Abstract
Lupus-prone female NZM2328 mice develop high titers of anti-nuclear and anti-dsDNA autoantibodies. Despite high expression of type I IFNs, these mice do not develop autoantibodies to the small nuclear ribonucleoprotein (snRNP) complex. Thus, additional genetic factors must regulate the generation of anti-snRNP autoantibodies. In contrast, despite much lower expression of type 1 IFNs, the diabetes-prone NOD mice spontaneously make anti-snRNP autoantibodies, albeit at a low incidence. To determine whether combination of high type I IFN response of NZM mice with appropriate susceptibility genes of NOD mice would result in anti-snRNP antibody response, cohorts of (NZM2328 × NOD) F1 mice were generated and characterized for development of autoimmunity.
In comparison with parental strains, the PBMCs from F1 mice showed intermediate expression of type I IFN responsive genes and augmented expression of IL-6 transcripts. TLR7 expression was similar in all strains. The F1 mice had very high incidence and titer of anti-snRNP autoantibodies, ANA and anti-dsDNA autoantibodies. The levels of anti-snRNP autoantibody correlated with the expression levels of type I IFN responsive genes. None of the F1 mice developed diabetes and only female mice developed severe renal disease.
Our data demonstrates that only in presence of appropriate susceptibility genes, anti-snRNP autoantibodies are induced and type I IFNs amplify this response. A synergy between IL-6 and type I IFNs might be critical for amplifying overall autoantibody responses in SLE. In NZM/NOD F1 mouse, genetic complementation between NZM and NOD genes leads to expression of phenotypes similar to those seen in certain lupus patients.
Introduction
Autoantibodies reactive with the snRNP complex are often found in lupus patients. Among these, antibodies reactive with the SmB/B' and SmD1 proteins, or anti-Sm antibodies dominate the autoimmune response. Anti-Sm autoantibodies are considered diagnostic for lupus (1). Also present in some patients are antibodies reactive with the U1RNA associated A, C and 70kDa, proteins. These are commonly referred to as anti-ribonucleoprotein (RNP) antibodies. Despite their diagnostic value, the incidence of anti-Sm/RNP autoantibodies in lupus patients is considerably lower when compared to the incidence of anti-dsDNA autoantibodies (2-4). This suggests that genetic factors or pathways regulating the development of different autoantibody specificities in lupus are distinct.
Recent studies have emphasized a significant role for TLR7 mediated type I IFN production in the generation of anti-snRNP autoantibodies (5-8). The binding of uridine rich RNA molecules from the snRNP complex with TLR7 results in the activation of TLR7 mediated signaling and production of type I IFNs (9, 10). While some studies have suggested this as a pathway for amplification of anti-snRNP autoantibody responses, others emphasize this to be the primary event for initiating anti-snRNP response. Regardless, data from lupus patients clearly suggests that factors other than type I IFN must be involved in generation of anti-snRNP autoantibody responses. Despite higher type I IFN signature, indicative of higher type I IFN production, anti-snRNP autoantibodies are not detectable in some lupus patients (11). In addition, evidence has been obtained to show that the levels circulating type 1 IFN is genetically determined in humans (12). Thus, high type I IFN does not necessarily translate into, nor is dependent on an anti-Sm/RNP autoantibody response.
The female NZM2328 mouse is a well established model for lupus-nephritis (13, 14). This mouse is representative of a population of lupus patients, who develop anti-dsDNA and ANA but lack autoantibodies reactive with the snRNP complex. NZM2328 mice show considerable upregulation in the expression levels for different type I IFN responsive genes. However, lack of anti-snRNP autoantibodies suggests that just heightened type I IFN is not sufficient to generate anti-snRNP antibody. Thus, the NZM2328 mouse lacks genetic susceptibility for spontaneous generation of anti-snRNP autoantibody response. In contrast, the diabetes prone NOD mouse seems to carry susceptibility genes for the development of anti-snRNP autoantibodies. The very low incidence (10-20%) of anti-snRNP autoantibody in this mouse strain suggests that these susceptibility genes are kept under control. This notion is supported by the findings that anti-Sm/snRNP antibodies can be induced in NOD mice, either by injecting Bacillus Calmette-Guerin (BCG) (15), or through genetic manipulations (16, 17).
To test the hypothesis that only in presence of appropriate susceptibility genes, type I IFNs will influence anti-snRNP autoantibody responses, female NZM2328 mice were crossed with male NOD mice. The NZM2328 contribute the high type I IFN response, whereas the NOD mice provide susceptibility genes for anti-snRNP antibody response. Our data shows that genetic complementation between NZM and NOD genes lead to an augmented anti-snRNP antibody response in the F1 mice. This antibody response was not associated with expression levels of TLR7. However, the correlation between anti-snRNP autoantibody level and type I IFN responsive genes supports the role of anti-snRNP antibodies in amplifying type I IFN responses through TLR7. The NZM/NOD F1 mouse is a novel model for lupus-nephritis, resembling a patient population with high titers of circulating anti-snRNP, ANA and anti-dsDNA autoantibodies.
Methods
Mice
All mouse experiments were approved by the Animal Care and Use Committee at the University of Virginia. NZM2328 female mice were crossed with NOD/LtJ male mice to generate the NZM2328/NOD F1 hybrid mice. NZM2328 mice are bred and housed in our facilities as previously described (13). NOD male mice were obtained from Jackson Laboratory, Bar Harbor, ME.
