Abstract
In spite of their relatively limited antigen receptor repertoire, CD1d-restricted NKT cells recognize a surprisingly diverse range of lipid and glycolipid antigens. Recent studies of natural and synthetic CD1d presented antigens provide an increasingly detailed picture of how the specific structural features of these lipids and glycolipids influence their ability to be presented to NKT cells and stimulate their diverse immunologic functions. Particularly for synthetic analogues of α-galactosylceramides which have been the focus of intense recent investigation, it is becoming clear that the design of glycolipid antigens with the ability to precisely control the specific immunologic activities of NKT cells is likely to be feasible. The emerging details of the mechanisms underlying the structure-activity relationship of NKT cell antigens will assist greatly in the design and production of immunomodulatory agents for the precise manipulation of NKT cells and the many other components of the immune system that they influence.
Keywords: Natural Killer T cell, CD1d, α-galactosylceramide, glycolipid, antigen presentation
1. Introduction
Natural Killer T cells (NKT cells) are a major population of innate-like T lymphocytes that perform a wide range of functions that are potentially relevant to host defense against microbial infections [1], the maintenance of immune tolerance [2] and the elimination or control of malignant cancers [3]. Originally defined as cells that co-express αβ T cell antigen receptors (TCRs) and markers characteristic of natural killer (NK) cells such as NK1.1 (CD161), they have more recently been subdivided into at least three subsets [4]. Among these, the most abundant and well-studied are known as type 1 NKT cells, also referred to as invariant NKT cells (iNKT cells) because of their expression of an invariant TCRα chain rearrangement (Vα24 with Jα18 in humans, or Vα14 with Jα18 in mice) [5]. Invariant NKT cells are dependent on the nonpolymorphic MHC class I-like CD1d molecule for their thymic selection and development, and recognize a variety of lipid and glycolipid antigens presented by this antigen presenting molecule to their TCRs [5,6]. A second population known as type II NKT cells has diverse TCRs, and also responds to antigens presumed to be lipid in nature that are presented by CD1d. A third population known as type III NKT cells shares some of the phenotypic properties with the other groups but is not CD1d dependent.
A characteristic feature of iNKT cells is that they circulate and populate organs such as the spleen and liver in a distinctive state of partial activation. Phenotypically, they resemble conventional effector memory T cells, with high surface levels of activation markers such as CD44 and CD69 and low expression of CD62L [6,7]. They display similar homeostatic maintenance requirements as antigen-experienced T cells for survival in the periphery [8], but in contrast to classical MHC-restricted memory T cells the iNKT cell active state does not require prior recognition of foreign antigens. Instead, this seems to be innately programmed by a type of controlled self-reactivity [9]. This innate self-reactivity may underlie the homeostatic function of iNKT cells in contributing to maintenance of immune tolerance [10,11]. The ability of iNKT cells to promote immune tolerance is believed to be related at least in part to their production of a variety of anti-inflammatory or regulatory cytokines, including high levels of several T helper-2 (Th2) type cytokines such as IL-4, IL-5 and IL-13 [12,13].
In the setting of microbial infection or possibly also in response to inflammation resulting from tumors, iNKT cells can be acutely activated to release IFNγ and other pro-inflammatory cytokines. This can be achieved either by TCR mediated recognition of specific microbial ligands, or by a combination of self-recognition plus signals delivered by inflammatory cytokines such as IL-12 [14]. Like effector memory T cells, iNKT cells do not require classical costimulatory signals to secrete cytokines following TCR engagement [15], and the constitutive expression of preformed cytokine-encoding mRNA transcripts in the cytoplasm of iNKT cells has been linked to their ability to mount extremely rapid effector responses [16]. This is also associated with the activation of a variety of other leukocytes including NK cells and dendritic cells (DCs) to express their effector functions. Through a combination of these and other activities, iNKT cells can exert major effects on early and delayed adaptive immunity to tumors and to a wide variety of infectious pathogens [1,17]. In addition NKT cell activating glycolipids have been used as adjuvants to improve antigen specific immune responses to vaccines in mice, against a wide variety of infectious disease [18–21].
The wide range of different functional activities associated with iNKT cells and the strong conservation of this T cell subset between mouse and human has led to considerable interest in exploring the therapeutic benefits that could be derived by modulating iNKT cell responses. It is believed that this might be achieved by the discovery or design of specific lipid antigens that precisely activate the different functions of iNKT cells. Here we review the substantial progress that has been made in recent years towards identifying both natural and synthetic lipid antigens recognized by iNKT cells and other CD1d-dependent T cell populations (i.e., type II NKT cells). The emerging picture of how ligand structure may control the expression of different iNKT cell functions is also discussed, along with implications for how this may contribute to the use of iNKT cell activators in the prevention or therapy of a broad range of different human diseases.
2. Natural Self lipids presented by CD1d
A characteristic feature of both mouse and human iNKT cells is that they can be stimulated in vitro by co-culture with antigen presenting cells expressing CD1d even without the addition of an exogenous lipid antigen [22,23]. This apparent CD1d-restricted autoreactivity is believed to be due to recognition of cellular lipids that normally associate with CD1d proteins within cells and are subsequently presented on the cell surface for recognition by the iNKT cell TCRs. The CD1d autoreactivity is considered a hallmark of mature iNKT cells, and may reflect their requirement for CD1d dependent positive selection in the thymus which most likely also involves the recognition of self lipid ligands [24]. Extensive research efforts have been devoted to discovering the specific self lipids that are involved in the thymic selection and peripheral activation of iNKT cells, and to a lesser extent type II NKT cells. Although significant progress has been made, this area remains to a great extent poorly defined and a number of controversies related to the identification of candidate natural lipid ligands have yet to be resolved, as summarized briefly below.
2.1. Self phospholipid antigens
Cellular glycosylphosphatidylinositols (GPIs) which bind to CD1d with high affinity through their phosphatidylinositol membrane lipid anchors have been proposed as potential natural self-ligands for iNKT cells [25]. This idea originated mainly with the finding that CD1d proteins purified from cells contained bound glycolipids that could be shown through a combination of mass spectroscopy and metabolic radiolabeling to most likely be GPIs. However, another recent study using a similar approach was unable to confirm an abundance of phosphatidylinositol (PI) or GPIs as the bound natural ligands of CD1d proteins extracted from cells, but instead found mainly phosphatidylcholine (PC) and sphingomyelin to be the major CD1d-associated cellular ligands [26]. Other studies using a functional approach to define the self lipids recognized by mouse iNKT cell hybridomas also found that polar lipids extracted from cells could activate murine iNKT hybridoma cells when incubated with plate-bound recombinant mCD1d [27]. This stimulatory activity was localized to the glycolipid and phospholipid enriched fractions and testing of a range of common cellular lipids revealed that purified or synthetic forms of PI, PC, phosphatidylglycerol (PG) and phosphatidylethanolamine (PE) could activate a substantial fraction of iNKT hybridomas in a CD1d dependent manner [27]. Furthermore, structural modifications in the acyl-chains of PE showed that the degree of activation of an NKT cell hybridoma was influenced by acyl chain unsaturation, as forms of PE with multiple double bonds showed increased binding to CD1d and were recognized better by iNKT cells than those with one double bond [28]. From these studies, it has been suggested that self-lipids comprising the majority of cell membrane lipids may be crucial in the folding and stabilization of CD1 molecules, and may also constitute targets for self-reactivity of iNKT cells.
More recently, lysophosphatidylcholine (LPC) has been identified as a natural phospholipid ligand for NKT cells by biochemical analysis of lipids extracted from the plasma of multiple myeloma patients. This monoacyl form of PC is elevated in allergic and autoimmune inflammation, asthma and human cancers such as multiple myeloma, and could represent a type of endogenous signal for cellular stress or damage. Recognition of LPC has been demonstrated for type II NKT cells, which can be directly visualized as a population of Vα24−Vβ11− T cells in human blood that bind LPC-loaded CD1d dimers [29]. An analysis of cellular lipids associated with human CD1d proteins purified from cultured cells also identified lysophospholipids to be among the bound natural lipid ligands [26].
