Abstract
We have previously proposed that the heterogeneous collapse of mitochondrial inner membrane potential (ΔΨm) during ischemia and reperfusion contributes to arrhythmogenesis through the formation of metabolic sinks in the myocardium, wherein clusters of myocytes with uncoupled mitochondria and high KATP current levels alter electrical propagation to promote reentry. Single myocyte studies have also shown that cell-wide ΔΨm depolarization, through a reactive oxygen species (ROS) -induced ROS release mechanism, can be triggered by global depletion of the antioxidant pool with diamide, a glutathione oxidant. Here we examine whether diamide causes mitochondrial depolarization and promotes arrhythmias in normoxic isolated perfused guinea pig hearts. We also investigate whether stabilization of ΔΨm with a ligand of the mitochondrial benzodiazepine receptor (4′-chlorodiazepam; 4-ClDzp) prevents the formation of metabolic sinks and, consequently, precludes arrhythmias. Oxidation of the GSH pool was initiated by treatment with 200μM diamide for 35 minutes, followed by washout. This treatment increased GSSG and decreased both total GSH and the GSH/GSSG ratio. All hearts receiving diamide transitioned from sinus rhythm into ventricular tachycardia and/or ventricular fibrillation during the diamide exposure: arrhythmia scores were 5.5 ± 0.5; n=6 hearts. These arrhythmias and impaired LV function were significantly inhibited by co-administration of 4-ClDzp (64μM): arrhythmia scores with diamide + 4-ClDzp were 0.4± 0.2 (n=5; P < 0.05 vs. diamide alone). Imaging ΔΨm in intact hearts revealed the heterogeneous collapse of ΔΨm beginning 20 minutes into diamide, paralleling the timeframe for the onset of arrhythmias. Loss of ΔΨm was prevented by 4-ClDzp treatment, as was the increase in myocardial GSSG. These findings show that oxidative stress induced by oxidation of GSH with diamide can cause electromechanical dysfunction under normoxic conditions. Analogous to ischemia-reperfusion injury, the dysfunction depends on the mitochondrial energy state. Targeting the mitochondrial benzodiazepine receptor can prevent electrical and mechanical dysfunction in both models of oxidative stress.
Keywords: ischemia, reperfusion, glutathione, arrhythmia, mitochondrial ion channels, oxidative stress, anti-oxidant, heart
Introduction
Sudden death from cardiac arrhythmias is a leading cause of mortality in the industrialized world. Despite advances in treatment, a paucity of information exists regarding the sub-cellular events that set the stage for arrhythmias at the organ level, thus limiting treatment strategies aimed at prevention of arrhythmogenesis.
The oxidative stress elicited by ischemia reaches a zenith during early reperfusion and is commonly associated with electromechanical dysfunction [1, 2]. In isolated myocytes, we have previously shown that oxidative stress (elicited by either laser flash or substrate deprivation) can lead to lability in action potential duration [3, 4]. The underlying cause of action potential heterogeneity was subsequently found to be due to oscillations in mitochondrial membrane potential (ΔΨm), which couple directly to cellular excitability via the activity of sarcolemmal ATP-sensitive potassium channels [3]. Treatment with ligands of the mitochondrial benzodiazepine receptor (mBzR) which are known to inhibit mitochondrial inner membrane anion channel (IMAC) activity (see [5] for review), stopped the ΔΨm oscillations in single cells, stabilizing ΔΨm in the polarized state [3]. Consistent with the hypothesis that rapid ΔΨm loss could activate sarcolemmal KATP channels and cause arrhythmias in the whole heart, 4-chlorodiazepam (4-ClDzp) successfully prevented reperfusion arrhythmias in intact guinea pig [6] and rabbit [7] hearts.
We have also shown that overwhelming the cellular antioxidant defenses with diamide, which chemically oxidizes the glutathione pool [8], leads to an accumulation of ROS in mitochondria of isolated cardiomyocytes. The accumulation of ROS ultimately reached a threshold level, resulting in oscillations in ΔΨm and NADH [9]. Similar to previous observations [3], 4-ClDzp prevented or reversed the oscillations induced by diamide treatment [9], providing further evidence that oxidative stress elicits oscillations in ΔΨm via the cyclical activation of IMAC.
In the intact heart, we recently observed oscillations in ΔΨm following diamide treatment [10]. Administration of diamide to the heart caused a gradual oxidation of the glutathione pool, ultimately leading to increased ROS production and ΔΨm collapse, resembling hearts exposed to ischemia/reperfusion [10]. However, we did not test whether diamide could induce arrhythmias, and if 4-ClDzp could prevent the collapse in ΔΨm evoked by diamide.
In light of our previous observations, here we examine whether overwhelming the cellular antioxidant defenses with diamide could induce arrhythmias in the intact heart under normoxic conditions. We also subjected hearts to a well-established model of oxidative stress, global ischemia and reperfusion, to show that these two distinct models of oxidative stress cause arrhythmias via the activity of the mBzR. We hypothesized that blocking the mBzR with 4-ClDzp would prevent the diamide-induced collapse in ΔΨm and prevent arrhythmias in guinea pig hearts.
Methods
Animals
Adult male guinea pigs (age 4-6 months) were housed in an animal facility with food and water provided ad libitum. All experiments were performed with prior approval from the Institutional Animal Care and Use Committee at both the Johns Hopkins University School of Medicine and East Carolina University, and were in accordance with guidelines established by the American Physiological Society.