Antibody analysis
Mice were bled at different time points through the tail vein and sera stored at -20°C till use. Antibody reactivity to the whole snRNP particle was assessed by immunoprecipitation using cell extracts from WEHI 7.1 cells metabolically labeled with 35S-methionine as described previously (18). In some experiments total radioactivity precipitated was measured by scintillation counting and results were expressed as CPM. ANA was detected by indirect immunofluorescence using HeLa cells and anti-dsDNA was determined by ELISA as previously described (14, 19). Serial dilutions of a purified mouse anti-dsDNA monoclonal antibody (R4A kind gift from Dr Betty Diamond) were used to generate a standard curve. Different dilutions of mouse sera were tested in the ELISA. Data are presented as antibody units/ml calculated from dilutions giving readings in the linear range of the standard curve. Total serum IgG and IgM levels were determined by a standard sandwich ELISA. Goat anti-mouse IgG or goat anti-mouse IgM antibodies were used as capture antibodies and peroxidase coupled goat anti-mouse IgG or peroxidase coupled goat anti-mouse IgM antibodies were used as detection antibodies respectively. Standard curves were constructed with purified mouse IgG and mouse IgM. All antibodies and standards were obtained from Southern Biotech (Birmingham, Al).
Analysis of gene expression
Mice were bled from the tail vein and blood was collected in heparinized tubes kept on ice. Following RBC lysis, the mononuclear cell pellet was used to prepare total RNA, using the RNAeasy mini kit (Qiagen Inc USA, Valencia, CA). During the RNA preparation, a step to remove genomic DNA was incorporated. Purified RNA was used to generate the first strand cDNA, using the SuperScript® III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen Corporation, Carlsbad. CA). Expression levels of PRKR (Eif2ak2), M×1, IL-6 and TNF- α were determined by Real Time PCR on MyiQ machine (Bio-Rad laboratories, Hercules, CA, USA) using the Taqman gene expression assays (Applied Biosystems, Foster City, CA, USA). The relative gene expression was calculated using the 2-Δ Δ C(T) method (20). A pooled RNA sample was used as reference calibrator and house keeping gene GAPDH was used as a control.
Staining for TLR7
Spleens from 2-3 month old female mice were removed and single cell suspensions prepared. RBCs were lysed and cells were suspended in PBS with 1%BSA and 0.1% sodium azide. Unless specified, antibodies used for flow cytometry were purchased from BD Biosciences (San Diego, CA). Cells were incubated with Fc Block and stained with PE conjugated antibodies to CD19 (1D3), CD11c (HL3), or CD11b (Mac-1α). Cells were incubated with rabbit anti-TLR7 (Imgenex Corporation, San Diego, CA) followed by biotinylated anti-rabbit IgG (Southern Biotech, Birmingham, Al) and neutravidin-FITC conjugate (Invitrogen Corp, Carlsbad, CA). Equal concentration of purified rabbit IgG was used as control. Data were acquired on a BD FACSCAN and analyses using Flow Jo Software.
Clinical and Histological Assessment
Mice were screened for proteinuria using Multistix 10 SG (Bayer Diagnostics Division, Elkhart, IN) starting at 3 months of age. Moribund mice showing urine protein concentrations of more than 300mg/dl for two consecutive screens were euthanized and kidneys removed for histological assessment. The surviving mice were euthanized at 12 months of age. Blood sugar was monitored using the Accu-Check comfort curve test strips and meter following manufacturer's instruction (Roche Diagnostics). The cohorts of NOD mice started to develop diabetes by 3 months of age. Moribund diabetic NOD mice were euthanized. Kidneys were collected in 10% buffered formalin and sections were stained with hematoxylin and eosin. Histological assessment for mesangial proliferation, glomerulosclerosis, interstitial inflammation, and tubular atrophy was performed as previously described by an independent observer in a blinded fashion (21). Kidneys were screened for IgG and C3 deposition by immunofluorescence as previously described (21).
Results
NZM/NOD F1 mice show intermediate level of expression of Type I IFN responsive genes
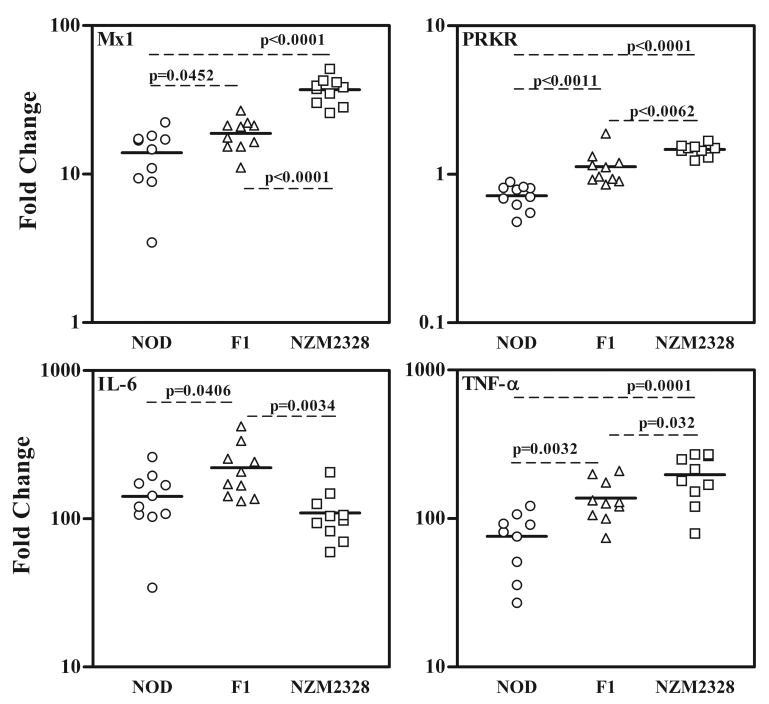
During an experiment to characterize a cohort of (NZM2328 × NOD) F1 × NZM2328 mice for a genetic linkage study, it was found that F1 mice produced high titers of anti-snRNP autoantibodies. This observation availed us the opportunity to study the role of type 1 IFN in the production of anti-snRNP antibodies. In lieu of determining the circulating IFN-α and IFN-β levels, we utilized the finding that the expression of genes responsive to type I IFNs are excellent indicators for type I IFN levels (22, 23). Analysis of type I IFN responsive genes PRKR (Eif2ak2) and M×1 in PBMCs was performed by real time PCR. (figure 1). The F1 mice had intermediate levels of PRKR and M×1 expression in comparison with the NOD (lowest expression) and NZM2328 mice (highest expression). These differences were statistically significant with p=0.0062 to p<0.0001.
