Abstract
Azlactone-functionalized polymers are used as reactive templates for the synthesis of a library of amine-functionalized polymers of interest in the context of DNA delivery and other applications.
Methods for the parallel synthesis and high-throughput screening of polymeric materials can accelerate dramatically the rates at which new and useful materials are identified in a variety of fundamental and applied contexts.1–3 The synthesis of libraries of synthetic cationic polymers, for example, has contributed significantly to the discovery of new agents for the delivery of nucleic acids in vitro and in vivo.1 The parallel synthesis/fabrication of spot-based arrays of polymers has also lead to the rapid identification of polymers that promote the adhesion and/or differentiation of stem cells and other cell types.2 Other examples of this approach in these contexts and a range of other potential applications have been reported recently.3
In addition to accelerating the rate at which new materials can be identified, approaches based on parallel synthesis and screening can also be used to identify key structural features that govern the behaviours of new classes of materials. Identifying structure/function relationships for libraries of synthetic polymers can be complicated, however, if systematic changes in some structural features (such as side chain or end group structure) cannot be made without holding other structural features (e.g., molecular weights or molecular weight distributions) constant. For example, step growth polymerization processes can be used to introduce systematic changes in side chain structure, but also often result in libraries of polymers with a broad range of molecular weights.1a–c,f Synthetic approaches that provide access to libraries of polymers that are structurally diverse – but that also have uniform molecular weights and molecular weight distributions – would provide opportunities to exploit further the potential of this approach and identify the key features of new materials that are important for function.
Several of the issues described above can be addressed, at least in part, by adopting a synthetic approach based on the functionalization of reactive ‘template’ polymers.4 The most apparent practical advantage of this approach is that the synthesis of multiple polymers using a single, ‘universal’ backbone can, in principle, provide access to libraries of structurally diverse polymers having one common molecular weight and one common polydispersity. Several different reactive polymers have been used to explore the feasibility of this approach.1h,3c,f,4 The work reported here sought to investigate the use of azlactone-functionalized polymers as reactive templates for parallel polymer synthesis.
 |
(Eq. 1) |
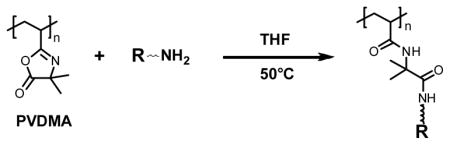
Polymers containing azlactone functionality appear well-suited to this approach because azlactones react readily with a variety of nucleophiles through simple ring-opening reactions.5a In the case of primary amine nucleophiles, these reactions proceed rapidly, in the absence of a catalyst, and without the generation of byproducts. We and others have reported the rational design of side-chain functionalized polymers by the reaction of poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) with different amine-based nucleophiles (Eq. 1).5 This investigation sought to investigate the suitability of this approach for the parallel synthesis of a small library of amine-functionalized polymers of potential interest in the context of DNA delivery and other applications for which cationic polymers are useful.
To explore the feasibility of this approach, we targeted the synthesis of a small test library of amine-functionalized polymers using PVDMA (Mn=74,000; PDI=2.65) and 12 different amine-functionalized small molecules. We selected compounds 1–12 possessing both primary and tertiary amine functionality for two reasons: (i) as stated above, primary amines react rapidly with azlactone functionality, and (ii) tertiary amines do not react with azlactone functionality.5a The library of polymers resulting from the reaction of PVDMA with these compounds would thus consist of tertiary-amine containing polymers (hereafter referred to as polymers P1–P12). Compounds 1–12 were selected on the basis of differences in steric environments and hydrophobicity as well as the resulting distance of the tertiary amines from the amide/amide linker in the backbone of the resulting polymer (e.g., two versus three carbons in the structures of polymers P1 and P2, etc).