Experimental Protocol
On each day of the experiment, animals were injected with pentobarbital (35 mg kg−1, ip injection) and hearts were excised with midline thoracotomy. The aorta was secured around a cannula of a modified Langendorff apparatus and retrogradely perfused with buffer consisting of (in mM): 118 NaCl, 24 NaHCO3, 1.2 KH2PO4, 4.75 KCl, 1.2 MgSO4, 2.0 CaCl2, and 10 glucose (equilibrated with 95/5 % O2/CO2), as described previously [7]. A latex balloon (Harvard Apparatus Balloon size #7) was inserted into the LV chamber through the mitral valve and on-line recordings of left ventricular pressure as well as volume-conducted ECG were obtained using the Powerlab System (A.D. Instruments), as previously described [7].
Diamide Treatment
After a 15 minute equilibration period, hearts were divided into one of two experimental groups: (i) normoxic perfusion for 35 minutes with the addition of 200μM diamide in the perfusate followed by a 15 minute washout (n = 6); (ii) normoxic perfusion with 64μM 4-ClDzp for 10 minutes, followed by the addition of 200μM diamide for 35 minutes and then 15 minute washout of both drugs (n = 5).
Arrhythmia scoring
Arrhythmias were characterized using the guidelines established by the Lambeth Conventions and scored using a system similar to that described by Curtis and Walker [11]. Arrhythmias were scored for the entire 35 min diamide exposure as follows: 0 = 0 – 49 ventricular premature beats; 1 = 50-499 ventricular premature beats; 2 = >499 ventricular premature beats and/or 1 episode of spontaneously reverting VT or VF less than 60 sec in total duration; 3 = > 1 episode of VT or VF that is <60 sec total duration; 4 = reverting VT or VF or both that is <120 seconds in total duration; 5 = VT or VT or both that is >120 sec in combined duration; 6 = non-reverting (fatal) VT or VF that began > 15 min after diamide; 7 = fatal VT/VF that began between 5 minutes and 15 minutes after diamide; 8 = fatal VT/VF that began less than 5 minutes after diamide.
Glutathione Content
For the glutathione experiments, an additional subset of hearts (n = 5) were perfused under normoxic conditions without diamide to serve as a time control group. At the end of each experiment, hearts treated with diamide (n=6) and diamide + 4-ClDzp (n=6) were snap frozen in liquid nitrogen for determination of myocardial glutathione status as previously described [12]. Briefly, frozen tissue was powdered with a nitrogen-cooled mortar and pestle, and 50-70mg of tissue were homogenized in the absence (for GSH determination) or presence (for GSSG determination) of 1-methyl-2-vinylpyridinium trifluoromethanesulfonate to scavenge reduced thiols. Total GSH and GSSG were determined using a commercially available kit (Oxis International), and final concentrations were determined by normalizing to protein content as determined by BCA Assay (Thermo Scientific).
Two-photon Imaging of Mitochondria in Intact Hearts
For 2-photon imaging, hearts were mounted on a custom-built cannula and imaged as previously described [10]. Fluorescence signals were transferred to a personal computer and analyzed using Image J software.
Ischemia-Reperfusion Protocol
A separate group of hearts were excised as described above and exposed to 30/30 min of global, no-flow ischemia and reperfusion under control conditions (n = 7) or in the presence of 64μM 4-ClDzp (n = 5).
Statistical analysis
All data are expressed as mean ± s.e.m. Analysis of glutathione content was performed using a one-way ANOVA, and significance between groups was determined using Tukey's post hoc tests. Left ventricular developed pressure data were analyzed with repeated-measures ANOVA. Between group differences in arrhythmia scores were compared using a 2-tailed Student's t-test. For all comparisons, statistical significance was noted when P < 0.05.