Figure 1. NZM/NOD F1 mice show intermediate level of gene expression for type I IFN responsive genes PRKR and M×1 in comparison with the parental strains. With respect to proinflammatory cytokines, while TNF-α shows intermediate expression, IL-6 expression in F1 mice is significantly higher.
Expression of PRKR, M×1, TNF-α and IL-6 in peripheral blood of 14 week old NOD, NZM2328 and NZM/NOD F1 mice (n=10 per strain) were determined by real time PCR using Taqman gene expression assays. Data are represented as fold change in gene expression over a pooled control sample and were calculated using the 2 -ΔΔ C(T) method. Levels of house keeping gene GAPDH were used to normalize the gene expression. The solid lines indicate mean level of gene expression and unpaired t-test was used to determine statistical significance of variance. A P<0.05 was considered significant. The hierarchy of gene expression for PRKR, M×1 and TNF-α are NZM2328>F1>NOD. For IL-6 the hierarchy was F1> NOD>NZM2328.
As a comparison to type 1 IFN, the gene expression levels of proinflammatory cytokines IL6 and TNF-α were measured in the same samples. A similar trend was observed for the expression of TNFα. Surprisingly, IL-6 gene expression showed a different trend. The F1 mice showed a moderate increase in IL-6 expression in comparison with the NZM2328 (2.01 fold, p=0.0034) and NOD (1.56 fold, p=0.0406) mice. However, circulating IL-6 was undetectable in sera obtained at same time points. These data suggest that heterozygosity in the F1 mice causes intermediate expression of type I IFNs and TNFα. Whereas increased IL-6 expression in the F1 mice is indicative of genetic complementation between NZM and NOD genes controlling the expression of IL-6.
NZM/NOD F1 mice have higher incidence and titer of anti-snRNP antibodies
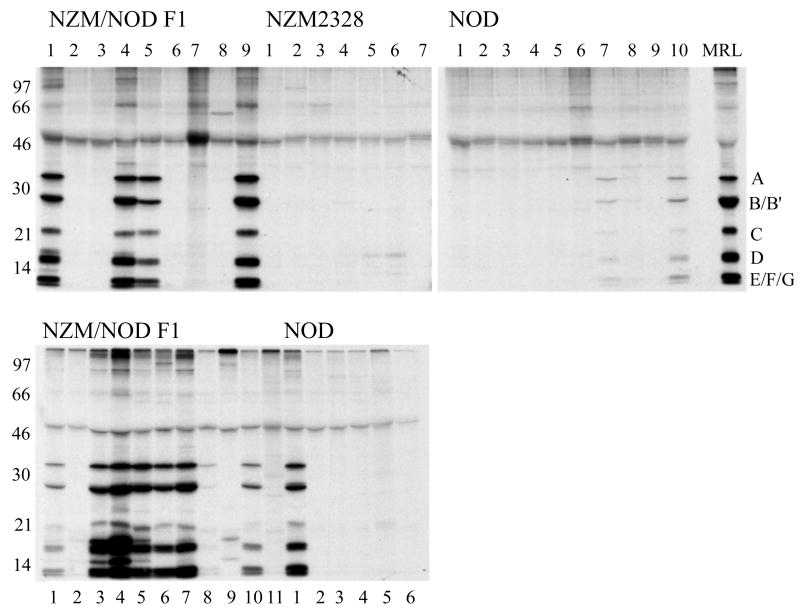
Sera obtained from the F1 mice and parental strains were used to immunoprecipitate the 35S-labeled snRNP particle. The top panel in figure 2 shows reactivity of sera obtained at 6 months of age. While none of the NZM2328 mice (0/7) had detectable anti-snRNP autoantibodies, the incidence in NOD mice was 20% (2/10), which is consistent with that reported by other investigators (16, 17). In contrast, 44% (4/9) of F1 mice showed the presence of anti-snRNP antibodies. Similar results were obtained in an additional cohort of F1 mice showing a 55% (6/11) incidence for anti-snRNP antibody production. Analysis of sera at terminal time points (moribund mice with ages between 6-12months), showed that 73% (8/11) of the F1 mice were positive for anti-snRNP antibody. In contrast only 17% (1/6) NOD mice had anti-snRNP antibodies (figure 2 bottom panel). None of the NZM2328 mice showed any anti-snRNP reactivity (data not shown). In comparison with the NOD mice the sera from the F1 mice immunoprecipitated more labeled snRNP, which is indicative of higher anti-snRNP antibody titers.