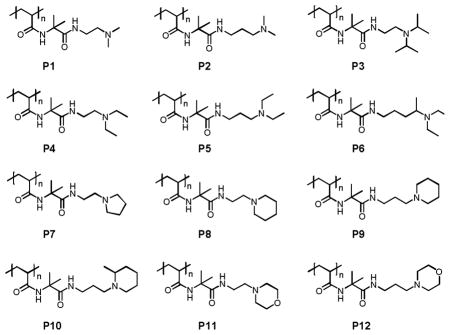
Exhaustive functionalization of PVDMA in 12 individual reactions resulted in polymers P1–P12 in near quantitative yield (additional details related to synthesis and characterization are included as Supporting Information). All polymers were soluble in aqueous media and organic solvents such as THF. Characterization of polymers P1–P10 by gel permeation chromatography (GPC) revealed average molecular weights (Mn) ranging from ~30,000 to ~43,000 with molecular weight distributions that were substantially overlapping (PDI’s ranging from 1.4–2.0; see Supporting Information). These differences are small compared to the large differences reported in past studies on the synthesis of polymer libraries using step growth methods,1a–c,f and could arise from precipitation steps used during isolation of the polymers or from small variations in the interactions of these polyamines with the GPC columns used to characterize molecular weight. The molecular weights of polymers P11 and P12 were significantly higher (Mn ~58,000) with higher PDI’s (PDI ~3.5). Although the reasons for these larger differences are not clear, these two polymers were also included in all subsequent experiments described below.
The results above demonstrate that azlactone-functionalized polymers can be used as reactive templates for the synthesis of a library of tertiary amine-functionalized polymers. As stated above, these polymers are of potential interest in a range of different applications for which polyamines or cationic polymers are commonly used. We sought to evaluate the potential of this small library to identify new polymers of interest in the context of DNA delivery and cell transfection.6 In addition to the potential to accelerate the discovery of new polymers for this application, this work is also of fundamental interest in this context because the ‘amide/amide’ side chain structural motif present in polymers P1–P12 has not, to our knowledge, been explored previously for the design of polymer-based DNA delivery agents.
Polymers P1–P12 were first characterized with respect to their ability to form electrostatic complexes with plasmid DNA using agarose gel electrophoresis retardation assays. Each polymer was used to formulate DNA/polymer complexes (or ‘polyplexes’) at 10 different DNA/polymer ratios (w/w) ranging from 1:1 to 1:10, as described in past studies.1a–c All 12 polymers formed complexes with DNA at levels sufficient to retard the migration of DNA at ratios as low as 1:1. The full results of these experiments are included as Supp. Info.
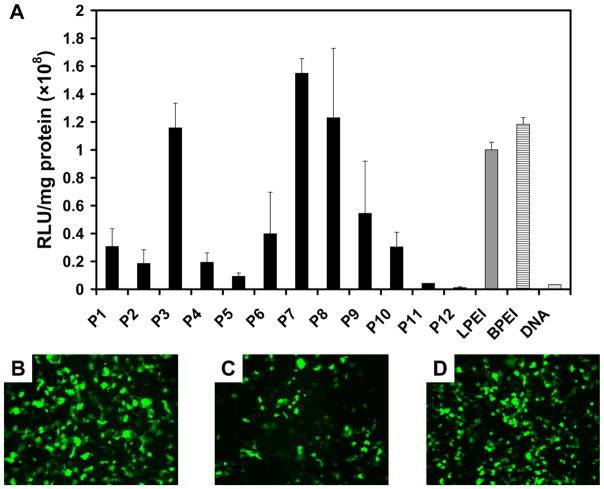
We next conducted a series of cell-based screening experiments to characterize the ability of polymers P1–P12 to promote cell transfection in vitro. These initial experiments were performed using COS-7 cells and polyplexes formed using a plasmid DNA construct (pCMV-Luc) encoding firefly luciferase to permit quantitative characterization of transgene expression using a bioluminescence-based assay.1a–c Fig. 1 shows the results (expressed as relative light units per mg of protein) of transfection experiments using polyplexes formed using DNA and polymers P1–P12 or linear or branched poly(ethyleneimine) (LPEI or BPEI; two well-established gene delivery polymers6 used here as positive controls). We note here that initial rounds of screening were performed in parallel to evaluate each polymer over a range of different DNA/polymer ratios (e.g., from 1:1 to 1:10). However, Fig. 1 shows only those results for DNA/polymer ratios identified to yield the highest levels of transfection for each polymer (i.e., the ‘optimal’ formulations observed for each polymer) after these initial rounds of screening. The results of initial screens and characterization of cytotoxicity are included as Supporting Information.
Figure 1.