Results
Myocardial glutathione content
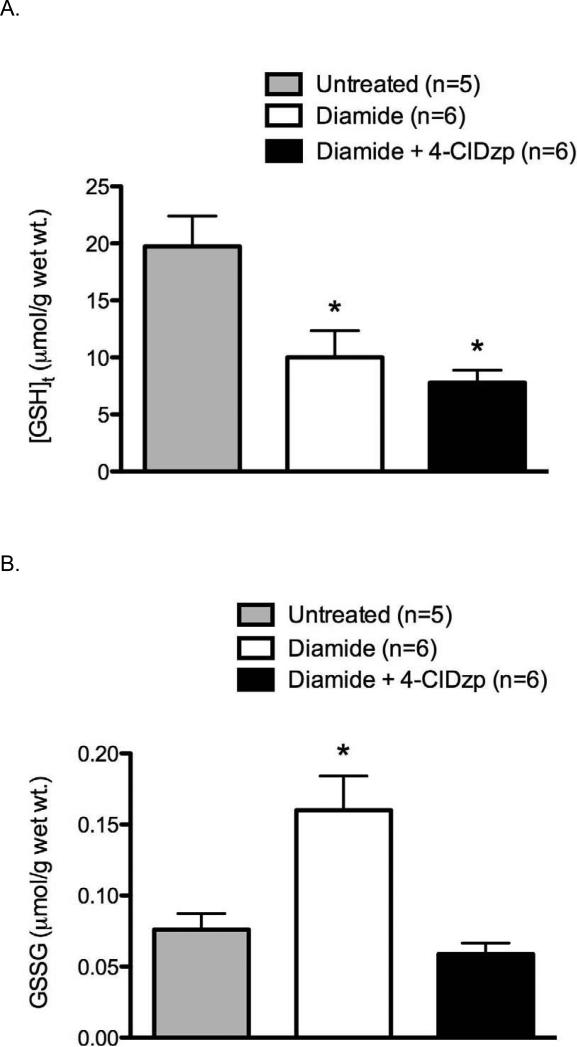
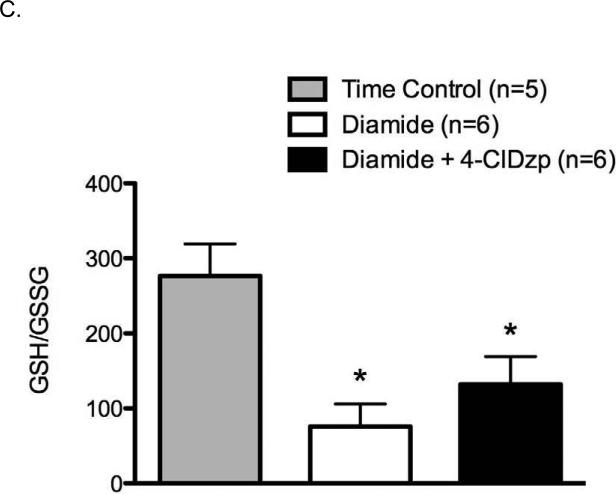
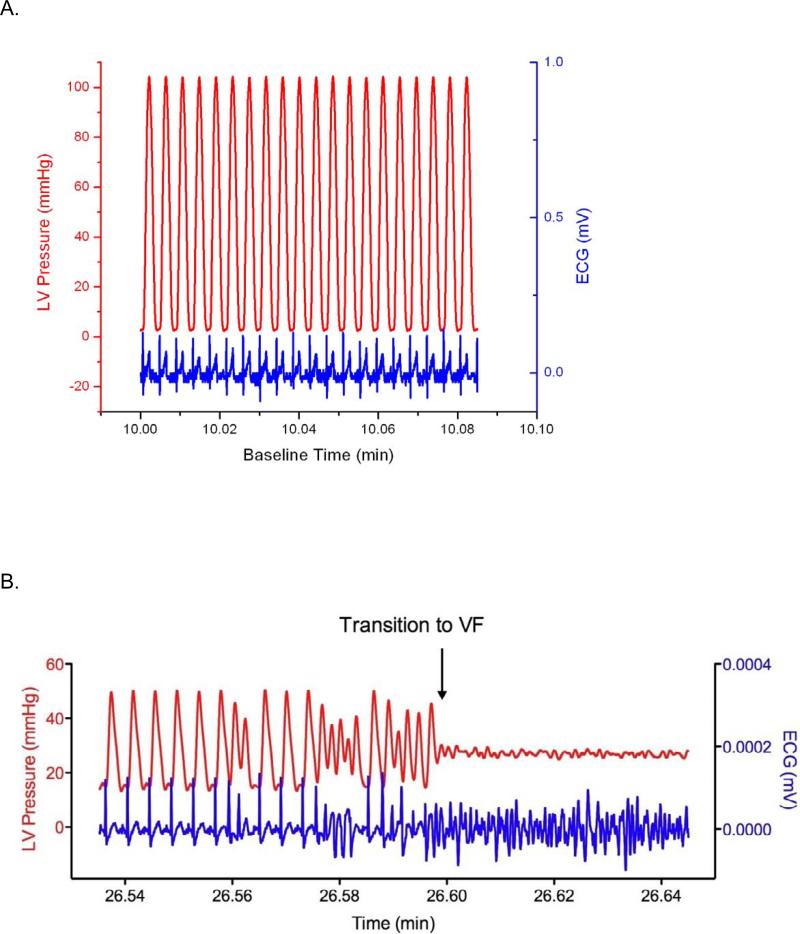
Diamide treatment led to a significant decrease in total glutathione (GSHt; Figure 1A; P < 0.05), and 4-ClDzp did not prevent the decreased GSHt. There were no differences in GSHt between diamide and diamide + 4-ClDzp groups (ANOVA). Administration of diamide resulted in a significant increase in GSSG (Figure 1B, P <0.05), and the presence of 4-ClDzp prevented the GSSG increase. The GSH/GSSG ratio in all diamide-treated hearts was significantly lower after diamide, and the decrease in GSH/GSSG was not significantly affected by the addition of 4-ClDzp (Figure 1C).
Figure 1.
Content of glutathione in guinea pig hearts. Control bars represent hearts that were perfused under normoxic conditions in the absence of diamide. A. Total myocardial glutathione content (GSHt); B. Content of oxidized glutathione (GSSG); C. Ratio of GSH/GSSG in hearts; *, P < 0.05 versus untreated (ANOVA).