Figure 2. The NZM/NOD F1 mice have higher incidence and titer of anti-snRNP antibodies.
Sera (5 μl) from NZM/NOD F1, NZM2328, and NOD mice were used to immunoprecipitate the 35S-labeled proteins from metabolically labeled WEHI7.1 cells. The pooled serum from MRL lpr/lpr mice used as a positive control shows the characteristic anti-snRNP pattern. A similar pattern is seen in the F1 mice and to some extent in the NOD mice. None of the sera from NZM2328 precipitate the snRNP particle. The top panel shows sera from all mice obtained at 26 weeks of age. Similar results were obtained in additional cohort of F1 mice. The lower panel shows reactivity of sera from F1 and NOD mice obtained at terminal time points.
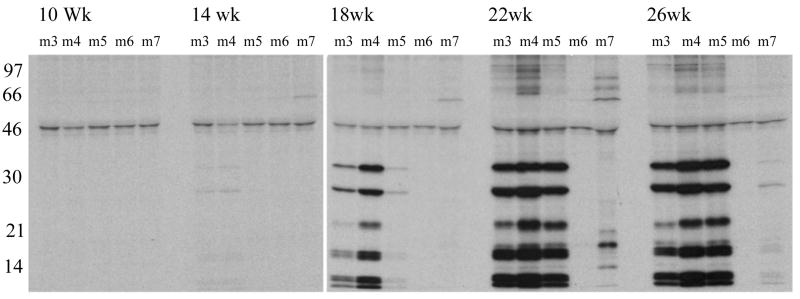
The kinetics of appearance of anti-snRNP antibodies in the F1 mice was studied. Sera from five representative F1 mice, (m3, m4, m5, m6 and m7 shown in figure 2) were used and data are shown in figure 3. As early as 14 weeks of age, a low level of anti-snRNP antibodies is seen in m3 and m4. These antibodies progressively increased in titer with age. In m5, the antibodies were first detected at 18 weeks and in m7 at 22 weeks. While m6 was negative for anti-snRNP antibody at 26 weeks, by 38 weeks of age the antibodies were readily detected (figure 2, lower panel). These data show a progressive increase in the incidence and titers of anti-snRNP antibodies in the F1 mice.
Figure 3. Kinetics of anti-snRNP autoantibody generation in NZM/NOD F1 mice.
Sera obtained at different time points from 5 representative F1 mice were used to immunoprecipitate the 35S-labeled proteins from metabolically labeled WEHI7.1 cells. A progressive increase in incidence and amount of anti-snRNP autoantibodies is seen in the F1 mice.
Anti-snRNP antibody production correlates with the expression of type 1 IFN response genes
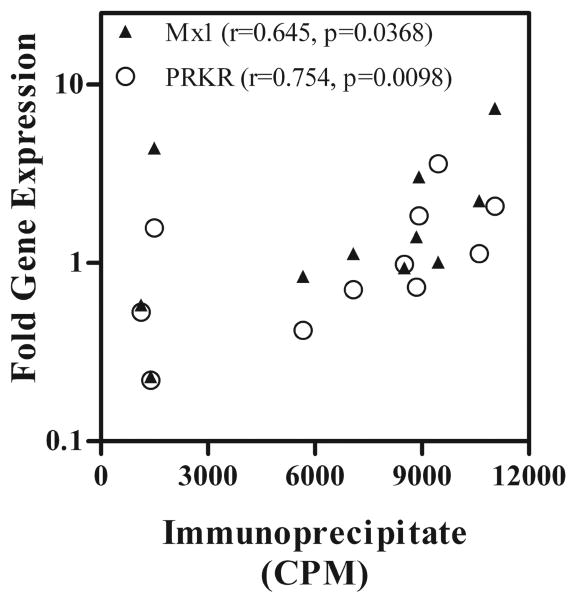
In comparison with the NOD mice, the F1 mice had significantly higher expression of type I IFN responsive genes. To determine, whether this correlated with levels of autoantibodies in the mice, we measured the amount of radioactivity immunoprecipitated by sera from F1 mice and compared it with expression levels of PRKR and M×1. Figure 4 shows that both PRKR (r=0.754, p=0.0098) and M×1(r=0.654, p=0.0368) expression levels correlated with autoantibody levels. PRKR gene expression, which is dominantly regulated by IFN-α had better correlation than M×1, which is regulated by IFN-β.
Figure 4. Expression levels of Type I IFN responsive genes PRKR and M×1 correlate with levels of circulating autoantibody in the sera of NZM/NOD F1 mice.
Mice were bled at terminal time points and RNA isolated from PBMCs used to determine gene expression levels for PRKR and M×1. Plasma isolated at same time point was used to immunoprecipitate proteins from cell extracts of metabolically labeled WEHI7.1 cells. The total amount of radioactivity in the immunoprecipitate was measured by scintillation counting. The CPM obtained was used as an index of autoantibody reactivity and correlation with fold change in gene expression of PRKR and M×1 was determined by Spearman correlation. Note the Spearman correlation coefficient for PRKR is 0.7545 (p=0.0098) and that for M×1 is 0.6455 (p=0.0368).
