (d). Wild type (○), Gsα (●), * P<0.05, Gsα vs. age-matched WT.
Cr is not made in the myocardium but accumulates via a saturable creatine transporter (17). Cr accumulation in WT hearts displayed the normal age-related increase for the myocardium, increasing from 5.0±0.4 nmol/mg wet weight in 2-week-old WT hearts to typical adult heart values of ∼14 nmol/mg wet weight in 6-week-old hearts. Although [Cr] in 2-week-old Gsα hearts was the same as for littermate WT hearts, the normal increase in [Cr] with age was blunted, reaching levels of only ∼11 nmol/mg wet weight. [Cr] in skeletal muscle was also measured. There was no difference in [Cr] between Gsα and age-matched WT mice at any age. Thus the failure to accumulate normal levels of Cr in Gsα mice was cardiac-specific. Importantly, the ratio of [PCr] to [Cr] was the same for both Gsα hearts and WT hearts for all age groups, independent of contractile performance or the development of cardiac dysfunction.
Normal Cytosolic [ADP], [AMP] and ΔG∼ATP in Gsα Mouse Hearts (Figure 6)
Based on the progressive decreases in [ATP] and [PCr] in Gsα hearts with age, it would be expected that cytosolic [ADP] and [AMP], as well as [Cr] and [Pi], would all increase. This was not observed. [ADP] and [AMP] for the three age groups of Gsα mouse hearts were either lower than or the same as for age-matched WT hearts. As a consequence, [ATP]/[ADP] and |ΔG∼ATP| was similar for all groups, ∼57 kJ/mol.
In Figure 6 we also show values for cytosolic [ADP], [AMP], [ATP]/[ADP] and ΔG∼ATP if cardiac [Cr] had been maintained at normal values for the age of the mouse. [ADP] would have increased by 1.6-, 1.7- and 2.0-fold, [AMP] would have increased by 2.4-, 2.9- and 3.9-fold, [ATP]/[ADP] would have progressively fallen to values half that observed, and |ΔG∼ATP| would have decreased by 1.9, 1.5, and 2.0 kJ/mol in 5-, 10- and 17-month Gsα hearts, respectively. Thus, because [Cr] was lower and [Pi] increased only slightly in chronically β-AR stimulated hearts, ΔG∼ATP was well preserved in both the hyperactive and cardiomyopathic states.
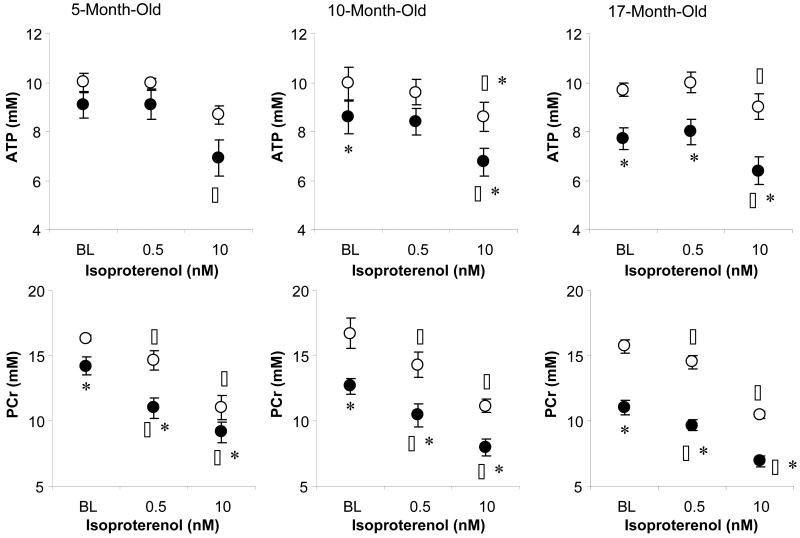
Energetic Response to Acute ISO Challenge (Table III, Figure 7)
Figure 7.
Changes in myocardial [ATP] and [PCr] in response to isoproterenol challenge among different age groups of Gsα and WT mouse hearts. The reduction of [ATP] and [PCr] at higher work load lower was significant with age in Gsα mouse and WT hearts, and fell only in the 17-month-old Gsα mice. Wild type (○), Gsα (●), * P<0.05, Gsα vs. age-matched WT, § P<0.05, vs. 5-month-old mouse hearts, BL (baseline).