Diamide-induced Arrhythmias
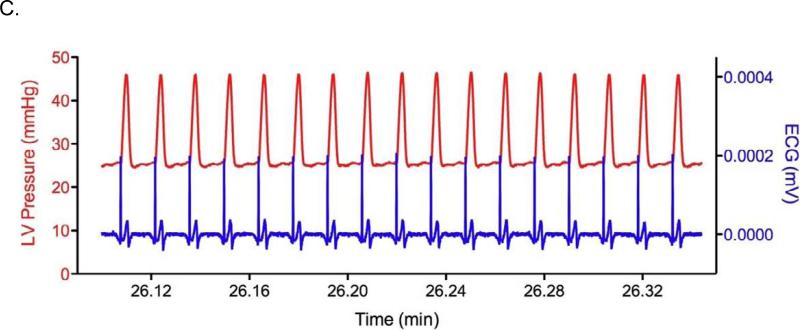
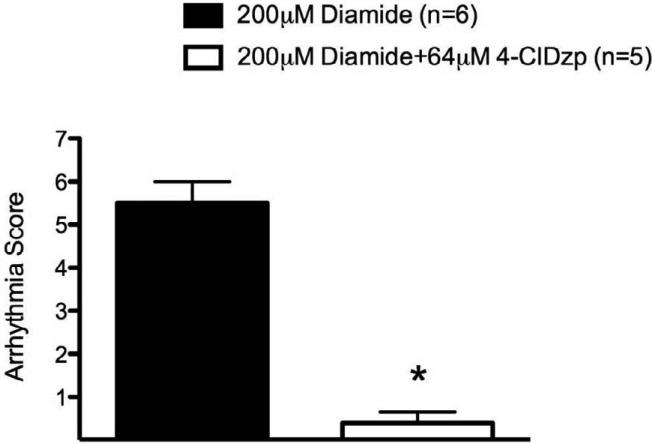
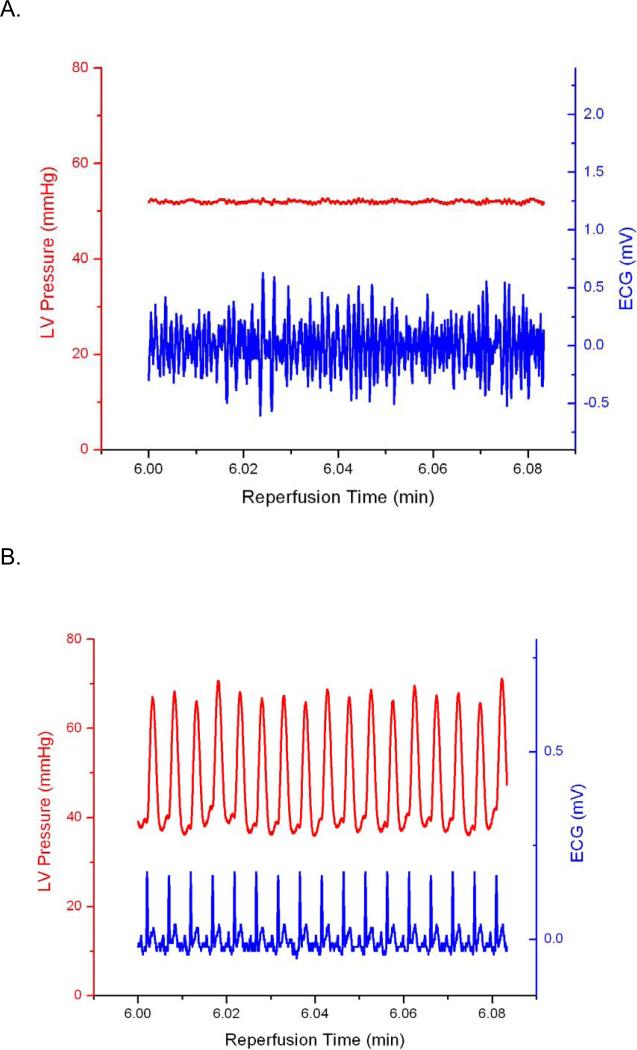
A representative trace of baseline LVDP and ECG is presented in Figure 2A. There were no significant differences in LVDP among the diamide or ischemia/reperfusion groups prior to infusion of drugs or ischemia. Representative ECG and LV Pressure traces recorded during diamide administration are presented in Figures 2B and 2C. Arrhythmia scores were quantified as described in Methods (Figure 3). Hearts perfused with diamide showed a significant propensity for arrhythmias (Figure 2B), with all hearts (6/6) displaying ventricular tachycardia and/or ventricular fibrillation during diamide. When 4-ClDzp was added to the perfusate, hearts were protected against arrhythmias induced by diamide (Figure 2C) and the arrhythmia scores were significantly lower than in hearts that received diamide alone (Figure 3). During the washout phase, only 2/6 diamide-treated hearts reverted out of VT/VF, and VT/VF was sustained for the duration of the protocol in the others. None (0/5) of the 4-ClDzp-treated hearts displayed marked VT or VF either during diamide treatment or following washout.
Figure 2.
Representative LV Pressure and ECG from hearts in the study. A. Control heart after 10 minutes of baseline perfusion with simultaneous LV Pressure (red) and ECG (blue). B. LV Pressure and ECG in a heart 6 minutes into the washout period following the diamide treatment showing the transition to VF (blue) with concomitant loss of pump function (red). C. LV Pressure and ECG in a heart 6 minutes into the washout period following diamide treatment plus 64μM 4-ClDzp.
Figure 3.
Arrhythmia scores for hearts during treatment with diamide and washout in the presence or absence of 64μM 4-ClDzp.