NZM/NOD F1 mice have stronger ANA and anti-dsDNA antibody responses
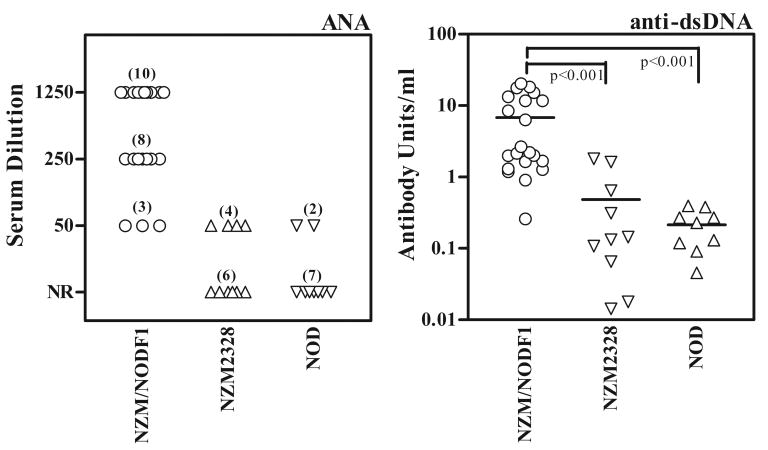
Anti-nuclear antibodies and anti-dsDNA antibodies are considered a hallmark of SLE. By indirect immunofluorescence (Figure 5, left panel), all F1 mice were positive for ANA by 8-10 months of age. In contrast only 40% of NZM2328 mice were positive for ANA at a 1:50 serum dilution. In a previous study we have reported the incidence of ANA in the NZM2328 mice to be 53% (24/45) (14). Analysis of sera from NOD mice showed that 22% were ANA positive. Thus the F1 mice had 100% incidence of positive ANA with much higher titers. Similar results were obtained regarding anti-dsDNA antibodies (Figure 5, right panel). The mean titer of anti-dsDNA antibodies in the F1 mice (6784±1487 mean antibody units/ml ± SEM) was a log scale higher than the NZM2328 mice (485±212) and NOD mice (214±71). The differences between the anti-dsDNA levels in the F1 mice and the two parental lines were statistically significant at p<0.001.
Figure 5. The NZM/NOD F1 mice develop high titers of ANA and anti-dsDNA antibodies.
Sera from mice (obtained at terminal time points) were screened for ANA titer by immunofluorescence (left panel). All F1 mice (n=21) were ANA positive with titers of 1:50 (n=3), 1:250 (n=8) and 1:1250 (n=10). NR means no reactivity detected at 1:50 dilution. The right panel shows reactivity of sera to dsDNA by ELISA. A mouse monoclonal anti-dsDNA antibody was used as a standard and results are expressed as anti-dsDNA antibody units. The solid lines indicate mean anti-dsDNA antibody units. Students t-Test was used to compare different groups and a p<0.05 considered statistically significant. Similar results were obtained in an additional experiment.
The augmented autoantibody responses in F1 mice were not a result of polyclonal B cell activation. The mean serum IgM levels did not show any significant difference between the NZM2328 (n=10, 0.97±0.08 mg/ml ± SEM) and F1 mice (n=10, 0.86±0.12 mg/ml ± SEM). At this time point the mean serum IgG levels in F1 mice (8.33±0.8 mg/ml ± SEM) were only modestly elevated (1.52 times, p=0.014) in comparison with the NZM2328 mice (5.46±0.68 mg/ml ± SEM).
TLR7 expression in NZM/NOD F1 mice is similar to that in the parental strains
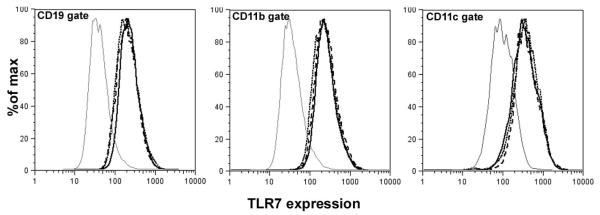
Recent studies have highlighted the role of TLR7, specifically the increased expression levels in autoantibody generation (5, 7). Thus, we analyzed the expression of TLR7 on B cells, dendritic cells and macrophages in F1 mice and those of the parental strains. Figure 6 shows that TLR7 expression between the F1 mice and the parental strains on different cell types was similar. These data suggest that in F1 mice, expression level of TLR7 does not contribute towards augmented anti-snRNP autoantibody response.
Figure 6. Level of TLR7 expression is similar between NZM2328, NOD and NZM/NOD F1 mice.
Spleen cells from 2-3 month old mice (n=3 per strain) were stained with anti-CD19 (B cells), anti-CD11c (dendritic cells), anti-CD11b (macrophages) and anti-TLR7. Figure shows TLR7 staining from representative NZM2328 (– –), NZM/NOD F1 (■ ■ ■ ■), and NOD (- - - -) mice in the CD19, CD11b and CD11c gates. The gray line represents staining with normal rabbit IgG. Similar results were obtained in an additional group of mice.