Abruptly increasing cardiac contractile performance in rodent hearts by supplying inotropic agents typically leads to decreased [PCr] with concomitant increased [ADP] and [Pi], with unchanged [ATP]. Changes in [ATP], [ADP] and [Pi] can be described by a single number, the free energy of ATP hydrolysis, ΔG∼ATP. Because ΔG∼ATP is a negative number, we describe how it changes using its absolute value, |ΔG∼ATP|., This value describes the energetic state of the heart and represents the chemical energy available for the ATPase reactions in the cell. |ΔG∼ATP| in the normal mouse heart varies between ∼60 kJ/mol at low work states to ∼53 kJ/mol at high work states (18,19).
As expected, |ΔG∼ATP| fell as work load increased. At low work states (baseline) in WT and Gsα mouse hearts at all ages, |ΔG∼ATP| was ∼57 kJ/mol. For the WT hearts, |ΔG∼ATP| fell ∼1.2 kJ/mol for low dose ISO and ∼ 3.8 kJ/mol for high dose ISO challenge. The decreases in |ΔG∼ATP| for Gsα hearts were greater: ∼2.2 kJ/mol at low ISO (P<0.05 vs. WT) and ∼4.3 kJ/mol at high ISO.
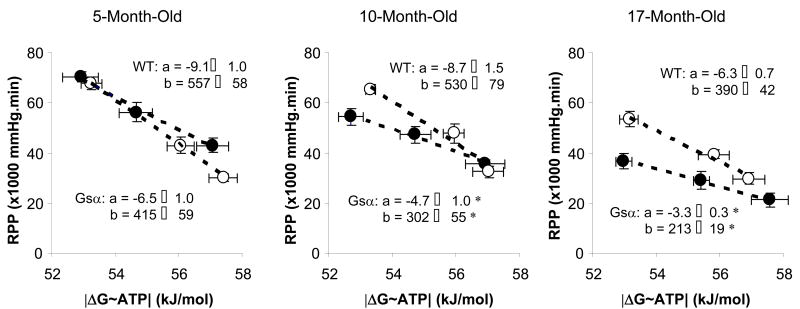
We constructed the relationships between RPP and ΔG∼ATP during ISO challenge for all groups (Figure 8). This relationship defines the change in contractile reserve as ΔG∼ATP changes; the inverse of the slope of this relationship provides a measure of the cost of increased contraction (kJ/mol per unit RPP). The regression lines for RPP and ΔG∼ATP in young hyper-contractile Gsα and young WT mouse hearts were similar, showing comparable cost of increasing contraction. In contrast, at any given |ΔG∼ATP|, RPP was lower (p<0.05) in old cardiomyopathic Gsα mouse hearts than for age-matched WT hearts, showing greater cost of increasing contraction in cardiomyopathic hearts. The cost of increasing contraction in old cardiomyopathic Gsα mouse hearts was nearly twice that compared to both young Gsα mouse hearts and age-matched WT hearts: 0.29 vs. 0.15 and 0.16 units, respectively. The values for WT hearts are in good agreement with other reports (18). For the transition state of contractile dysfunction prior to cardiomyopathy, hearts had normal |ΔG∼ATP| at low workloads but used more free energy from ATP hydrolysis to support a smaller increase in work upon inotropic challenge. Thus, the cost of increasing contraction progressively increased with the development of cardiomyopathy in Gsα mouse hearts.
Figure 8.
Relationship between rate-pressure product (RPP) and free energy from ATP hydrolysis (|ΔG∼ATP |) for Gsα and WT mouse hearts, which was constructed by linear regressions (y = ax + b). The slopes and y-intercepts were significantly different among 10- and 17-month-old Gsα mouse hearts, indicating a shifting of the RPP - ΔG∼ATP relationship, compared to the WT mouse hearts. Wild type (○), Gsα (●), * P<0.05, Gsα vs. age-matched WT.