2.2. Self glycosphingolipid antigens
Several cellular glycosphingolipids have been implicated as possible self ligands for iNKT cells. An initial report showing that a β-glucosylceramide synthase mutant cell line was defective in iNKT cell stimulation suggested that the natural ligands of NKT cells might include lysosomal glycosphingolipids [30]. A detailed subsequent analysis of mouse mutants with defects in various glycosphingolipid synthesis or degradation pathways pointed to isoglobotrihexosylceramide (iGb3) as a putative self glycolipid antigen for mouse and human iNKT cells [31]. In support of this possibility, it was shown that both mouse and human iNKT cells mounted modest CD1d-restricted responses to purified or synthetic iGb3 in vitro. Furthermore, since iGb3 can be generated by the processing of isoglobotetrahexosylceramide (iGb4) by lysosomal hexosaminidases, the finding that mice lacking the enzyme β-hexoaminidase B have a pronounced defect in iNKT cell development gave support to the hypothesis that iGb3 is a major selecting ligand for iNKT cells in vivo [31].
A number of studies using a synthetic approach have provided further detail on the CD1d presentation and iNKT cell stimulatory properties of iGb3. A structure-activity relationship study between structural analogues of iGb3 and an iNKT cell hybridoma revealed that hydroxyl groups at specific positions (2’ and 3’) on the terminal galactose played a crucial rule for recognition of iGb3 by iNKT cells, while other positions (4’ and 6’) were not found to be as important for this recognition [32]. In the glycosphingolipid extracts from mammalian cell membranes, iGb3 exists in minute amounts mixed with its isomer globotrihexosylceramide (Gb3) present in larger amounts, and the recent development of improved mass spectrometry methods, based on permethylation and ion trap MS, has made the sensitive quantitation of isoglobotriaosylceramide in the presence of isomeric components in limited biological samples feasible [33]. The influence of the stereochemistry of the glycosidic linkage between the ceramide and the proximal sugar, which in natural iGb3 is in the beta configuration, has also been evaluated using a synthetic approach [34]. This showed that synthetic α-anomers of iGb3 and Gb3 are active as iNKT cell ligands, and stimulate iNKT cell responses in vitro with somewhat greater potency than the natural iGb3 containing the beta linkage. A crystal structure of iGb3 bound to CD1d has been reported, providing detailed structural insight into the possible mode of recognition of this putative self antigen [35].
However, despite the considerable evidence that iGb3 can serve as a stimulatory ligand for iNKT cell responses in vitro, several recent studies have challenged the relevance of iGb3 as a natural selecting ligand for iNKT cells in vivo. Analysis of mice with a variety of different enzyme deficiencies other than lysosomal hexosaminidase that all lead to accumulation of lysosomal glycosphingolipids showed that these almost uniformly caused deficiencies in iNKT cell development [36]. This suggests that the defect originally observed in hexosaminidase B deficient mice may have reflected a general dysregulation of endosomal function resulting from lipid storage disease, rather than the specific loss of iGb3. In addition, the development of iNKT cells and their distribution in various organs were observed to be normal in iGb3 synthase deficient mice [37]. Although iGb3 synthase mRNA has been detected in many murine tissues, the human and mouse thymus have been reported to be devoid of iGb3 [38]. The finding that iGb3 cannot be detected using a highly sensitive HPLC based assay in the thymus or dendritic cells of mice and humans has further strengthened the conclusion that this glycolipid may not be a physiological selecting-ligand for normal NKT cell development [38].
A few other glycosphingolipids besides iGb3 have been studied as potential natural ligands for iNKT cells, particularly in the setting of cancer or other diseases. The ganglioside GD3, which is highly expressed on tumors of neuroectodermal origin, has been identified as a potential natural ligand for mouse iNKT cells. CD1d-restricted iNKT cells were shown to respond when either a GD3+ human melanoma or syngeneic APCs loaded with this lipid were injected into mice [39]. Glycosphingolipids may also play a role as antigenic targets in autoimmune diseases such as multiple sclerosis and Guillan–Barré syndrome where self-lipid reactive CD1-restricted T cells may occur [40]. In particular, sulfatide (or 3'-sulfogalactosyl ceramide) which is a major glycolipid present in myelin of the nervous system has been shown to be recognized by CD1d-restricted type II NKT cells. Sulfatide specific NKT cells were shown to be present in detectable numbers in the central nervous system during experimental autoimmune encephalomyelitis (EAE), and treatment of mice with sulfatide was shown to suppress EAE [41]. When synthetic analogues of sulfatide with different fatty acid chain lengths (C16–C24) were analyzed, the cis-tetracosenoyl (C24:1, monounsaturated fatty acid) was found to be the most active species and therefore the potentially immunodominant sulfatide species [42].
3. Microbial glycolipids that activate NKT cells
Multiple studies support the view that iNKTcells have a broad antigen specificity against microbial glycolipids that includes both ceramide-based lipids as well as diacylglycerol-based microbe-derived antigens. The first natural iNKT cell microbial antigen to be reported was a tetramannosylated form of phosphatidylinositol purified from M. bovis BCG [43]. This compound, designated PIM4, was shown to activate only a small fraction of iNKT cells that produced IFNγ but little or no IL-4, and displayed cytolytic activity [43]. Similarly, a nonphosphorylated diacylglycerol based glycolipid derived from the spirochete Borrelia burgdorferi (αMGalD) was shown to be recognized by a fraction of mouse type I NKT cells, and also by a human type I NKT cell line. The iNKT cell responses to this antigen were also characterized by a preferential stimulation of IFNγ rather than IL-4 [44].
The recent discovery of α-glycuronosylceramides, including α-galacturonosyl and α-glucuronosylceramide, in Sphingomonas species bacteria as CD1d-presented iNKT cell antigens, provided further evidence of a significant role for iNKT cells in antimicrobial defense [45–47]. α-Glucuronosylceramides are 6-carboxylated α-glycosylceramides expressed by bacteria of the Sphingomonas or Ehrlichia genuses. CD1d molecules bind these glycolipids and present them to iNKT cells. It was found that a fraction of iNKT cells react to α-glucuronosylceramides in mice, and all human NKT cell lines tested recognized such antigens. These antigens induced strong secretion of both IFNγ and IL-4 in both human and murine NKT cells. The presentation of Sphingomonas glycolipids appears to have the potential to significantly modulate the outcome of infection, since high-dose Sphingomonas infection of wild-type mice resulted in septic shock caused by inflammatory cytokines rapidly released by activated iNKT cells. On the other hand, infection of mice that lack iNKT cells can be associated with a delay in bacterial clearance [44,47]. Evaluation of synthetic forms of glycosphingolipids (GSLs) from the Sphingomonadaceae family revealed that among the mono-, di-, tri- and tetragylycosylceramides (GSL-1 to GSL4), only the GSL-1 is a potent stimulator of iNKT cells [48]. This could be explained by the observation that the oligoglycosylceramides are not truncated to GSL-1 in lysosomes, possibly due to the fact that these higher order GSLs are poor substrates for lysosomal acyltransfer enzymes [49].
Murine iNKT cells have been reported to recognize the lipophosphoglycan (LPG or LD1S) derived from the protozoal pathogen Leishmania donovani. A small fraction of iNKT cells recognize LD1S and these produce IFNγ but no IL-4 [50]. Recently iNKT cells have been shown to be critical for protection against amoebic liver abscess in mice. A lipopeptidophosphoglycan (EhLPPG) in the membrane of Entamoeba histolytica was identified as a natural ligand for activation of iNKT cells which results in protective immunity [51].