(A) Levels of transgene expression as a function of polymer structure for COS-7 cells treated with polyplexes formed using DNA encoding firefly luciferase. Light units are arbitrary and normalized to total cell protein; experiments were performed in triplicate. Results correspond to experiments using polyplexes formed at DNA/polymer ratios of 1:2 for P2, P5, P10, and P11; 1:4 for P8; 1:5 for P1 and P3; 1:8 for P4, P6, P7, and P12; 1:9 for P9 chosen on the basis of initial broader screens (see text). Experiments performed in the absence of polymer (DNA only) and using polyplexes formed using LPEI and BPEI as controls are shown for comparison. (B–D) Fluorescence micrographs showing EGFP expression in cells treated with polyplexes formed using DNA encoding EGFP and polymer P3 (B), P7 (C), or P8 (D) at the DNA/polymer ratios used in (A).
The data in Fig. 1 reveal that polymers P1–P12 can promote cell transfection, but at levels that vary significantly as a function of polymer structure. Three polymers in this library (P3, P7, and P8) mediated levels of transfection that were on the order of those mediated by BPEI and LPEI. Figs. 1B–D show additional representative fluorescence microscopy images of cells treated with polyplexes formed using these three polymers and plasmid DNA encoding enhanced green fluorescent protein (EGFP).
Fig. 1 also demonstrates that all other polymers mediated levels of transfection that were lower than BPEI, LPEI, or polymers P3, P7, and P8. However, further inspection of these data permits the identification of structural motifs that appear to influence the gene delivery properties of these materials. For example, the best performing polymers in this library all have side chains with tertiary amine groups that are, in general, (i) more sterically hindered (e.g., the isopropyl groups or cyclic structures of P3, P7, and P8), and (ii) positioned two carbons away from the amide/amide linker to the backbones of the polymers. The apparent importance of this latter feature is further suggested by a comparison of the results for polymers P1 and P2, P4 and P5, P8 and P9, which each differ in structure only by the number of carbons between the tertiary amine group and the amide/amide linker. It is difficult to draw quantitative conclusions using the results for these less effective polymers. However, the data in Fig. 1, when combined, suggest that polymers with amine groups two carbons removed from the amide/amide linker promote levels of transfection that are, in general, higher than those with amine groups that are three carbons removed.
Our results demonstrate that the addition of amine-containing functionality to azlactone-functionalized polymers can be used to design cationic polymers useful for the delivery of DNA to cells. Our initial screens using this relatively small polymer library have also uncovered structural features that appear important with respect to the gene delivery behaviours of this new class of materials. It is important to note, however, that the synthesis and additional screening of larger and more diverse polymer libraries will be required to establish these and other structure/property relationships more clearly. In this context, the azlactone-based approach described here presents a robust platform for the elaboration of larger, more diverse libraries of polymers by selecting potential side chain functionality from (i) a larger pool of primary amine-functionalized molecules, and/or (ii) a range of other alcohol-or thiol-based nucleophiles (which can also react readily with azlactones).5a This latter approach would also permit comparisons of the properties of polymers bearing the amide/amide structure of polymers P1–P12 with those having analogous amide/ester or amide/thioester structures, as well as the combinatorial synthesis of copolymers having mixtures of different side chain functionality.1h
Finally, we note that the synthesis of azlactone-functionalized polymers can be performed under living/controlled conditions7 that give rise to reactive polymers with specified molecular weights and lower polydispersities (thereby increasing the potential ‘structural space’ that could be designed into a given polymer library). Initial characterization of analogs of polymers P3, P7, and P8 synthesized using lower molecular weight PVDMA (Mn=5,800; PDI=1.11) demonstrated that these polymers do not mediate high levels of transfection (see Supp. Info.), suggesting that molecular weight also plays an important role in governing the ability of these polymers to transfect cells. In addition to the structural influences noted above, differences in the pKa values of the amines in polymers P1–P12 could lead to differences in endosomal escape or the release of DNA that could also lead to differences in transfection. Additional characterization of polyplexes arising from these libraries with respect to other physicochemical properties (e.g., size, zeta potential) known to be important in the context of cell transfection6 will provide additional insight into factors that govern the gene delivery behaviours of these new materials. More generally, access to libraries of new polymers with well defined structures should prove useful in a wide range of other fundamental and applied contexts.