Left ventricular developed pressure
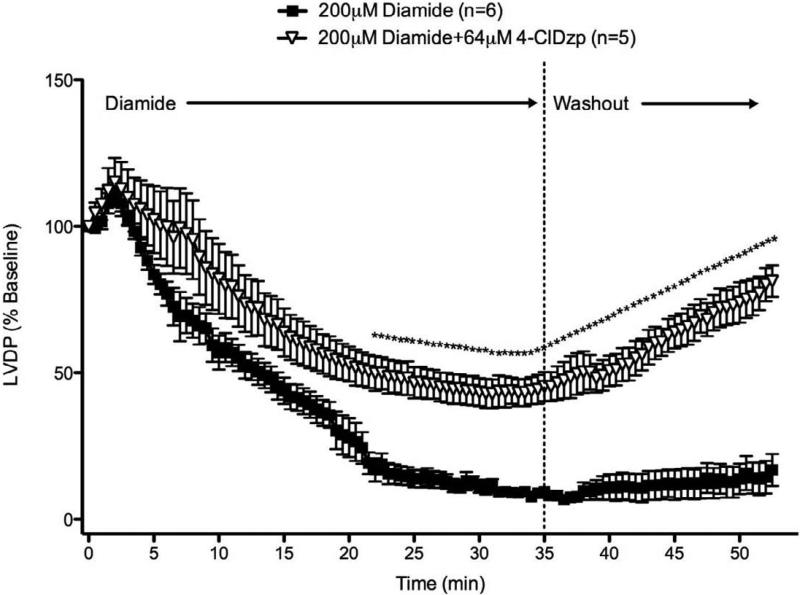
Since both diamide and 4-ClDzp have been shown to influence the inotropic state of the heart [7, 13, 14], left ventricular developed pressure (LVDP) in each heart was normalized to the baseline LVDP measurement taken 30 sec before the onset of diamide or ischemia (Figure 4 and 6, respectively). During the diamide perfusion, LVDP showed a steady decline in the control group. The decline in LVDP was attenuated with 4-ClDzp, with 4-ClDzp treated hearts having significantly higher LVDP from 22 minutes after diamide through the duration of the protocol (Figure 4). By the end of the washout period, LVDP in hearts that received 4-ClDzp during the diamide treatment almost returned to pre-diamide levels.
Figure 4.
Left ventricular developed pressure (LVDP) for hearts in the study. Hearts were perfused with diamide for 25 minutes in the presence or absence of 64μM 4-ClDzp and then washed out for 15 minutes. LVDP values are normalized to baseline values.
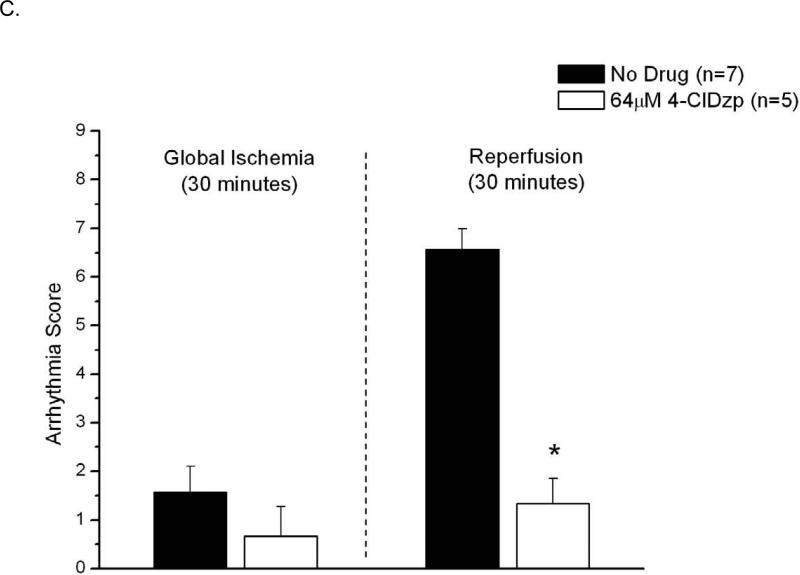
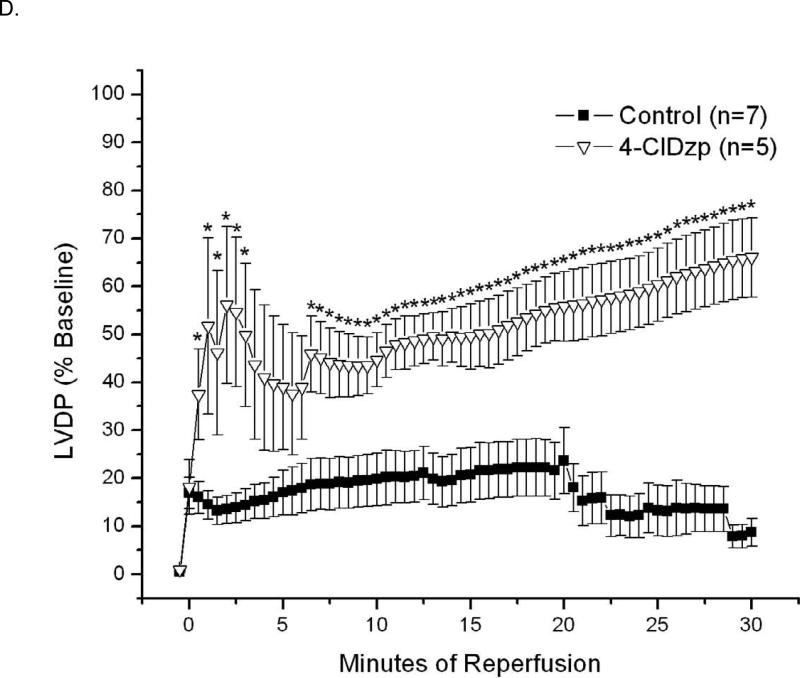
Figure 6.