4. Synthetic analogues of α-galactosylceramide
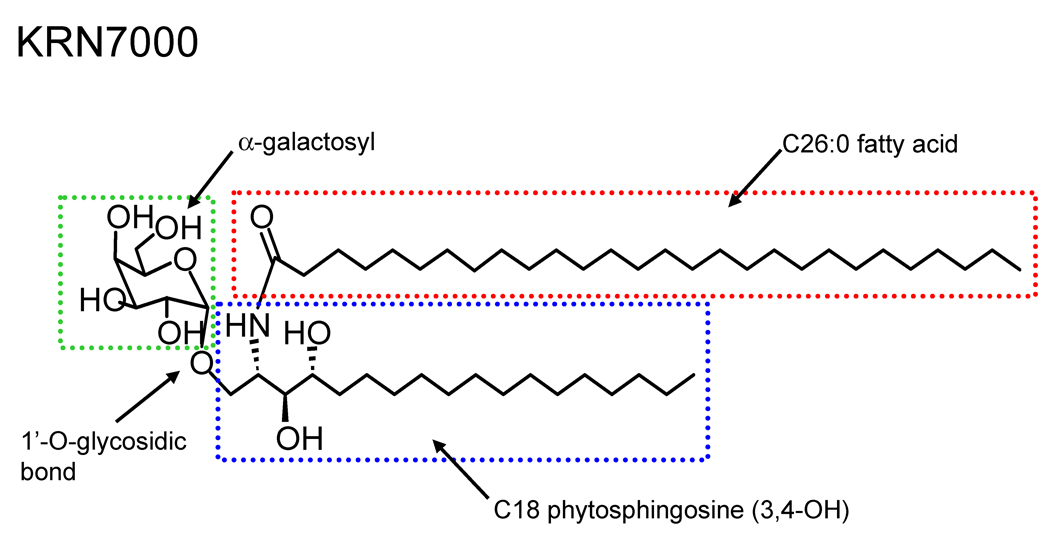
The discovery that α-galactosylceramides possess remarkably potent iNKT cell activating properties has resulted in profound advances in the study of this lymphocyte population. Initially identified as the immune activator present in extracts of the marine sponge Agelas mauritianus, the development of a synthetic form of α-galactosylceramide (αGalCer) known as KRN7000 [(2S,3S,4R)-1-O-(α-D-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol ] provided for the first time a well characterized ligand that could be used to identify and activate virtually the entire population of iNKT cells (Fig. 1) [52]. KRN7000, which contains a C18 phytosphingosine base and a fully saturated C26 N-linked fatty acyl chain, was shown to have marked anti-tumor activities in mice bearing melanomas and other types of cancers [53]. Although most mammalian ceramide glycolipids contain proximal sugars in the β-anomeric form, KRN7000 is unusual in that the sugar moiety is linked in the α-anomeric configuration. This glycolipid generates a high affinity complex for the TCRs of most iNKT cells when presented by CD1d, and this has enabled construction of fluorescently labeled CD1d tetramers loaded with KRN7000 or other closely related glycolipids for use in the identification of human or murine NKT cells in flow cytometry [10,54,55].
Fig 1.
Structure of the prototypical synthetic iNKT cell antigen KRN7000, which is an αGalCer with a C26:0 amide linked acyl chain and a C18 saturated phytosphingosine base. Key structural components that have been systematically modified to create analogues of this potent iNKT cell antigen are indicated by the boxed areas.
Numerous studies have shown that KRN7000 is a potent activator of both mouse and human iNKT cells, and that it elicits production of a complex mixture of cytokines that includes those typically associated with both Th1- and Th2-type responses [56]. However, the fact that KRN7000 induces a mixed Th1 and Th2 cytokine response may be a disadvantage in many settings in which a pure polarization of the adaptive immune response is required. For example, proinflammatory Th1-type cytokines such as IFNγ or TNFα induced by KRN70000 are not desirable when treating certain autoimmune conditions where Th1 cytokines are implicated in pathogenesis [10]. Conversely, Th2-type cytokines that are also induced by KRN7000 such as IL-4, IL-13 and IL-5 may be deleterious in the setting of cancer immunotherapy or allergic disease such as asthma. As a result, a major research effort has been focused in recent years on developing synthetic analogues of KRN7000 that are able to induce a either a pure Th1 or Th2 type cytokine response. Several studies have shown that modifying the basic lipid structure of KRN7000 can result in significant changes in the profiles of cytokine production following iNKT cell activation [57–63].
4.1‥ Sphingosine base derivatives
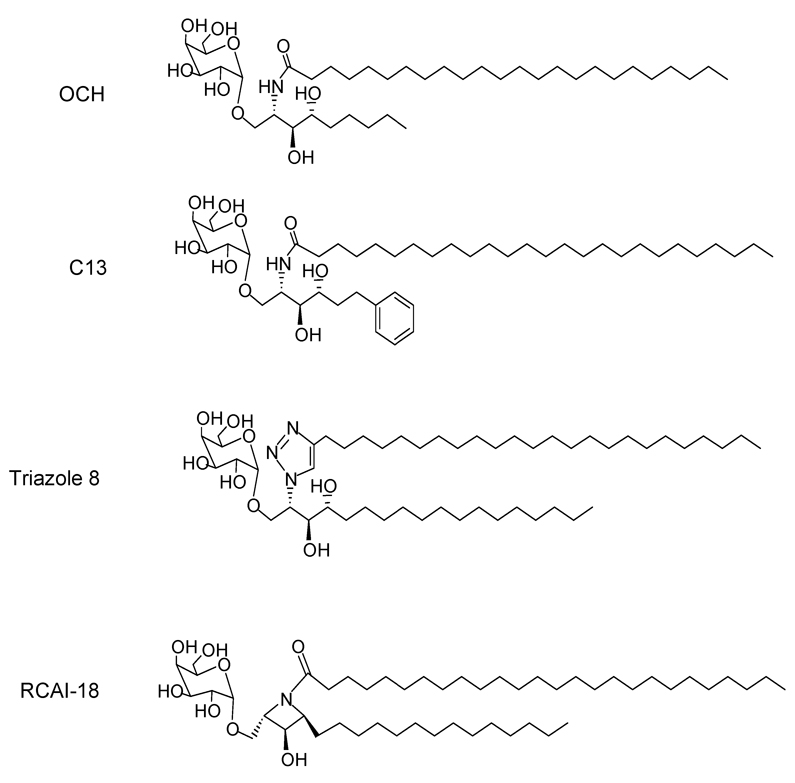
The first analogue of αGalCer shown to have distinct cytokine polarizing properties was a modified version of KRN7000 designated OCH, in which the sphingoid base was truncated to C9 and the the fatty acid chain slightly shortened to C24:0 (Fig. 2) [57]. This analogue when injected into mice induces a rapid IL-4 response with no detectable IFNγ. The proposed mechanism for this Th2 bias in cytokine response is that the transcription factor c-Rel, which belongs to the NF-κB family of transcriptional regulators and a key component for IFNγ release, is transcribed inducibly by iNKT cells following stimulation by KRN7000 but not by OCH [64]. Truncation of the phytosphingosine chain has been reported to increase the release of IL-4 from both murine and human iNKT cells [59], although this point has not been strongly confirmed in the literature in the case of human iNKT cell responses. Conversely, the attachment of an aromatic ring to the end of a truncated sphingosine chain (e.g., αGalCer C13) has been suggested to result in a potent iNKT cell activator that induces a more Th1-biased cytokine response (Fig. 2) [65].
Fig 2.
Four examples of sphingosine chain analogues of KRN7000 with iNKT cell activating properties. OCH is a Th2-cytokine biasing iNKT cell activator, which has a markedly truncated C9 phytosphingosine and a slightly shortened N-acyl chain (C24:0) compared to KRN7000. C13 is an example of a truncated sphingosine derivative in which incorporation of an aromatic ring led to increased iNKT cell activating potency. Triazole 8 has a 24 carbon alkyl chain linked to the sphingoid base by triazole ring, which is a highly stable rigid linking unit that mimics the topological and electronic features of an amide bond. RCAI-18 demonstrates how a trans-2,4-dialkylated azetidine ring can be incorporated in the ceramide portion to restrict flexibility while retaining the ability to stimulate iNKT cell responses. See text for references.
Modifications in the phytoshingosine scaffolds have been used as one approach to making analogues with differential ability to skew NKT cell responses. Since sphinganine-analogues are more easily prepared than the phytosphingosine-containing forms of αGalCer, such sphinganine analogues of KRN7000 and OCH were evaluated in comparison with the parent compounds. The relative potencies of these analogues for activation of murine iNKT cells in vitro showed that they were as potent as the parent lipids, and that they also closely resembled the phytosphingosine-containing forms of αGalCer in terms of cytokine induction [66]. Consistent with these functional properties, surface plasmon resonance studies showed that there was negligible loss of iNKT cell TCR binding to αGalCer-mCD1d complexes due to lack of the phytospingosine 4-hydroxyl group, whereas the 3-hydroxyl group on the phytoshingosine chain was essential for TCR binding [67]. In another study, the amide of the ceramide base of αGalCer was replaced with a 1,2,3-triazole group, a rigid linking unit that mimics the topological and electronic features of an amide bond and has extremely high stability to hydrolysis and oxidative/reductive conditions [68]. A series of GalCer analogues in which the triazole-linked lipid chain lengths were incrementally varied showed that isosteric replacement of the amide moiety of αGalCer with triazole could yield highly stimulatory iNKT cell antigens. The stimulatory effect was influenced by the length of the attached chain, with the long chain triazole analogues having a potency for iNKT cell stimulation comparable to KRN7000 (e.g., triazole 8 containing a 24 carbon saturated alkyl chain linked to the triazole group; Fig. 2). Significantly increased production of IL-4 relative to IFNγ by responding iNKT cells was observed with some compounds in this series with in vitro and in vivo analysis of mouse iNKT cell responses [68].