Female NZM/NOD F1 mice develop severe proteinuria
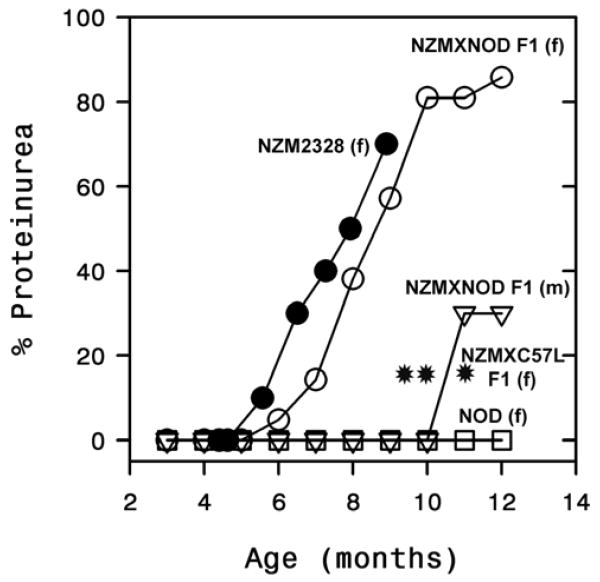
To determine the consequences of augmented autoantibody responses on renal disease, two independent cohorts of female F1 mice (n=10 and n=11) were monitored for development of proteinuria and the cumulative incidence is shown in figure 7. Our previous studies in the NZM2328 mice have shown that development of severe proteinuria (>300mg/dl) correlated strongly with the presence of chronic glomerulonephritis and impaired renal function (21). The F1 mice started to develop severe proteinuria by 6-7 months of age and by 10 months almost 80% of the mice had severe proteinuria. In contrast, only 3/10 male mice showed evidence of renal disease by 11 months of age. Despite augmented autoantibody responses, the incidence of proteinuria in the female F1 mice was very similar to that seen in the female NZM2328 mice. Female NOD mice did not develop any proteinuria, instead by 3 months of age they started to progressively develop severe diabetes and moribund mice had to be euthanized at different time points. By 12 months, the surviving NOD mice that did not develop diabetes were still negative for proteinuria.
Figure 7. Female NZM/NOD F1 mice develop severe proteinuria.
Cumulative incidence of severe proteinuria (>300mg/dL) in 21 female (○) and 10 male (▽) NZM/NOD F1 mice shows a higher incidence in the female mice. By 10-12 months of age the incidence of severe proteinuria in female mice was around 80%. Also shown for comparison is the incidence in female NZM2328 (●) (n=10), and female NOD mice (□) (n=7). The incidence of severe proteinuria in female NZM2328 × C57L/J F1 mice (n=30) was reported previously (14) and is shown at 10-12 months (*). Several of the moribund diabetic NOD mice had to be sacrificed starting at 4 months of age. By 12 months, 3 non diabetic NOD mice did not develop proteinuria.
None of the F1 mice showed evidence for diabetes. Histological examination of pancreas showed that few mice had mild focal perivascular lymphocytic infiltrates. However, the islets appeared normal and the blood sugar levels in all F1 mice were similar to those observed in the NZM2328 mice. Submandibular glands from some F1 mice (n=9) were collected in 10% buffered formalin and sections were stained with hematoxylin and eosin. Several mice (66%, 6/9) showed lymphocytic infiltrates within the salivary glands and they were very similar to those observed in the NOD mice (data not shown). A detailed characterization of salivary and lacrimal gland inflammation in the F1 mice and its effect of glandular function is being done.
Characterization of renal disease in the NZM/NOD F1 mice shows immunoglobulin and C3 deposits; and development of glomerulonephritis
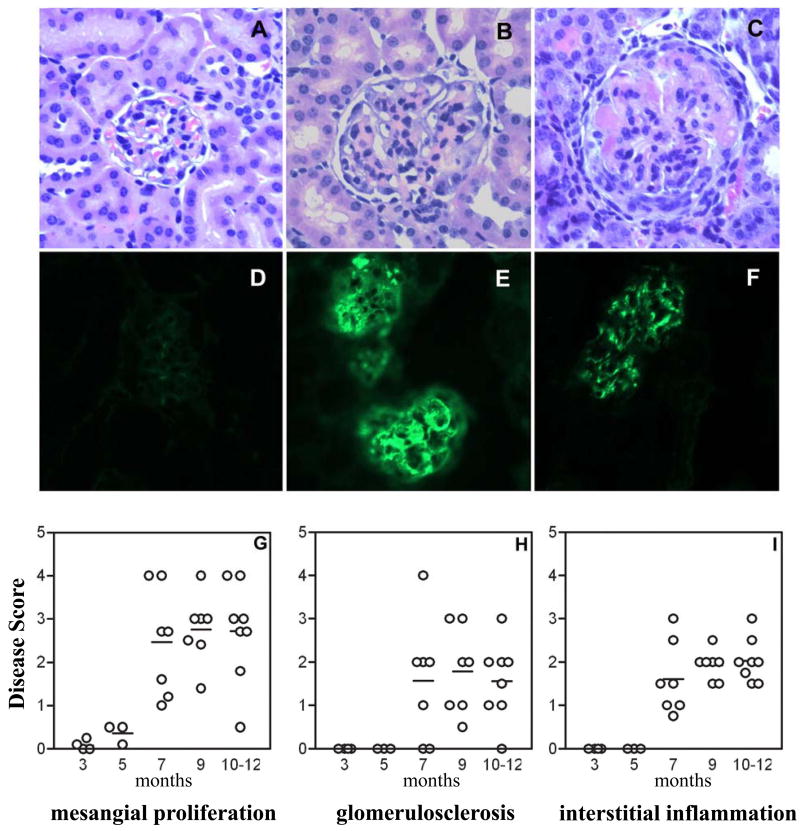
Renal pathology and immune-complex deposition was studied in a cohort of F1 mice euthanized at different ages (3, 5, 7, 9 and 10-12 months of age) or when they developed persistent, severe proteinuria (>300mg/dl for 2 consecutive readings). Thus, in this study mice were not monitored for mortality. Kidneys from NOD mice were studied at time of sacrifice (moribund diabetic or non diabetic at 12 months). A representative normal glomerulus in 12 month old female NOD mice is shown in figure 8A. In contrast, the F1 kidney shows both acute proliferative GN with mesangial expansion and cellular infiltrate (figure 8B), and chronic GN with glomerulosclerosis and interstitial inflammation (figure 8C). NOD kidneys had no detectable IgG (figure 8D), or C3 deposits (data not shown), whereas kidneys from F1 mice were positive for IgG and C3 deposits by direct immunofluorescence.