Discussion
To define the sequence of changes in energy metabolites and to test whether loss of creatine is an early and persistent marker of contractile dysfunction requires measuring both energetic parameters and contractile performance in a longitudinal model of heart failure. The model we chose for this study is the mouse with selective overexpression of cardiac Gsα leading to chronically enhanced β-adrenergic signaling. These hearts slowly made the transition from hyperactivity at 5 months of age to cardiomyopathy by 17 months of age. Here we studied three distinct contractile states in the isolated perfused Gsα mouse heart: 1) an early stage with a hyper-contractile baseline contractile function with normal contractile reserve; 2) a transition stage with a reduced contractile reserve despite apparently compensated baseline contractile function; and 3) a dysfunctional stage, with lower baseline LV contractile performance as well as decreased contractile reserve associated with hypertrophy, intracellular edema and fibrosis (9,10,11). The latter stage was considered heart failure.
Progressive Fall in [ATP]
An important observation made here is that [ATP] was normal in young hyper-contractile Gsα hearts and progressively fell in parallel with the age-related decrease in isovolumic contractile performance and impaired contractile reserve. We previously reported that myocardial [ATP] was lower in a canine model of pacing-induced heart failure during the phase of cardiac dysfunction even prior to congestive heart failure (2). Since [ATP] fell even before the development of heart failure in these two very different models, the progressive decrease in myocardial [ATP] is likely to be a characteristic of the heart during prolonged cardiac dysfunction as well as cardiomyopathy.
Progressive Fall in [PCr] and [Cr]
Decreases in [PCr] and in [PCr]/[Cr] in response to supply/demand mismatch such as during acute ischemia or with increased inotropic stimulation are well known (20,21). Unlike those cases where [PCr]/[Cr] is lower because [PCr] is used to support ATP synthesis and [Cr] is not lost from the cell, [PCr] was lower in Gsα mouse hearts because [Cr] was lower. Gsα hearts failed to accumulate as much Cr as age-matched WT hearts and the failure to accumulate normal Cr levels began during (or perhaps before) the hyper-contractile phase, prior to hypertrophy and cardiomyopathy. [Cr] in Gsα hearts was lower than for age-matched WT hearts as early as 6 weeks postpartum. Importantly, as observed for the canine model of pacing-induced heart failure (2), decreases in [Cr] occurred earlier and to a greater degree than that observed for [ATP].
We also measured the total Cr pool in skeletal muscle from both Gsα and WT mice, and found no difference at any age. These observations make it unlikely that there was a defect in Cr synthesis in Gsα mice or that blood levels of [Cr] were abnormal. Instead, the reduction of myocardial [Cr] was tissue-specific. Failure to accumulate normal levels of Cr could be due to a down-regulation of the Cr transporter protein synthesis, decreased trafficking of the transporter to the sarcolemma or to altered kinetics of the transporter. In support of the results presented here, Spindler at al (22) studying non-failing 4-month-old Gsα mice over-expressing the β1-adrenergic receptor found concordant decreased [PCr] and [Cr] with no change in [ATP], and decreased Cr transporter protein levels.
The progressive decreases in [PCr] and [ATP] in the Gsα hearts studied here suggest that chronic activation of the β-adrenergic signaling pathway is so energy costly that normal [PCr] and [ATP] cannot be maintained. The [PCr] to [ATP] ratio has been used in both basic and clinical studies as an index of impaired cardiac energetics and a predictor of mortality (23,24). It is important to point out that this ratio was nearly normal in 10-month-old Gsα hearts, with apparently normal contractile function at baseline but decreased contractile reserve, even though both [ATP] and [PCr] were reduced. Thus, the using the PCr/ATP ratio can underestimate the presence of impaired energetics.
Consequences of Maintaining Near Normal Levels of Cytosolic [Pi], [ADP] and |ΔG∼ATP|
An inverse relationship between [Pi] and [PCr] has been well studied in many physiologic and pathophysiologic conditions. During a twitch in skeletal muscle (25) and in many examples of acute hypoxia and ischemia in the heart (26,27), the increase in [Pi] matched the decrease in the sum [PCr] + [3×ATP]; the sum of phosphate-containing molecules was constant. This was not observed in the model of chronic activation of β-adrenergic signaling pathway studied here. Instead, we observed a progressive fall in [PCr] and [ATP] without a concomitant increase in [Pi]; [Pi] increased only modestly, by ∼40-50 %. These results suggest that net Pi efflux occurred in Gsα hearts.