Data from hearts exposed to 30/30 min of ischemia/reperfusion. Representative LV Pressure (red) and ECG (blue) traces for control (A) and 4-ClDzp-treated (B) hearts. C. Arrhythmia scores for hearts exposed to ischemia and reperfusion in the absence or presence of 64μM 4-ClDzp. D. LV Developed Pressure for hearts after 30 minutes of global ischemia.
Two-photon Imaging of Mitochondria in Intact Hearts
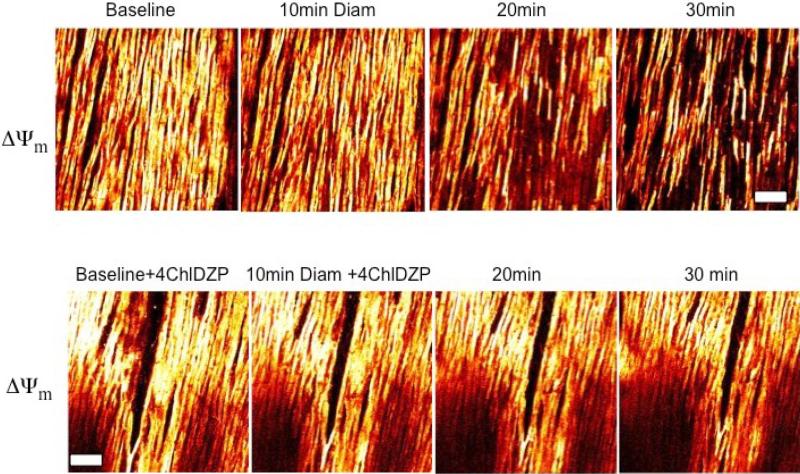
□□□□ fluorescence from intact hearts are presented in Figure 5. During diamide treatment, mitochondria remained polarized until approximately 20 minutes of diamide exposure, at which point ΔΨm began to heterogeneously depolarize. Co-administration of 4-ClDzp during diamide treatment prevented the collapse of ΔΨm.
Figure 5.
Imaging of ΔΨm in intact guinea pig hearts using 2-photon microscopy after exposure to diamide (top) and diamide + 4-ClDzp. Bar is equal to 100μM.
Global Ischemia-Reperfusion
Hearts that underwent 30 minutes of ischemia and reperfusion are presented in Figure 6. Hearts showed a high propensity for arrhythmias at the onset of reperfusion (Figure 6A), and hearts that were perfused with 4-ClDzp before ischemia showed significantly lower incidence of arrhythmias (Figure 6B). Arrhythmia scores were significantly lower in hearts that were treated with 4-ClDzp (Figure 6C, P < 0.05).
LVDP for hearts in the I/R groups are presented in Figure 6D. As globally ischemic hearts do not beat, LVDP is presented only for the 30 minute reperfusion period for hearts in the I/R groups. In hearts that underwent ischemia, LVDP was significantly higher in hearts that were treated with 4-ClDzp than in control hearts for the duration of the reperfusion (Figure 6D).
Discussion
In this study, we hypothesized that overwhelming the antioxidant defenses with diamide in normoxic perfused hearts would trigger mitochondrial criticality [15], leading to lethal arrhythmias in isolated hearts. Diamide treatment mediated the collapse of ΔΨm, increased arrhythmias, and initiated mechanical dysfunction. A ligand of the mBzR, 4′-chlorodiazepam (4-ClDzp), prevented the collapse in ΔΨm evoked by diamide and protected guinea pig hearts from electromechanical dysfunction. The protection provided by 4-ClDzp was similar to that observed after ischemia/reperfusion. To the best of our knowledge, no previous study has described the pro-arrhythmic effects of diamide treatment or the protection of diamide-induced arrhythmias afforded by 4-ClDzp.
Depletion of Cellular Glutathione Pool Causes Arrhythmias
It is widely known that reactive oxygen species (ROS) play a role in cardiac electromechanical dysfunction following ischemia/reperfusion. Bursts of ROS are commonly observed within the first few minutes of reperfusion and have been associated with increased propensity for arrhythmias [2] and mechanical dysfunction [1]. Significant evidence suggests that increased ROS scavenging ability decreases reperfusion injury. Up-regulation of mitochondrial antioxidant capacity, either by direct infusion of ROS scavengers [16] or increased protein expression [17, 18] results in cardioprotection.
The reduced glutathione pool represents the largest capacity thiol buffer in mammalian cells [19] and it is a key player in the detoxification of H2O2 via glutathione peroxidase. Decreased glutathione would therefore contribute to oxidative stress by altering the balance of ROS production to ROS scavenging. As originally described by Kosower [8], diamide chemically oxidizes the GSH pool without generating ROS during the reaction, which allowed us to alter the antioxidant status without increasing the ROS load directly (as would H2O2 treatment). Administration of either diamide under normoxic conditions [10, 13], or global ischemia/reperfusion [20], increases the oxidized glutathione in heart. The negative consequences of oxidized glutathione are also evident from studies showing that infusion of reduced glutathione during early reperfusion can completely eliminate the incidence of ventricular fibrillation in the isolated heart [2].