Figure 8. Characterization of renal disease in the female NZM/NOD F1.
Top panel: Representative photomicrographs of H&E-stained kidney sections of F1 mouse showing acute proliferative GN (B) and chronic GN (C). Shown for comparison is normal kidney from a NOD mouse (A). Immune-complex and C3 deposition in kidneys was studied by direct immunofluorescence staining. Representative pictures for IgG (E) and C3 (F) deposits in the F1 mice are shown. IgG deposition in NOD mice (D) was not seen. Lower Panel: Female mice were euthanized at different ages and kidney sections stained with H&E were evaluated for renal pathology. All parameters were scored on a scale of 0-5 with 0 = no disease and 5 = maximal severity. Each data point represents one mouse.
Kidneys from F1 female mice were graded for severity of glomerulonephritis by an independent observer, blind to experimental detail. The kidneys were evaluated for percent glomeruli affected, severity of mesangial expansion, glomerulosclerosis, tubular atrophy, interstitial fibrosis, and inflammation. Each parameter was scored for increasing severity from 0 to 5. As shown in figure 8G-I, mice at 3 and 5 months of age showed little to no pathologic changes in the kidney. By 7 months, most mice showed changes characteristic of lupus nephritis with mesangial expansion, glomerulosclerosis, and interstitial inflammation. This also coincided with the significant increase in incidence of proteinuria and was observed in all mice studied at 9 and 10-12 months of age. These data establish the NZM/NOD mice as a valid model for lupus-nephritis. Based on the onset of proteinuria (figure 7) and renal pathology (figure 8), the F1 mice do not show evidence for accelerated nephritis. The renal pathology and kinetics of renal disease in the F1 mice are similar to that observed in the parental NZM2328 mouse strain as reported previously (21).
Discussion
Autoantibodies reactive with the snRNP complex are often found in lupus patients and those reactive with the Sm proteins are an important diagnostic marker for the disease (1). Recent studies have indicated a role for IFN-α in the generation of the anti-snRNP antibody response (8, 23). A reduced incidence of anti-Sm/RNP antibodies is observed in MRL.lpr/lpr mice deficient in TLR7 (6). The RNA moiety associated with the snRNP complex can activate TLR7 and induce type I IFN production (9, 10). These studies implicate RNA within the snRNP particle engages TLR7 segregated in endosomal compartment resulting in the production of IFN-α, which in turn plays a role in the production of anti-snRNP antibodies. Recent findings with respect to TLR9, which may be pivotal in production of anti-dsDNA antibodies, suggest that formation of autophagosome brings together TLR9 and B cell receptor bound ligand to the endosomal location (24, 25). Whether a similar mechanism is operative for TLR7 is not known. The RNA moiety within the snRNP particle can gain access to TLR7 through the uptake and internalization of immune complexes containing snRNP particle and induce type I IFN production (10). However, it should be noted that despite the presence of high type I IFN, not all lupus patients develop anti-Sm/RNP autoantibodies. Thus, additional genetic factors contribute towards the generation anti-snRNP autoantibodies. Our results from the present investigation add support to this hypothesis.
In this study, we have shown genetic complementation in generation of the anti-snRNP antibody response. This conclusion is drawn from the observation that NZM/NOD F1 mice have very high titers and high incidence of anti-snRNP antibodies. The parental lines, NOD mice have low titers and low incidence of anti-snRNP antibodies, whereas NZM2328 mice fail to produce such antibodies. It is of significant interest that NZM2328 strain is high in type 1 IFN expression, NOD is low in type 1 IFN expression and the F1 mice have intermediate level of expression. Although there is a general correlation between type 1 IFN expression and anti-snRNP antibody levels, the type 1 IFN expression in F1 mice does not exceed that in NZM2328. The F1 mice also have much higher ANA and anti-dsDNA antibody titers than the NZM2328 mice. These data indicate type I IFN responses might be better regulated by genetic predisposition than just by levels of anti-snRNP and anti-dsDNA autoantibodies. These observations provide new insights in the relationship of IFN expression and anti-snRNP antibody production. At 23 weeks of age, the mean serum IgM levels between the F1 mice and NZM2328 mice were not significantly different and only a modest elevation in mean serum IgG level (1.52 times, p=0.014) was seen in the F1 mice in comparison with the NZM2328 mice. In contrast, the titers of ANA or anti-dsDNA antibodies in F1 mice were a log scale higher than the parental strains. In addition, in younger mice (10-12 weeks of age) we did not see any evidence for splenomegaly and the total spleen cell numbers in the F1 and NZM2328 mice were very similar (data not shown). These data suggest that augmented autoantibody responses in F1 mice are not a result of generalized polyclonal activation of B cells. However, whether B cells from F1 mice respond better to signals from helper T cells needs to be determined.
A similar observation of genetic complementation has been reported in the SWR × SJL F1 hybrid mice (26). Sera from parental strains were negative for the presence of anti-snRNP autoantibodies. However, by 40 weeks of age, 70% of the F1 mice were positive for anti-snRNP antibodies. Genetic complementation between the SWR and SJL strains of mice was considered as a mechanism for the observed phenotype. The role of type I IFN in this model was not investigated.