The major goal of this study was to test the hypothesis that the decrease in [Cr] is a compensatory mechanism blunting the expected increase in cytosolic [ADP] in the dysfunctional failing heart. If this hypothesis is correct, it would have two important consequences: 1) by maintaining low [ADP], the purine nucleotide pool and [ATP] should be better preserved; and 2) by maintaining low [ADP], the free energy of ATP hydrolysis, ΔG∼ATP, should remain near normal, despite decreased [ATP]. This theory of the energetics of the failing heart is supported by the results presented here. We observed that [Cr] failed to accumulate to normal levels in these hearts and that [ADP] was near normal in both the hyperactive and failing states. Importantly, the apparent loss of Cr occurred early in the evolution of heart failure, prior to the loss of ATP.
Based on the creatine kinase and adenylate kinase reactions, the fall in [PCr] without a fall in [Cr] would have led to an increased cytosolic [ADP] and [AMP]. Because [Cr] was lower in both young and old Gsα hearts, calculated cytosolic [ADP] and [AMP] did not increase. As a consequence, activation of the cytosolic AMP-specific 5′-nucleotidase, the primary gatekeeper for purine loss from the myocyte, by ADP and AMP (24) was minimized, and [ATP] fell slowly during the evolution of cardiomyopathy. If [Cr] had been normal in the Gsα mouse hearts, [ADP] would have increased by 2-fold and [AMP] by as much as 4-fold. The increased [AMP] would have activated 5′-nucleotidase, leading to a greater loss of purines.
The variable terms in the expression for ΔG∼ATP are [ATP]/[ADP] and 1/[Pi]. As a result of the apparent loss of Cr coupled with the less than expected increase in Pi from the failing Gsα mouse hearts, [ATP]/[ADP] and [Pi] were maintained near normal, and combine to maintain a nearly normal ΔG∼ATP despite lower [ATP] and substantial molecular remodeling. A normal free energy of ATP hydrolysis ensured that the contractile apparatus and ATP-dependent Ca2+ and Na+ pumps continue to function in hyperactive, hypertrophied and dysfunctional myocardium (5,6). If these compensatory changes had not occurred, |ΔG∼ATP| would have been ∼2 kJ/mol lower.
The importance of maintaining a normal [PCr[/[Cr] is shown by experiments in which myocardial [Cr] was increased in mouse hearts by increasing Cr transporter accumulation genetically (28,29). [Cr] increased ∼2-fold but, unexpectedly, [PCr]/[Cr] was half normal. As a consequence, cytosolic [ADP] increased, |ΔG∼ATP| decreased and the hearts develop hypertrophy, dilatation and contractile dysfunction.
Increased Cost of Contraction in Gsα Mouse Hearts
Because Gsα mouse hearts have enhanced adrenergic signalling and the β-receptors do not desensitize (30), ISO infusion effectively induced cardiac inotropic response in all age groups of Gsα mouse hearts. In response to an acute inotropic challenge in the normal myocardium, myocardial [Pi] increases as [PCr] decreases but [ATP] remains constant. This pattern was observed for both WT and Gsα hearts with the low dose of ISO. However, with high dose of ISO, [ATP] decreased, showing that [ATP] production failed to meet ATP utilization. The decrease in [ATP] was significantly greater in Gsα mouse hearts, indicating a defect in ATP supply in these hearts.
The relationship between RPP and ΔG∼ATP defines the cost of increasing contraction, an index of the efficiency of the coupling between ATP supply and utilization (5,18). The cost of increasing contraction progressively increased with the development of cardiomyopathy in Gsα mouse hearts. For young hyper-contractile Gsα mouse hearts, the RPP - ΔG∼ATP relationship was the same as for WT hearts; however, for old cardiomyopathic Gsα mouse hearts, a large change in ΔG∼ATP supported less cardiac work. The cost of increasing contraction (kJ/mol per unit RPP) was twice as high in cardiomyopathic hearts as for hyper-contractile hearts and for WT hearts, demonstrating lower energetic efficiency during inotropic challenge in the Gsα failing heart, despite nearly normal ΔG∼ATP.