In an effort to examine the effects of conformational restriction in the ceramide part, analogues were synthesized with azetidine or pyrrolidine rings. Among these, the compound designated RCAI-18 with a trans-2,4-dialkylated azetidine ring was shown to be a potent inducer of cytokines from murine NKT cells (Fig. 2). The activity of RCAI-18 was observed to be dose-dependent with a Th2 type cytokine skewing activity at lower doses, thus making it a potential candidate for clinical applications [69]. Synthetic galactosyl serine-type ceramide analogues have also been synthesized and tested in in vitro murine splenocyte proliferation and cytokine assays. These lipids were generally found to be less potent than ceramide-based α-galactosyl glycolipids [70].
Two groups have reported synthesis of epimers of αGalCer in which the stereochemistry of chiral carbons 2, 3 and 4 of the KRN7000 phytosphingosine base (i.e., 2S, 3S, 4R) have been systematically altered [71,72]. Although functional studies of these epimers have not yet been reported in detail, the preliminary analyses indicate that the 3-D spatial orientations of C2–NH2 group and C3-OH group of phytosphingosine both have an important impact on iNKT cell stimulation, with the configuration of C2–NH2 group being more critical to recognition than that of C3-OH group. In contrast, stereochemical variation of the C4-OH group seems to have relatively minor if any impact on iNKT cell responses.
4.2. N-acyl derivatives
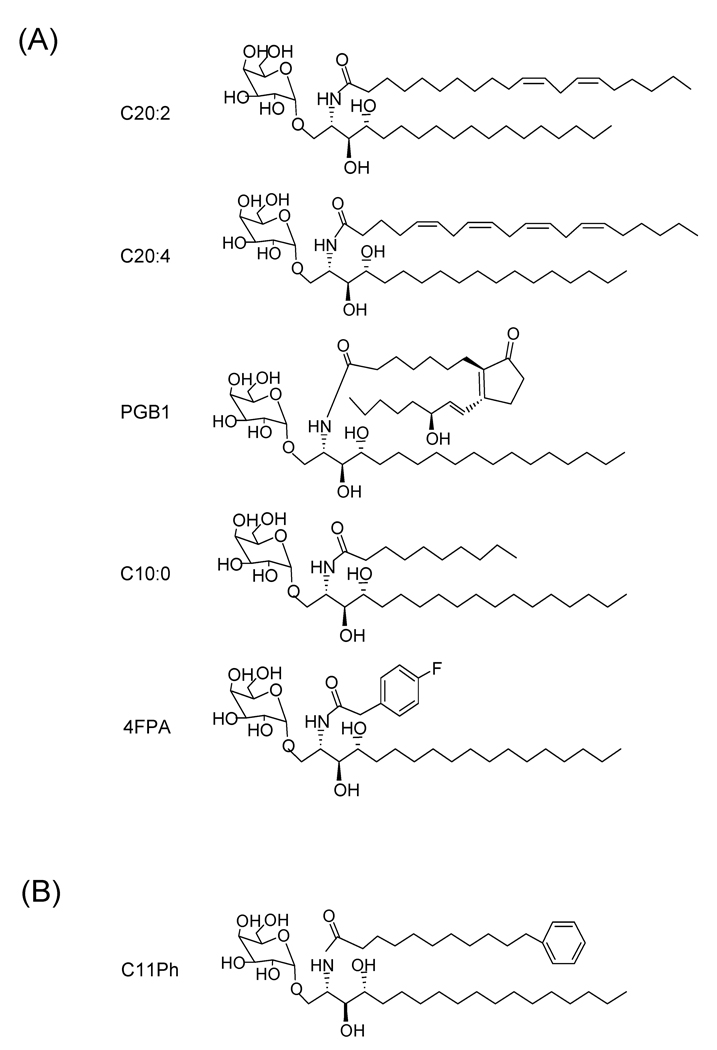
Analogues of αGalCer produced by alteration of the length and extent of unsaturation of their N-acyl substituents have been tested for their ability to activate iNKT cells. The shortening of the fatty-acid chain in αGalCer has been reported to reduce potency of iNKT cell activation, and the ratio of IL-4 to IFNγ was found to be higher in analogues with shorter chain lengths [59]. Compounds with 20-carbon acyl chains with varying degrees of unsaturation coupled to the sphingosine core structure were screened, and it was observed that a dienoic analogue with unsaturations at carbons 11 and 14 (αGalCer-C20:2; 11,14-cis-diunsaturated C20 fatty acid) resulted in a very potent analogue (Fig. 3A). The C20:2 analogue induced a Th2-biased and potentially anti-inflammatory cytokine response. Analysis of this altered cytokine response suggested that it was due to an alteration in the downstream cascade of events triggered by iNKT cell activation, including the dampening of secondary activation of IFNγ producing NK cells [60].
Fig 3.
Structures of representative N-acyl chain analogues of KRN7000. (A) Five examples are shown of glycolipids that induce Th2-biased iNKT cell responses. Although the structures vary widely, they all contain acyl chain modifications that introduce increased polarity such as unsaturations, chain truncation or incorporation of oxygen atoms. (B) An example of a highly active analogue in which the acyl chain is terminated by an aromatic ring. Such compounds have been reported to be potent iNKT cell activators and have been suggested to favor a more Th1-biased cytokine response. See text for references.
Interestingly, αGalCer-C20:2 differed significantly from KRN7000 in its requirements for loading into CD1d. Whereas KRN7000 requires endosomal localization of CD1d for efficient loading and presentation, this was not required for αGalCer-C20:2 [60]. Recently, several other N-acyl derivatives of the α-galactosyl C18 phytosphingosine base have been described which also show a lack of endosomal loading requirement and stimulate a Th2-cytokine biased response. These contain generally more polar or less hydrophobic N-acyl substitutions compared to KRN7000, such as C20 prostaglandin B1 chain (αGalCer-PGB1) or arachidonic acid (αGalCer-C20:4), a saturated C10 chain (αGalCer-C10:0), or a 4-fluorophenylacetate group (αGalCer-4FPA) (Fig. 3A). These findings suggest that the ability to associate with CD1d directly at the cell surface without a requirement for endosomal loading correlates with the ability to polarize iNKT cell cytokine production to a Th2 bias [73].
Conversely, a few N-acyl derivatives of αGalCer have been described that polarize the cytokine response in the opposite direction, with IFNγ predominating over IL-4 production. The introduction of terminal aromatic rings into the acyl chain of αGalCer has been reported to induce a Th1 biased cytokine response when used to stimulate human NKT cells. Analogues in which the fatty acyl chain contained a 6-phenylhexanoyl (C6Ph), 8-phenyloctanoyl (C8Ph), or the 11-phenylundecanoyl (C11Ph) group showed enhanced potency for overall cytokine production with a possible Th1 bias (Fig. 3B) [61]. A subsequent follow up study of αGalCer analogues containing an aromatic ring substitution in their acyl chains suggested that these could induce a potent Th1 biased cytokine response in vivo when injected into mice [65].