Our data are consistent with a number of studies indicating that oxidative stress results in a marked increase in GSSG, a decrease in the GSH/GSSG ratio, and decreased total GSH [21-23]. The GSH/GSSG ratio was lower in diamide-treated hearts, and 4-ClDzp blunted the increase in GSSG seen with diamide alone. In single cell studies, both ΔΨm and NAD(P)H redox potential were maintained in the presence of 4-ClDzp following diamide treament [9], and GSSG was shown to be the key factor correlating with ΔΨm loss and ROS accumulation. Our experiments here in the whole heart corroborate the single-cell studies, given that diamide decreased total glutathione to a similar extent in both treatment groups, yet the 4-ClDzp-mediated protection was associated with much lower levels of GSSG.
The observed protection by 4-ClDzp during diamide treatment was very similar to the attenuation of ischemia/reperfusion injury. The protection against LV dysfunction by 4-ClDzp after ischemia-reperfusion are novel findings in the guinea pig, and are in agreement with our previous work in the rabbit heart [7]. Although we did not measure glutathione in ischemic hearts from this study, the ~60% decrease in GSH/GSSG that we observed following diamide is similar to other studies examining changes in glutathione after ischemia [23-25]. Treatment of hearts with 4-ClDzp completely prevented episodes of VT or VF observed in early reperfusion and led to dramatically better LV function. In this study, both diamide and ischemia led to a drastic reduction in mechanical function (largely due to incidence of ventricular arrhythmia), and administration of 4-ClDzp led to recoveries of function that were 81% and 67% for diamide and ischemia, respectively (Figures 4 and 6D). The similar cardioprotection evoked by 4-ClDzp in the two distinct models of oxidative stress (diamide and ischemia/reperfusion) suggests that this compound is targeting a common pathway activated during ROS bursts, namely the mitochondrial ROS-induced ROS release mechanism.
Mechanistic Link Between ROS, ΔΨm, and Arrhythmias
In the present study, diamide-induced arrhythmias and mechanical dysfunction could be attenuated with a ligand of the mitochondrial benzodiazepine receptor (mBzR). Previous work by our group has indicated that the phenomenon of ROS-induced ROS release can increase the activity of energy-dissipating mitochondrial ion channels, in particular, inner membrane anion channels (IMAC) modulated by mBzR. Activation of these energy-dissipating channels, in turn, influences the electrical excitability of the cardiac tissue. In a series of studies, we showed that cardiac myocytes exposed to oxidative stress exhibit oscillations in ΔΨm that activate sarcolemmal ATP-sensitive potassium channels [3], likely via phosphotransfer networks [26]. Oscillating membrane K+ currents were observed under several different conditions in isolated myocytes exposed to substrate deprivation [4] or laser flash [3]. According to the model we have previously described, KATP current activation driven by mitochondrial instability induces lability in the cellular action potential duration and alters the refractory period, which increases the dispersion of repolarization in the tissue and promotes reentrant arrhythmias through the formation of metabolic sinks [6, 7]. The ability of ligands to the mBzR to stabilize ΔΨm and prevent arrhythmias [6, 7] is reinforced here using a very different method to induce oxidative stress in the absence of ischemia.
Interestingly, occurring within the same time window as the period of maximum susceptibility to arrhythmias, two-photon laser scanning fluorescence imaging of the whole heart showed that gradual oxidation of the cellular glutathione pool caused a heterogeneous collapse in ΔΨm during diamide. Administration of 4-ClDzp prevented ΔΨm loss during diamide and prevented the diamide-induced arrhythmias.
Our observations in the intact heart compliment several recent cellular experiments where the redox status of the cell influenced the propensity for ΔΨm collapse. In isolated myocytes exposed to graded ischemia, Ganetkevich et al. noted that the collapse of ΔΨm observed under low O2 tensions could be prevented by the administration of glutathione [27]. Our group recently reported that treatment of isolated cardiac myocytes with diamide triggers oscillations in ΔΨm that could be reversed by 4-ClDzp [9]. In addition, when myocytes were partially permeabilized and treated with decreasing ratios of reduced glutathione/glutathione disulfide, depolarization of ΔΨmwas prominent at GSH/GSSG ratios less than 100:1. In the present study, hearts receiving diamide showed high susceptibility to arrhythmias and had GSH/GSSG less than 100:1 (mean GSH/GSSG was 76:1). Hearts that received 4-ClDzp showed higher GSH/GSSG ratio (mean 132:1), and did not display marked VT/VF at any time during diamide exposure or washout. Although the decrease in GSH/GSSG was slightly blunted with 4-ClDzp, it was not significantly different from diamide alone, indicating that a decrease in the GSH/GSSG per se is not the only factor required to induce criticality. The diamide-induced increase in GSSG, however, was markedly blocked with 4-ClDzp, suggesting that the GSSG concentration itself appears to be a main regulator of IMAC [9].
Importantly, both the collapse of ΔΨm and arrhythmias are blocked by ligands of the mBzR complex, and not by cyclosporine A, the classical permeability transition pore (PTP) inhibitor, indicating a graded redox sensitivity of the target mitochondrial ion channels [6, 7]. In cells, more extensive depletion of the glutathione pool by diamide, or lowering the GSH/GSSG ratio to less 50:1 in permeabilized cell, eventually induces PTP opening, as evidenced by an irrevocable ΔΨm depolarization and loss of matrix markers [9]. These findings stress the importance of targeting mBzR/IMAC as a harbinger of more severe and permanent mitochondrial damage, which, through the ROS-induced ROS release mechanism, primes the opening of the PTP.