Comparison with Ischemia
Comparison of the energetic phenotypes of the failing and ischemic myocardium reveals many similarities. For both, [PCr] falls before and to a greater extent than [ATP]; phosphoryl synthesis, turnover and utilization are all down regulated (31,32,33,34), and the cost of increasing contractile work is higher than normal (32,33). Comparing cardiac hypoxia, hypo-perfusion and failure shows that the changes in [PCr] and [Pi] can be modest, and [ATP] is often well preserved. For example, unlike the continued fall in ATP with severe ischemia with time, [ATP] fell by ∼30 % early during ischemia but changed little even after 5 hrs of persistent moderate ischemia in the canine heart (35). In another example, in sustained hypo-perfusion in the swine heart, [ATP] was well preserved; initially [Pi] increased from 4 to 8 mM and |ΔG∼ATP| fell from 58 to 52 kJ/mol; however, with time, these returned to normal (32,33). In these examples, the new energetic steady state is well tolerated provided [ATP] had not fallen below ∼70% of normal. Maintaining a low [Pi] in the failing heart as reported here and the return to normal [Pi] as observed in the swine heart study of hypoperfusion (32) both lead to near normal |ΔG∼ATP|, suggesting that regulation of [Pi] efflux is a previously unappreciated aspect of energetics of the chronically stressed myocardium. We suggest that chronic mismatch in ATP supply and utilization, whether due to persistent ischemia or heart failure, leads to a common energetic phenotype.
Summary
Chronic activation of the β-adrenergic signaling pathway due to selective over expression of cardiac Gsα in the mouse led to progressive decreases in isovolumic contractile performance, contractile reserve and the tissue contents of PCr, Cr, Pi and, with time, ATP. We suggest that the decreased [Cr] combined with net efflux of Pi are adaptive, limiting the loss in the purine pool and maintaining a near normal ΔG∼ATP in spite of a reduced [ATP] in failing hearts. Despite this adaptation, the cost of increasing contraction was higher in the failing heart. We suggest that this energetic phenotype is a common feature of the failing mammalian myocardium and that interventions designed to reduce the cost of increasing contraction would be effective therapy.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health: HL059139, HL033107, AG014121, HL069020, AG027211, HL069752, HL093481, HL095888 and AG023137 to Vatner SF and Vatner DE, and HL 075619 and HL 52320 to Ingwall JS.
Footnotes
Disclosure Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Starling RC, Hemarce DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem. 1998;180:171–177. [PubMed] [Google Scholar]
- 2.Shen W, Asai K, Uechi M, Mathier MA, Shannon RP, Vatner SF, et al. Progressive loss of myocardial ATP due to a loss of total purine during the development of heart failure in dogs. Circulation. 1999;100:2113–2118. doi: 10.1161/01.cir.100.20.2113. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall JS. Energy metabolism in heart failure and remodeling. Cardiovasc Res. 2009;81:412–9. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol. 1996;270:H1207–H1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 6.Kammermeier H. Phosphorylation potential and free energy of ATP. Adv Organ Biology. 1998;4A:159–169. [Google Scholar]
- 7.Bak MI, Ingwall JS. Regulation of cardiac AMP-specific 5′-nucleotidase during ischemia mediates ATP resynthesis on reflow. Am J Physiol. 1998;274:C992–1001. doi: 10.1152/ajpcell.1998.274.4.C992. [DOI] [PubMed] [Google Scholar]
- 8.Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, et al. Overexpression of Gsα protein in the hearts of transgenic mice. J Clin Invest. 1995;95:1676–1683. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, et al. Adverse effects of chronic endogenous sympathetic drive induced by cardiac Gsα overexpression. Circ Res. 1996;78:517–524. doi: 10.1161/01.res.78.4.517. [DOI] [PubMed] [Google Scholar]
- 10.Geng YJ, Ishikawa Y, Vatner DE, Wagner TE, Bishop SP, Vatner SF, et al. Apoptosis of cardiac myocytes in Gsα transgenic mice. Circ Res. 1999;84:34–42. doi: 10.1161/01.res.84.1.34. [DOI] [PubMed] [Google Scholar]
- 11.Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, Ishikawa Y, et al. β-Adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic Gsα mouse. J Clin Invest. 1999;104:551–558. doi: 10.1172/JCI7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spindler M, Saupe KW, Christe ME, Sweeney Lee, Seidman CE, Seidman JG, et al. Diastolic dysfunction and altered energetic in the MHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101:1775–1783. doi: 10.1172/JCI1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saupe KW, Spindler M, Hopkins JC, Shen W, Ingwall JS. Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. Reaction kinetics of the creatine kinase isoenzymes in the intact heart. J Biol Chem. 2000;275:19742–6. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 14.Lawson JWR, Veech RL. Effect of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolysis and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- 15.Veech RL, Lawson JWR, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem. 1979;254:6538–6547. [PubMed] [Google Scholar]