Supplementary Material
Acknowledgments
Support was provided by the Arnold and Mabel Beckman Foundation, the NIH (EB006820), and the Alfred P. Sloan Foundation. M.E.B was funded in part by an NIH Chemistry Biology Interface Training Grant (NIGMS T32 GM008505).
Footnotes
Electronic Supplementary Information (ESI) available: Details of polymer synthesis and characterization; results of gel electrophoresis, cell transfection, and cytotoxicity assays.
Notes and references
- 1.(a) Lynn DM, Anderson DG, Putnam D, Langer R. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]; (b) Akinc A, Lynn DM, Anderson DG, Langer R. J Am Chem Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]; (c) Anderson DG, Lynn DM, Langer R. Angew Chem, Int Ed. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]; (d) Anderson DG, Peng WD, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. Proc Natl Acad Sci U S A. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen DJ, Majors BS, Zelikin A, Putnam D. J Controlled Release. 2005;103:273–283. doi: 10.1016/j.jconrel.2004.11.028. [DOI] [PubMed] [Google Scholar]; (f) Gan L, Olson JL, Ragsdale CW, Yu LP. Chem Commun. 2008:573–575. doi: 10.1039/b714278a. [DOI] [PubMed] [Google Scholar]; (g) Barua S, Joshi A, Banerjee A, Matthews D, Sharfstein ST, Cramer SM, Kane RS, Rege K. Mol Pharm. 2009;6:86–97. doi: 10.1021/mp800151j. [DOI] [PubMed] [Google Scholar]; (h) Wong SY, Sood N, Putnam D. Mol Ther. 2009;17:480–490. doi: 10.1038/mt.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Anderson DG, Levenberg S, Langer R. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]; (b) Bailey SN, Sabatini DM, Stockwell BR. Proc Natl Acad Sci U S A. 2004;101:16144–16149. doi: 10.1073/pnas.0404425101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]; (d) Tweedie CA, Anderson DG, Langer R, Van Vliet KJ. Adv Mater. 2005;17:2599–2604. [Google Scholar]
- 3.(a) Brocchini S, James K, Tangpasuthadol V, Kohn J. J Am Chem Soc. 1997;119:4553–4554. [Google Scholar]; (b) Brocchini S, James K, Tangpasuthadol V, Kohn J. J Biomed Mater Res. 1998;42:66–75. doi: 10.1002/(sici)1097-4636(199810)42:1<66::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (c) Pedone E, Li XW, Koseva N, Alpar O, Brocchini S. J Mater Chem. 2003;13:2825–2837. [Google Scholar]; (d) Rickerby J, Prabhakar R, Patel A, Knowles J, Brocchini S. J Controlled Release. 2005;101:21–34. doi: 10.1016/j.jconrel.2004.07.021. [DOI] [PubMed] [Google Scholar]; (e) Yang Y, Bolikal D, Becker ML, Kohn J, Zeiger DN, Simon CG. Adv Mater. 2008;20:2037–2043. [Google Scholar]; (f) Gibson MI, Frohlich E, Klok HA. J Polym Sci, Part A: Polym Chem. 2009;47:4332–4345. [Google Scholar]
- 4.Theato P. J Polym Sci, Part A: Polym Chem. 2008;46:6677–6687. [Google Scholar]
- 5.(a) Heilmann SM, Rasmussen JK, Krepski LR. J Polym Sci, Part A: Polym Chem. 2001;39:3655–3677. [Google Scholar]; (b) Guichard B, Noel C, Reyx D, Thomas M, Chevalier S, Senet JP. Macromol Chem Phys. 1998;199:1657–1674. [Google Scholar]; (c) Zhang JT, Lynn DM. Adv Mater. 2007;19:4218–4223. [Google Scholar]; (d) Kinsinger MI, Buck ME, Campos F, Lynn DM, Abbott NL. Langmuir. 2008;24:13231–13236. doi: 10.1021/la803376u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Messman JM, Lokitz BS, Pickel JM, Kilbey SM. Macromolecules. 2009;42:3933–3941. [Google Scholar]
- 6.(a) Pack DW, Hoffman AS, Pun S, Stayton PS. Nat Rev Drug Discovery. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]; (b) Putnam D. Nat Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 7.Fournier D, Pascual S, Fontaine L. Macromolecules. 2004;37:330–335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.