4.3. Glycosidic bond derivatives
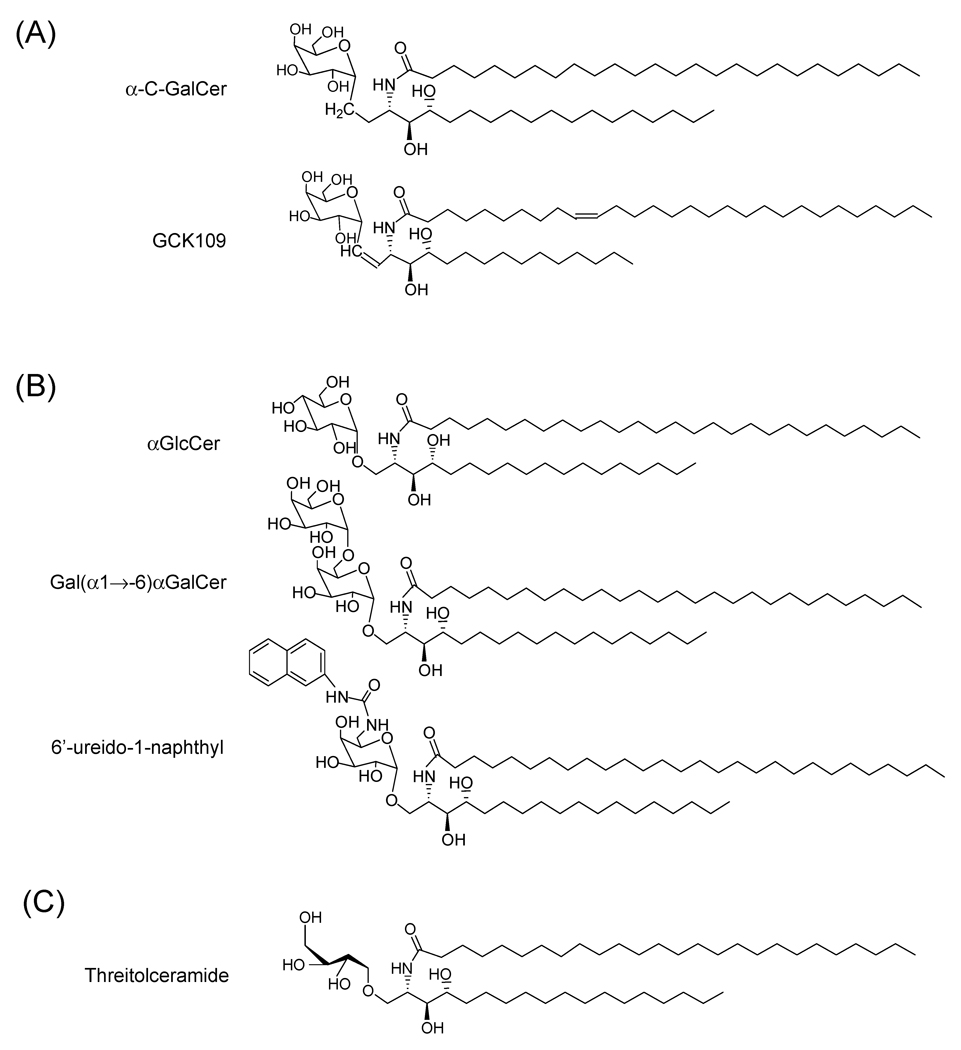
One analogue of αGalCer that has created tremendous interest among NKT cell biologists is the α-C-GalCer where the glycosidic oxygen, a polar hydrogen bond acceptor, is replaced by a nonpolar CH2 group. This analogue has been demonstrated to induce a potent and sustained Th1 cytokine response in mice and was shown to be as much as 1000 fold more potent than KRN7000 for inducing protection against malaria and metastasis of melanoma in mice. The α-C linkage is speculated to increase the stability of the lipid as this substitution renders it resistant to degradation by α-galactosidase in vivo (Fig. 4A) [58].
Fig 4.
Structures of representative carbohydrate and glycosidic bond derivatives of KRN7000. (A) Glycosidic bond substitution based derivatives including the original C-glycoside of KRN7000 (α-C-GalCer with a methylene unit (-CH2-) in place of oxygen in the glycosidic linkage, and GCK109 which is a highly active E-alkene linked analogue of α-C-GalCer. (B) Carbohydrate modification based derivatives with replacement of galactose by α-linked glucose, di-galactose (Gal(α1→6)αGalCer) or 1-naphthyl group linked to the 6'-ureido-6'deoxy-αGalCer. (C) An active non-glycosidic derivative represented by threitolceramide. See text for references.
A panel of E-alkene-linked C-glycoside analogues was tested recently for activation of human iNKT cells in vitro and they were reported to be potent ligands for both mouse and human iNKT cells and to induce a Th1-type biased response. In this study the E-alkene analogue GK127 was further modified by adding an aromatic ring in the tail of a shorter alkyl chain (compound GK152) or by adding an olefin linkage in the alkyl chain (compounds GK109 and GK151), and these analogues were found to induce a higher level of Th1 cytokine induction as compared to GK127 (Fig. 4A) [63]. It was proposed that insertion of the E-alkene linker in place of the CH2 glycosidic linkage may fix the position of the sugar close to the optimal conformation for iNKT cell TCR recognition [63]. In addition the steric repulsion energy calculated for the E-olefin linker for this analogue was estimated to be much smaller than those of the O-glycoside and C-glycoside linkers of αGalCer and α-C-GalCer respectively, thereby causing less repulsion within the proximal portion of the hydrophobic CD1d groove where it would be expected to fit [63].
Contrary to the general impression that β-anomeric galactosylceramides do not activate iNKT cells, one study that investigated the activity of four β-anomers which differed in the length of their acyl chain showed that two of these, β-GCa12 and β-GCa26, activated NKT cells in vivo associated with induction of IFNγ and IL-4. Although these analogues were found to be less potent than αGalCer, they were shown to induce transactivation of NK cells [74]. However another study which showed activation of NKT cells by β-anomeric GalCer was unable to find evidence for NK cell transactivation [75].
4.4. Carbohydrate modification based derivatives
Analysis of a variety of glycoside analogues in mouse spleen iNKT cell stimulation assays showed that the activity of α-glucosylceramide (αGlcCer) was similar to that of αGalCer (Fig. 4B). Since αGlcCer and αGalCer differ only in their 4-hydroxyl group, this indicated that the 4-hydroxyl configuration of the sugar was not essential for bioactivity. In contrast, α-mannosylceramide (αManCer) with the 2-hydroxyl group in an axial configuration as compared to an equatorial bond on αGalCer or αGlcCer, did not show any activity indicating the importance of the configuration of the 2-hydroxyl group on the sugar moiety [52]. A number of disaccharide analogues have also been synthesized and tested for iNKT cell activating properties. In one study, responses to two digalactosyl derivatives of KRN7000, Gal (α1 →2)αGalCer and Gal(α1→6)αGalCer were analyzed in an APC-free T cell stimulation system using plate coated murine CD1d to determine if processing of the carbohydrate was required for presentation by CD1d. The Gal (α1→2)αGalCer was indeed unable to stimulate NKT hybridoma cells in this cell-free presentation system, whereas Gal(α1→6)αGalCer induced stimulation similar to KRN7000 (Fig. 4B). Using presentation by intact APCs, the presentation of Gal(α1→2)αGalCer was demonstrated to be dependent upon the trafficking of CD1d to lysosomes and the presence of lysosomal α-galactosidase A for generation of a monosaccharide epitope [76]. This study established the principle that enzymatic processing of oligosaccharides could be associated with certain types of CD1d-presented antigens.
Several analogues of KRN7000 with the galactose 6’-position modified have been tested for their ability to activate mouse iNKT cells. Among these the 6′-O-methylated analogue, RCA-61, was found to be a strong inducer of IFNγ in mice in vivo [77]. By introducing an acetamide group in the C6 position of galactose and a cisdouble bond in the acyl chain of KRN7000, a highly soluble analogue (PBS57) was shown to activate both human and murine NKT cells more efficiently than KRN7000 [78]. The fact that galactose 6’-OH is the only sugar alcohol which is not involved in any hydrogen bonding between αGalCer and CD1d motivated the synthesis of analogues with more elaborate modifications at this position, with the goal of potentially enabling more interactions between CD1d and the substituents of the 6’ position of the hexose ring. With this goal in mind, a series of 6′-amide and 6′-ureido analogues of 6′-deoxygalactosylceramide analogues was synthesized and tested for stimulation of systemic cytokine responses [62]. Although the functional analysis was quite limited, the in vivo functional screen for activity in mice showed that the 6'-amide derivatives evoked variable levels of serum cytokines consistent with iNKT cell stimulatory activity and tended to induce a bias toward IFNγ with reduced IL-4 secretion relative to KRN7000. The 6'-ureido analogues (such as compound 19 reported by Trappeniers et al. [62], with a bulky 1-naphthyl group linked to the 6'-ureido-6'deoxy-αGalCer; Fig. 4B) generally showed IFNγ stimulation comparable or possibly greater than KRN7000 with reduced IL-4 production, also indicative of a Th1-biased cytokine response. These compounds represent potentially promising Th1-type iNKT cell agonists, and will be of great interest to subject to further detailed immunological analysis.
With the discovery that hydrogen bonds between the anomeric oxygen and the hydroxyl group at positions 2 and 3 are important for binding of the polar head group to CD1d, non-glycosidic analogues containing the l-threo configuration of the galactosyl residue at C2 and C3 were tested in comparison with KRN7000. One of the threitolceramide analogues induced lower levels of cytokines and less killing of dendritic cells than KRN7000, and also displayed adjuvant activity for a model antigen (Fig. 4C) [79].