- 16.Gibbs C. The cytoplasmic phosphorylation potential. Its possible role in the control of myocardial respiration and cardiac contractility. J Mol Cell Cardiol. 1985;17:727–731. doi: 10.1016/s0022-2828(85)80034-1. [DOI] [PubMed] [Google Scholar]
- 17.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 18.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J. 2007;93:1834–44. doi: 10.1529/biophysj.107.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer K, Krenz M, Robbins J, Ingwall JS. Shifts in the myosin heavy chain isozymes in the mouse heart result in increased energy efficiency. J Mol Cell Cardiol. 2007;42:214–21. doi: 10.1016/j.yjmcc.2006.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittl JA, Balschi JA, Ingwall JS. Effect of norepinephrine infusion on myocardial high-energy phosphate content and turnover in the living rat. J Clin Invest. 1987;79:852–1859. doi: 10.1172/JCI113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bache RJ, Zhang J, Path G, Merkle H, Hendrich K, From AH, et al. High-energy phosphate responses to tachycardia and inotropic stimulation in left ventricular hypertrophy. Am J Physiol. 1994;266:H1959–H1970. doi: 10.1152/ajpheart.1994.266.5.H1959. [DOI] [PubMed] [Google Scholar]
- 22.Spindler M, Engelhardt S, Niebler R, Wagner H, Hein L, Lohse MJ, et al. Alterations in the myocardial creatine kinase system precede the development of contractile dysfunction in beta(1)-adrenergic receptor transgenic mice. J Mol Cell Cardiol. 2003;35:389–97. doi: 10.1016/s0022-2828(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzerith C, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 24.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolism in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801. doi: 10.1016/0002-8703(91)90527-o. [DOI] [PubMed] [Google Scholar]
- 25.Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscles. Am J Physiol. 1985;248:C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- 26.Bak MI, Ingwall JS. Acidosis during ischemia promotes adenosine triphosphate resynthesis in postischemic rat heart, In vivo regulation of 5′-nucleotidase. J Clin Invest. 1994;93:40–49. doi: 10.1172/JCI116974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer S, Camacho SA, Gober J, Obregon RG, DeGroot MA, Botvinick EH, et al. Response of myocardial metabolites to graded regional ischemia: 31P NMR spectroscopy of porcine myocardium in vivo. Circ Res. 1989;64:968–76. doi: 10.1161/01.res.64.5.968. [DOI] [PubMed] [Google Scholar]
- 28.Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, et al. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation. 2005;112:3131–9. doi: 10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- 29.ten Hove M, Makinen K, Sebag-Montefiore L, Hunyor I, Fischer A, Wallis J, et al. Creatine uptake in mouse hearts with genetically altered creatine levels. J Mol Cell Cardiol. 2008;45:453–9. doi: 10.1016/j.yjmcc.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatner DE, Asai K, Iwase M, Ishikawa Y, Wagner TE, Shannon RP, Homcy CJ, Vatner SF. Overexpression of myocardial Gsalpha prevents full expression of catecholamine desensitization despite increased beta-adrenergic receptor kinase. J Clin Invest. 1998;101:1916–22. doi: 10.1172/JCI1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bittl JA, Balschi JA, Ingwall JS. Contractile failure and high-energy phosphate turnover during hypoxia: 31P-NMR surface coil studies in living rat. Circ Res. 1987;60:871–8. doi: 10.1161/01.res.60.6.871. [DOI] [PubMed] [Google Scholar]
- 32.Martin C, Schulz R, Rose J, Heusch G. Inorganic phosphate content and free energy change of ATP hydrolysis in regional short-term hibernating myocardium. Cardiovasc Res. 1998;39:318–26. doi: 10.1016/s0008-6363(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 33.Guth BD, Schulz R, Heusch G. Time course and mechanisms of contractile dysfunction during acute myocardial ischemia. Circulation. 1993;87(5 Suppl):IV35–42. [PubMed] [Google Scholar]
- 34.Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78:893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- 35.Neill WA, Ingwall JS. Stabilization of a derangement in adenosine triphosphate metabolism during sustained, partial ischemia in the dog heart. J Am Coll Cardiol. 1986;8:894–900. doi: 10.1016/s0735-1097(86)80432-6. [DOI] [PubMed] [Google Scholar]