Genes contributing towards the development of anti-snRNP antibodies have not been clearly defined. From the aforementioned discussion, TLR7 is obviously one of the contenders. Studies from BXSB mice have demonstrated that increased expression of TLR7 due to gene duplication contributes towards the generation of antibodies reactive with ribonucleoproteins (5). In our study, comparison of TLR7 expression between the F1 mice and parental strains did not show any differences. Similarly we did not see any differences in TLR9 expression between these strains (data not shown). These findings are important as a recent study failed to demonstrate any significant association between relative TLR7 gene copy number and autoantibody profiles in lupus patients (27). Also, there was considerable variation in the relative gene copy number of TLR7 in both healthy controls and SLE patients, without any significant increase in the patients. Thus, expression level of TLR7 does not seem to be a major determining factor for generation of anti-snRNP autoantibodies in our model system. However, in the NZM/NOD F1 mice the level of autoantibodies correlated with the gene expression levels type I IFN responsive genes PRKR and M×1. These data suggest that both TLR7 and TLR9 must contribute towards the amplification of type I IFN responses in the F1 mice.
Genetic manipulation of NOD mice was shown to induce production of anti-snRNP antibodies (16). Presence of B10 mouse derived Idd9.3 locus on the NOD background (NOD.B10Idd9.3) was sufficient to increase the penetrance of anti-Sm antibodies (17). It was proposed that CD137 might be one of the candidate genes playing an important role in this autoantibody production through modulation of costimulatory signals. However, analysis of data from different crosses involving the NOD congenic mice shows multigenic regulation of anti-snRNP autoantibody responses. Our study also supports the notion of polygenic control of autoantibody production as heterozygosity with NZM genes was sufficient to induce a very high incidence of anti-snRNP antibodies. Whether in addition to genetic complementation, suppression of resistance genes plays a role in the F1 mice remains to be determined. The increased IL-6 transcript levels in F1 mice suggest that genes within this pathway might also be important for anti-snRNP, ANA and anti-dsDNA autoantibody generation. Clearly, the NZM/NOD F1 mouse model will be a valuable tool to identify these genes as well as to investigate the pathogenic potential of anti-Sm/RNP autoantibodies in lupus-nephritis.
Acknowledgments
This study was supported by grants from National Institutes of Health: P01-AR-45222, R01-AR-47988, R01-AR-49449 (S.M.F); R01-DK069769 (H.B); K01AR051391 (U.S.D) and grants from Lupus Research Institute (U.S.D) and Alliance for Lupus Research Award #68164 (S.M.F.).
References
- 1.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 2.Barada FA, Jr, Andrews BS, Davis JS, 4th, Taylor RP. Antibodies to Sm in patients with systemic lupus erythematosus. Correlation of Sm antibody titers with disease activity and other laboratory parameters. Arthritis Rheum. 1981;24:1236–1244. doi: 10.1002/art.1780241003. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Garcia E, Schur PH, Lahita R, American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests. Arthritis Rheum. 2004;51:1030–1044. doi: 10.1002/art.20836. [DOI] [PubMed] [Google Scholar]
- 4.Font J, Cervera R, Ramos-Casals M, Garcia-Carrasco M, Sents J, Herrero C, del Olmo JA, Darnell A, Ingelmo M. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33:217–230. doi: 10.1053/s0049-0172(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 5.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Scienc. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 6.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunit. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunit. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, Kellner ES, Nacionales D, Barker T, Kelly-Scumpia K, van Rooijen N, Kumar H, Kawai T, Satoh M, Akira S, Reeves WH. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 12.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 14.Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, Tung KS, Fu SM. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawke CG, Painter DM, Kirwan PD, Van Driel RR, Baxter AG. Mycobacteria, an environmental enhancer of lupus nephritis in a mouse model of systemic lupus erythematosus. Immunolog. 2003;108:70–78. doi: 10.1046/j.1365-2567.2003.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koarada S, Wu Y, Fertig N, Sass DA, Nalesnik M, Todd JA, Lyons PA, Fenyk-Melody J, Rainbow DB, Wicker LS, Peterson LB, Ridgway WM. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 17.Irie J, Wu Y, Sass DA, Ridgway WM. Genetic control of anti-Sm autoantibody production in NOD congenic mice narrowed to the Idd9.3 region. Immunogenetic. 2006;58:9–14. doi: 10.1007/s00251-005-0066-1. [DOI] [PubMed] [Google Scholar]
- 18.Deshmukh US, Bagavant H, Sim D, Pidiyar V, Fu SM. A SmD peptide induces better antibody responses to other proteins within the small nuclear ribonucleoprotein complex than to SmD protein via intermolecular epitope spreading. J Immunol. 2007;178:2565–2571. doi: 10.4049/jimmunol.178.4.2565. [DOI] [PubMed] [Google Scholar]
- 19.Bagavant H, Tung KS. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175:944–950. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177:8258–8265. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 22.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 23.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunit. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe K, Tsubata T. Autophagy connects antigen receptor signaling to costimulatory signaling in B lymphocytes. Autophag. 2009;5:108–110. doi: 10.4161/auto.5.1.7278. [DOI] [PubMed] [Google Scholar]
- 26.Vidal S, Gelpi C, Rodriguez-Sanchez JL. (SWR × SJL)F1 mice: a new model of lupus-like disease. J Exp Med. 1994;179:1429–1435. doi: 10.1084/jem.179.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum. 2007;56:3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]