5. Molecular basis for recognition of diverse ligands by NKT cells
A remarkable feature of NKT cell responses is the range of different lipid structures that can be recognized, given the relative lack of diversity of the TCRs of these cells and the single antigen presenting molecule involved. Crystal structures of mouse and human CD1d clearly show the basic mechanism by which these proteins are able to bind and present a diverse range of lipid ligands. The membrane distal α1 and α2 domains of CD1d contain a hydrophobic ligand binding site that is comprised of two connected pockets, designated A' and F' [80]. Crystal structures of αGalCer in complex with murine and human CD1d have confirmed that this binding site is occupied by the lipid tails of bound ligands such as αGalCer, and that the hydrophobic interactions among these lipid tails and the nonpolar surface of the binding groove mainly contribute to the binding. In the case of αGalCer binding, the A’ pocket of hCD1d was shown to be filled by the acyl chain and the F’ pocket with the sphingosine chain [81]. The crystal structure of the complex of mCD1d with the most active synthetic analogue of sulfatide (cis-tetracosenoyl sulfatide) revealed that the longer fatty acid chain fully occupied the A’ pocket while the sphingosine chain filled the F’ pocket of the CD1d binding groove owing to precise hydrogen bonding in the center of the groove [82]. The crystal structure of mCD1d in complex with PBS-25, a short chain (C8) form of αGalCer, showed a similar orientation of lipid binding and also revealed the presence of a "spacer lipid" in the distal portion of the A’ pocket [42]. Density consistent with a similar spacer lipid was also found in the A' pocket of the crystal structure of the mCD1d complex with a Sphingomonas α-galacturonosylceramide [83], and also in the crystal structure of mCD1d in complex with the putative self glycolipid antigen iGb3 [35]. The presence of such spacer lipids to fill empty spaces in the CD1d groove is likely to be a general mechanism by which the binding of lipids with relatively short alkyl chains can be stabilized, thus increasing further the range of different lipid structures that can be presented.
Considerable flexibility also exists in the positioning of the exposed portions of bound lipid ligands by CD1d, and in the TCR recognition of these. As highlighted by the crystal structure of the ternary complex of hCD1d complexed with αGalCer and bound to the iNKT cell TCR, the α-anomeric galactose group of this antigen is held intimately against the surface of the CD1d protein by a network of hydrogen bonds [84]. This allows an extremely close approach of the TCR to the surface of CD1d, resulting in multiple areas of direct contact between the proteins. In contrast, the β-linked sugars of sulfatide or iGb3 appear to project up and away from the binding pocket due to its β-linkage and was highly exposed for recognition by the TCR [35,42]. A similar projection of the carbohydrate groups away from the surface of CD1d has also been suggested by the crystal structure of mCD1d in complex with phosphatidylinositol dimannoside [85]. In such cases, TCR contacts may mostly be with the carbohydrate groups of the bound antigen, and direct TCR interactions with the surface of CD1d may be limited or relatively unimportant. In the case of the C-glycoside α-C-GalCer, the oxygen atom of the αGalCer glycosidic bond (a polar hydrogen acceptor) has been replaced with a non-polar CH2 that cannot participate in hydrogen bonding. This has been proposed to cause the glycolipid to sit differently in the CD1d groove thereby changing the structure of the complex and its affinity for the iNKT TCR, and may also allow for free rotation of the glycoside bond between galactose and the ceramide [58]. These findings highlight the extraordinary flexibility of the iNKT cell TCR, which in spite of its relatively limited diversity appears to be capable of different modes of recognition that enable responses to a wide range of CD1d presented ligands.
6. Mechanisms by which ligand structure controls NKT cell response
iNKT cells are capable of a generating markedly divergent immune responses, ranging from stimulation of protective immunity to the generation of immune tolerance. Particularly in the case of responses to structural variants of αGalCer, it is clear that the structure of the glycolipid antigen can have a profound effect on the nature of the iNKT cell response that is generated. This is readily apparent from the many studies of the different patterns of cytokines that can be generated by different αGalCer analogues, ranging from a mixture of Th1 and Th2 type cytokines (e.g., with KRN7000) to a more pure Th2-like response (e.g., with OCH or αGalCer-C20:2) or pure Th1-like response (e.g., with α-C-GalCer). The mechanisms accounting for the stimulation of qualitatively different iNKT cell responses remains poorly understood, although a number of studies have recently addressed this important point and several hypotheses are now receiving attention.
6.1. TCR affinity and control of iNKT cell responses
Initial studies comparing KRN7000 to the pure Th2-type agonist OCH suggested the straightforward hypothesis that the affinity of the iNKT cell TCR might be the critical factor that determines the quality of the response. Whereas KRN7000 generates complexes with CD1d that have high TCR affinity, it is clear that OCH generates complexes with markedly lower affinity. Human and murine CD1d molecules loaded with the truncated sphingosine analogue OCH have been shown to have a low affinity for iNKT cell TCR [86,87]. Using surface plasmon resonance to measure the binding of a soluble human iNKT cell TCR to hCD1d loaded with αGalCer analogues, the equilibrium dissociation constant (Kd) for TCR binding to complexes loaded with a C9 sphingosine analogue similar to OCH was approximately two orders of magnitude greater than that for αGalCer-C26:0 loaded complexes [87]. Recently the TCR binding avidity of OCH-loaded mCD1d tetramers were shown to be at least an order of magnitude lower than that of αGalCer-C26:0 loaded mCD1d tetramers, and with the hCD1d tetramers the avidity was too low to measure [73].
Based on the weak binding, an affinity threshold model has been proposed to account for the Th2-cytokine biasing activity [64,87]. This model predicts that the high level of sustained IFNγ production induced by ligands like KRN7000 is a consequence of the high affinity interaction of the TCR, which results in different intracellular signaling than recognition of weak agonists such as OCH [57]. Along these lines, one study has reported that the transcription factor c-Rel which belongs to the NF-κB family of transcriptional regulators and a key component for IFNγ release, is transcribed inducibly by iNKT cells that are stimulated by KRN7000 and not by OCH [64]. In addition, synthetic analogues of αGalCer with an aromatic ring in either the acyl or sphingosine tail that stimulated a higher in vitro Th1-cytokine/chemokine induction, TCR activation and human iNKT cell expansion as compared to αGalCer were shown to bind to TCR with higher affinity and induce phosphorylation of CD3ε, ERK1/2, or CREB [65].
However, studies using tetramer binding assays and surface plasmon resonance to assess iNKT cell TCR binding to a number of other glycolipid ligands have not been consistently supportive of a simple TCR affinity threshold model as an explanation for variations in the type of cytokine response observed. For example, the αGalCer-C20:2 analogue, which gives a Th2-type cytokine bias similar to that seen with OCH stimulation, was found to have a binding affinity when complexed with mCD1d or hCD1d that was very similar to that observed for complexes loaded with αGalCer-C26:0 [73,87]. Recent studies of other Th2-biasing analogues have shown these to have a range of binding avidities for mouse and human iNKT cell TCRs that are generally intermediate between those of OCH and KRN7000, thereby demonstrating that Th2-biasing analogues can have a low, intermediate or high avidity for iNKT TCRs [73]. Thus, while TCR affinity may account for some aspects of the quality of the iNKT cell response, this appears not to be the only factor involved and may not even be the dominant factor for potent Th2-biasing αGalCer analogues such as αGalCer-C20:2.
6.2. Presentation by alternate types of APCs
Another possibility that has been considered to explain the different patterns of cytokines produced in response to various glycolipid antigens relates to differences in uptake and presentation of these antigens. It has been shown that KRN7000 is loaded into CD1d mainly in endosomes after uptake by endocytosis through a process that is facilitated by extracellular lipid carrying proteins such as apolipoprotein E [88], and that the loading is dependant upon acidic pH and lipid transfer proteins (LTPs) such as saposins and GM2 activator protein [89–92]. However, the N-acyl variant of KRN7000, αGalCer-C20:2 which induces a predominant Th2 response, was found not to require any of these factors as it could directly load into surface CD1d suggesting that these differences could lead to qualitative and quantitative changes in the outcome of Vα14 iNKT cell activation [60]. This property of direct cell surface loading of CD1d was found to be a general feature of multiple Th2-biasing analogues analyzed in a recent study. All these lipids were presented rapidly by APCs and were able bind to cell surface CD1d molecules without requiring intracellular loading of CD1d, unlike KRN7000 which necessitated intracellular loading and was presented much more slowly [73].