In summary, these experiments have reinforced the strategy of protecting the heart by targeting mitochondrial ion channels in early reperfusion or under other conditions of oxidative stress (e.g., during chronic cardiac disease progression). Overwhelming the mitochondrial antioxidant defenses with diamide resulted in collapse of ΔΨm, cardiac arrhythmias, and mechanical dysfunction. A ligand of the mBzR prevented arrhythmias induced either by ischemia/reperfusion or by treatment with diamide, emphasizing the important role played by the reduced glutathione pool in maintaining ROS balance in the heart.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, et al. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4695–9. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward B, Zakaria MN. Effect of some free radical scavengers on reperfusion induced arrhythmias in the isolated rat heart. J Mol Cell Cardiol. 1985 May;17(5):485–93. doi: 10.1016/s0022-2828(85)80053-5. [DOI] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. The Journal of biological chemistry. 2003 Nov 7;278(45):44735–44. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994 Aug 12;265(5174):962–6. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 5.Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999 Dec;51(4):629–50. [PubMed] [Google Scholar]
- 6.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. The Journal of clinical investigation. 2005 Dec;115(12):3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4'-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008 Jul 1;79(1):141–9. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosower NS, Kosower EM, Wertheim B, Correa WS. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–6. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- 9.Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. The Journal of biological chemistry. 2007 Jul 27;282(30):21889–900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slodzinski MK, Aon MA, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. Journal of molecular and cellular cardiology. 2008 Nov;45(5):650–60. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovascular research. 1988 Sep;22(9):656–65. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 12.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of clinical investigation. 2009 Feb 2; doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antolini M, Debetto P, Trevisi L, Luciani S. Diamide: positive inotropic effect in isolated atria and inhibition of Na+/Ca2+ exchange in cardiomyocytes. Pharmacol Res. 1991 Feb;23(2):163–72. doi: 10.1016/s1043-6618(05)80118-5. [DOI] [PubMed] [Google Scholar]
- 14.Mestre M, Carriot T, Belin C, Uzan A, Renault C, Dubroeucq MC, et al. Electrophysiological and pharmacological characterization of peripheral benzodiazepine receptors in a guinea pig heart preparation. Life Sci. 1984 Aug 27;35(9):953–62. doi: 10.1016/0024-3205(84)90661-1. [DOI] [PubMed] [Google Scholar]
- 15.Aon MA, Cortassa S, Akar FG, O'Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006 Feb;1762(2):232–40. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konya L, Kekesi V, Juhasz-Nagy S, Feher J. The effect of superoxide dismutase in the myocardium during reperfusion in the dog. Free radical biology & medicine. 1992 Nov;13(5):527–32. doi: 10.1016/0891-5849(92)90147-9. [DOI] [PubMed] [Google Scholar]
- 17.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size following ischemia-reperfusion in rat heart. J Appl Physiol. 2003 Aug 22;95:2510–8. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 18.Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. American journal of physiology. 2003 Jan;284(1):H277–82. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- 19.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free radical biology & medicine. 2001 Jun 1;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 20.Bindoli A, Cavallini L, Rigobello MP, Coassin M, Di Lisa F. Modification of the xanthine-converting enzyme of perfused rat heart during ischemia and oxidative stress. Free radical biology & medicine. 1988;4(3):163–7. doi: 10.1016/0891-5849(88)90024-x. [DOI] [PubMed] [Google Scholar]
- 21.Mallet RT, Squires JE, Bhatia S, Sun J. Pyruvate restores contractile function and antioxidant defenses of hydrogen peroxide-challenged myocardium. Journal of molecular and cellular cardiology. 2002 Sep;34(9):1173–84. doi: 10.1006/jmcc.2002.2050. [DOI] [PubMed] [Google Scholar]
- 22.Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002 Jan;51(1):174–81. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 23.Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of beta-adrenergic inotropism in stunned myocardium. Journal of molecular and cellular cardiology. 1999 Oct;31(10):1863–72. doi: 10.1006/jmcc.1999.1020. [DOI] [PubMed] [Google Scholar]
- 24.Werns SW, Fantone JC, Ventura A, Lucchesi BR. Myocardial glutathione depletion impairs recovery of isolated blood-perfused hearts after global ischaemia. Journal of molecular and cellular cardiology. 1992 Nov;24(11):1215–20. doi: 10.1016/0022-2828(92)93088-2. [DOI] [PubMed] [Google Scholar]
- 25.Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O. The role of glutathione status in the protection against ischaemic and reperfusion damage: effects of N-acetyl cysteine. Journal of molecular and cellular cardiology. 1988 Jan;20(1):5–13. doi: 10.1016/s0022-2828(88)80174-3. [DOI] [PubMed] [Google Scholar]
- 26.Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. Faseb J. 1998 May;12(7):523–9. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 27.Ganitkevich V, Reil S, Schwethelm B, Schroeter T, Benndorf K. Dynamic responses of single cardiomyocytes to graded ischemia studied by oxygen clamp in on-chip picochambers. Circulation research. 2006 Jul 21;99(2):165–71. doi: 10.1161/01.RES.0000232321.89714.0e. [DOI] [PubMed] [Google Scholar]