A general feature of most or all Th2-biasing αGalCer analogues is that they have either substantially shortened N-acyl chains, or contain polar substitutions such as double bonds or oxygen atoms within the N-acyl group. These structural attributes contribute to increased polarity and reduced hydrophobicity compared to αGalCer-C26:0, possibly resulting in increased solubility and therefore greater access to the lipid binding site of cell surface expressed CD1d molecules [73]. Interestingly, a recent study has shown that αGalCer analogues with relatively short acyl chains are actually excluded from intracellular loading within late endosomes, even though they are transported efficiently to this site [93]. This provides an extremely strong bias toward direct cell surface loading of CD1d by such glycolipid antigens (Fig. 5).
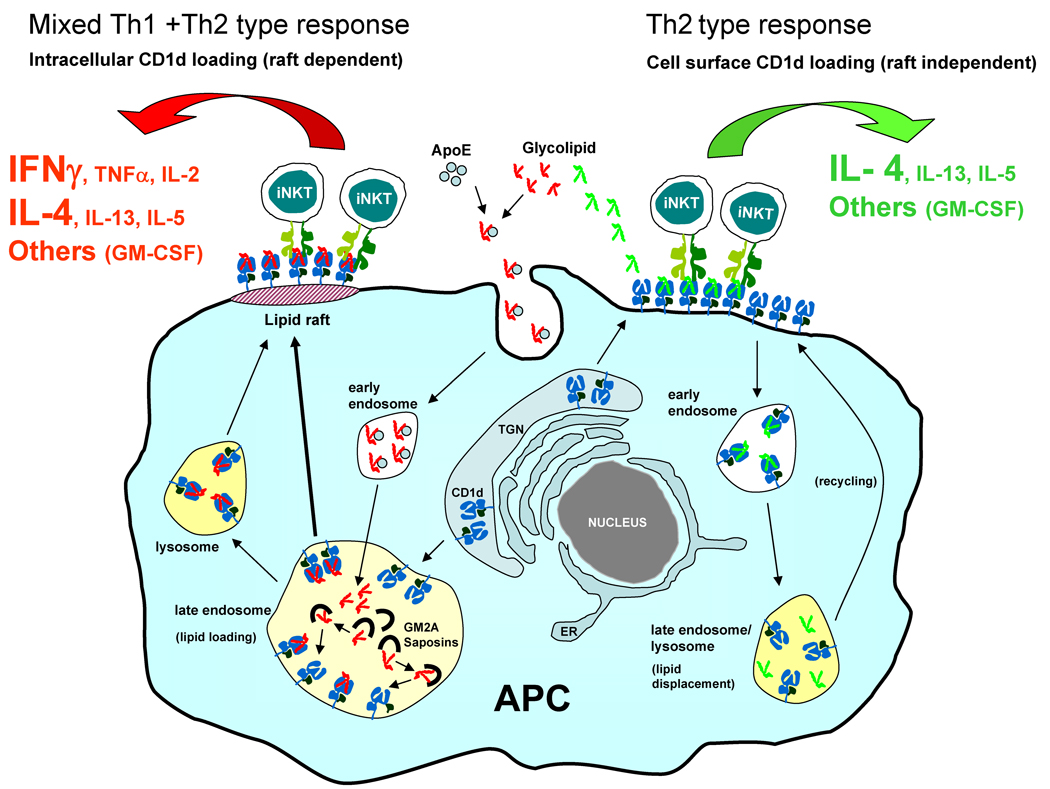
Fig 5.
Model for mechanism leading to stimulation of biased cytokine responses by analogues of αGalCer. The αGalCer analogues such as KRN7000 (shown in red) that generate mixed cytokine responses comprised of both Th1 and Th2 type cytokines are endocytosed after binding to extracellular lipid carrier proteins such as ApoE and loaded into CD1d intracellularly in endosomes where this process is facilitated by low pH and the presence of lipid transfer proteins such as the GM2 activator protein (GM2A) and saposins. These intracellularly generated CD1d-lipid complexes are transported to lipid raft microdomains on the plasma membrane and activate strong and prolonged iNKT cell responses that are characterized by a mixture of Th1 and Th2 type cytokines with prominent and sustained IFNγ production. Note that the majority of IFNγ generated in this situation is most likely not a direct product of the activated iNKT cells, but is generated by the secondary activation of NK cells. In contrast, glycolipids that are rapidly loaded into surface CD1d (shown in green) activate iNKT cells to induce responses that are dominated by Th2 type cytokines without stimulating prolonged secretion of IFNγ or other proinflammatory Th1 type cytokines. Glycolipids of this type are unloaded from CD1d in the late endosome and lysosome, so that intracellular complexes containing these are inhibited from forming and therefore do not undergo transport into plasma membrane lipid rafts. TGN, trans Golgi network; ER, endoplasmic reticulum. See text for more detailed explanation and references.
The differences in the requirement for antigen uptake by APCs for presentation between KRN7000 and most or possibly all Th2-biasing type analogues of αGalCer suggested the possibility that the latter compounds could be presented preferentially by non-phagocytic CD1d+ APCs such as B cells. The presentation of KRN7000 and Th2 – biasing glycolipids by different types of APCs was envisioned to lead to the different patterns of cytokine production, perhaps because of the lack of essential costimulatory molecules on cell types serving as the dominant APCs for the Th2-biasing agonists. This hypothesis was supported by an in vitro analysis that compared responses of human iNKT cell clones stimulated with DCs or with CD1d+ Schwann cells as APCs [12]. In addition, one study of mouse iNKT cell responses found that presentation of KRN7000 by B cells led to a skewing of the cytokine production toward increased IL-4 relative to IFNγ secretion [94]. However, other recent studies indicate that CD11c+ DCs are likely to be the predominant APC type for Th2-biasing analogues of αGalCer analogues as well as KRN7000 [73], and depletion of CD11c+ cells leads to loss or marked diminution of iNKT cell responses to all αGalCer analogues at least for in vitro cultures of mouse splenocytes (S. Porcelli, unpublished).
6.3. Lipid raft localization of CD1d and altered iNKT cell responses
It has been demonstrated that a fraction of plasma membrane CD1d molecules are localized in detergent insoluble plasma membrane microdomains, also known as lipid rafts [95–97]. In one recent study, it has been shown that mCD1d molecules that are loaded intracellularly by KRN7000 accumulate in lipid rafts on the plasma membrane of APCs, whereas mCD1d loaded with the Th2-biasing analogues αGalCer-C20:2 or αGalCer-C10:0 are predominantly located outside of lipid rafts [73]. Given the known involvement of lipid raft domains in cell signaling, this suggests that the extent of lipid raft associated presentation may be an important factor controlling the outcome of iNKT cell stimulation. This idea has strong precedents in the study of peptide specific MHC class II restricted T cells, for which raft-associated presentation favors Th1 responses while presentation by MHC class II molecules outside of rafts stimulates Th2 responses [98]. Thus, the structure dependent sorting of glycolipid ligands for preferential loading into CD1d molecules in either the lipid raft or the non lipid raft regions may be an important mechanism that steers iNKT cell activation towards a pro-inflammatory (mixed Th1 and Th2 type) or the tolerogenic (pure Th2 type) response (Fig. 5).
8. Conclusion
The repertoire of lipid and glycolipid ligands that can be presented by CD1d to activate NKT cells continues to grow in number and in complexity. The currently available data reviewed here underscore the remarkable flexibility of the NKT cell responses, which allows the recognition of many diverse antigens through the use of a single antigen presenting molecule and a collection of TCRs with relatively low variability. Although our understanding of the mechanisms by which different glycolipid structures are able to stimulate qualitatively different iNKT cell responses is still imperfect, continued development of this area of research is likely to enable the design of agonists that will allow a more precise control of the many potential activities of these lymphocytes for a broad range of applications.
Acknowledgements
The authors are supported by grants from the NIH/NIAID (AI45889 and AI063537), and by funding provided by the Collaboration for AIDS Vaccine Discovery of the Bill and Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 3.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 5.Bricard G, Porcelli SA. Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity. Cell Mol Life Sci. 2007;64:1824–1840. doi: 10.1007/s00018-007-7007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Ann Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda JL, Gapin L, Fazilleau N, Warren K, Naidenko OV, Kronenberg M. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor beta repertoire and small clone size. Proc Natl Acad Sci U S A. 2001;98:12636–12641. doi: 10.1073/pnas.221445298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JYT, Ceredig R, et al. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 10.Yu KOA, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Kojo S, Harada M, Ohkohchi N, Taniguchi M, Seino K. Mechanism of NKT cell-mediated transplant tolerance. Am J Transpl. 2007;7:1482–1490. doi: 10.1111/j.1600-6143.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 12.Im JS, Tapinos N, Chae GT, Illarionov PA, Besra GS, DeVries GH, et al. Expression of CD1d molecules by human schwann cells and potential interactions with immunoregulatory invariant NK T cells. J Immunol. 2006;177:5226–5235. doi: 10.4049/jimmunol.177.8.5226. [DOI] [PubMed] [Google Scholar]
- 13.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–3462. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 14.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 15.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan YF, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: Costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 19.Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol. 2009 doi: 10.4049/jimmunol.0900858. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, et al. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol. 2008;38:706–719. doi: 10.1002/eji.200737660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, Franck RW. α-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine. 2009;27:3766–3774. doi: 10.1016/j.vaccine.2009.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 Recognition by mouse NK1+ T-lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 23.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen NR, Garg S, Brenner MB. Lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 25.Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, et al. Natural ligand of mouse CD1d1: Cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 26.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 28.Rauch J, Gumperz J, Robinson C, Skold M, Roy C, Young DC, et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanic AK, De Silva AD, Park JJ, Sriram V, Ichikawa S, Hirabyashi Y, et al. Defective presentation of the CD1d1-restricted natural Vα14Jα18 NKT lymphocyte antigen caused by β-D-glucosylceramide synthase deficiency. Proc Natl Acad Sci U S A. 2003;100:1849–1854. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 32.Chen WL, Xia CF, Wang JH, Thapa P, Li YS, Nadas J, et al. Synthesis and structure activity relationship study of isoglobotrihexosylceramide analogues. J Org Chem. 2007;72:9914–9923. doi: 10.1021/jo701539k. [DOI] [PubMed] [Google Scholar]
- 33.Li YS, Zhou DP, Xia CF, Wang PG, Levery SB. Sensitive quantitation of isoglobotriaosylceramide in the presence of isobaric components using electrospray ionization-ion trap mass spectrometry. Glycobiology. 2008;18:166–176. doi: 10.1093/glycob/cwm127. [DOI] [PubMed] [Google Scholar]
- 34.Yin N, Long XT, Goff RD, Zhou DP, Cantu C, Mattner J, et al. Alpha anomers of iGb3 and Gb3 stimulate cytokine production by natural killer T cells. ACS Chem Biol. 2009;4:191–197. doi: 10.1021/cb800277n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, et al. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J Exp Med. 2006;203:2293–2303. doi: 10.1084/jem.20060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speak AO, Salio M, Neville DCA, Fontaine J, Priestman DA, Platt N, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Libero G, Mori L. Structure and biology of self lipid antigens. Curr Top Microbiol Immunol. 2007;314:51–72. doi: 10.1007/978-3-540-69511-0_3. [DOI] [PubMed] [Google Scholar]
- 41.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zajonc DM, Cantu C, Mattner J, Zhou DP, Savage PB, Bendelac A, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 45.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U S A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinjo Y, Wu D, Kim GS, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 47.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, Zhou DP, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 48.Kinjo Y, Pei B, Bufali S, Raju R, Richardson SK, Imamura M, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long X, Deng S, Mattner J, Zang Z, Zhou D, McNary N, et al. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007;3:559–564. doi: 10.1038/nchembio.2007.19. [DOI] [PubMed] [Google Scholar]
- 50.Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lotter H, Gonzalez-Roldan N, Lindner B, Winau F, Isibasi A, Moreno-Lafont M, et al. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434. doi: 10.1371/journal.ppat.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawano T, Cui JQ, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 54.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–753. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 58.Schmieg J, Yang GL, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goff RD, Gao Y, Mattner J, Zhou DP, Yin N, Cantu C, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 60.Yu KOA, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujio M, Wu DG, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: Tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 62.Trappeniers M, Van Beneden K, Decruy T, Hillaert U, Linclau B, Elewaut D, et al. 6'-derivatised α-GalCer analogues capable of inducing strong CD1d-mediated Th1-biased NKT cell responses in mice. J Am Chem Soc. 2008;130:16468–16469. doi: 10.1021/ja8064182. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ndonye RM, Izmirian DP, Dunn MF, Yu KO, Porcelli SA, Khurana A, et al. Synthesis and evaluation of sphinganine analogues of KRN7000 and OCH. J Org Chem. 2005;70:10260–10270. doi: 10.1021/jo051147h. [DOI] [PubMed] [Google Scholar]
- 67.Sidobre S, Hammond KJL, Benazet-Sidobre L, Maltsev SD, Richardson SK, Ndonye RM, et al. The T cell antigen receptor expressed by Vα14 iNKT cells has a unique mode of glycosphingolipid antigen recogniton. Proc Natl Acad Sci U S A. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee T, Cho M, Ko SY, Youn HJ, Baek DJ, Cho WJ, et al. Synthesis and evaluation of 1,2,3-triazole containing analogues of the immunostimulant α-GalCer. J Med Chem. 2007;50:585–589. doi: 10.1021/jm061243q. [DOI] [PubMed] [Google Scholar]
- 69.Fuhshuku K, Hongo N, Tashiro T, Masuda Y, Nakagawa R, Seino K, et al. RCAI-8, 9, 18, 19, and 49-52, conformationally restricted analogues of KRN7000 with an azetidine or a pyrrolidine ring: Their synthesis and bioactivity for mouse natural killer T cells to produce cytokines. Bioorg Med Chem. 2008;16:950–964. doi: 10.1016/j.bmc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Gang-Ting Fan, Yi-shin Pan, Kuo-Cheng Lu, Yu-Pei Cheng, Wan-Chen Lin, Steven Lin, et al. Synthesis of α-galactosyl ceramide and the related glycolipids for evaluation of their activities on mouse splenocytes. Tetrahedron. 2005;61:1855–1862. [Google Scholar]
- 71.Trappeniers M, Goormans S, Van Beneden K, Decruy T, Linclau B, Al Shamkhani A, et al. Synthesis and in vitro evaluation of α-GalCer epimers. Chemmedchem. 2008;3:1061–1070. doi: 10.1002/cmdc.200800021. [DOI] [PubMed] [Google Scholar]
- 72.Park JJ, Lee JH, Ghosh SC, Bricard G, Venkataswamy MM, Porcelli SA, et al. Synthesis of all stereoisomers of KRN7000, the CD1d-binding NKT cell ligand. Bioorg Med Chem Lett. 2008;18:3906–3909. doi: 10.1016/j.bmcl.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 75.Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 76.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 77.Tashiro T, Hongo N, Nakagawa R, Seino K, Watarai H, Ishii Y, et al. RCAI-17, 22, 24–26, 29, 31, 34–36, 38–40, and 88, the analogs of KRN7000 with a sulfonamide linkage: their synthesis and bioactivity for mouse natural killer T cells to produce Th2-biased cytokines. Bioorg Med Chem. 2008;16:8896–8906. doi: 10.1016/j.bmc.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Goff RD, Zhou DP, Mattner J, Sullivan BA, Khurana A, et al. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Meth. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 79.Reddy BG, Silk JD, Salio M, Balamurugan R, Shepherd D, Ritter G, et al. Nonglycosidic agonists of invariant NKT cells for use as vaccine adjuvants. Chemmedchem. 2009;4:171–175. doi: 10.1002/cmdc.200800354. [DOI] [PubMed] [Google Scholar]
- 80.Zeng ZH, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 81.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 82.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, et al. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci U S A. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 85.Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- 86.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, et al. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 87.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, et al. The length of lipids bound to human CD1d molecules modulates the affinity threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 89.Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, et al. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. 2004;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- 90.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 91.Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, et al. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci U S A. 2007;104:5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou D, Cantu C, III, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci U S A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, et al. Distinct roles of dendritic cells and B cells in Vα14Jα18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 95.Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of α-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112:386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem Biophys Res Comm. 2005;327:1143–1154. doi: 10.1016/j.bbrc.2004.12.121. [DOI] [PubMed] [Google Scholar]
- 97.Peng W, Martaresche C, Escande-Beillard N, Cedile O, Reynier-Vigouroux A, Boucraut J. Influence of lipid rafts on CD1d presentation by dendritic cells. Mol Membrane Biol. 2007;24:475–484. doi: 10.1080/09687680701359408. [DOI] [PubMed] [Google Scholar]
- 98.Buatois V, Baillet M, Becart S, Mooney N, Leserman L, Machy P. MHC class II-peptide complexes in dendritic cell lipid microdomains initiate the CD4 Th1 phenotype. J Immunol. 2003;171:5812–5819. doi: 10.4049/jimmunol.171.11.5812. [DOI] [PubMed] [Google Scholar]