Abstract
Purpose
Nonhuman primates reared with daily alternating monocular occlusion (AMO) during their first few months of life develop large horizontal strabismus, A/V patterns and dissociated vertical deviation (DVD). In addition, these animals often alternate or switch the fixating eye during binocular viewing. The purpose of this study was to characterize the alternating fixation behavior of these animals during visually guided saccade tasks.
Methods
Binocular eye movements were measured in two monkeys with AMO-induced exotropia as they performed a visually guided saccade task (random target presentation over a ±15° grid horizontally and vertically) during either monocular or binocular viewing.
Results
During binocular viewing, large target steps into the temporal hemifield of the nonfixating eye (nasal retina of the nonfixating eye) produced fixation switches. Target steps into the nasal hemifield of the nonfixating eye (temporal retina of the nonfixating eye) tended not to produce a fixation switch. There were no significant differences in the amplitude–peak velocity or amplitude– duration main sequence relationships between alternating (binocular viewing) and nonalternating saccades (monocular or binocular viewing). Saccade latency tended to be greater during binocular viewing than during monocular viewing.
Conclusions
This study shows that the AMO model for strabismus may be used for studying neural circuits involved in generating alternating fixation and alternating saccade behavior. Since patterns of alternating fixation are likely to be influenced by patterns of visual suppression, alternating saccade behavior may also be used as a probe to study mechanisms of visual suppression in strabismus.
Developmental strabismus is a significant public health problem, as it occurs in as many as 5% of all children.1–3 Although the exact etiology of strabismus is often unknown, disruption of binocular vision early in postnatal development leads to strabismus. Previous studies have shown that monkeys specially reared using an alternate monocular occlusion (AMO) paradigm develop strabismus.4,5 In addition to horizontal misalignment, these strabismic animals display A/V patterns and dissociated vertical deviation (DVD), all commonly observed in humans with strabismus. These studies establish that AMO rearing of monkeys results in a suitable animal model to examine various properties of strabismus.
A phenomenon of strabismus that we observed in this animal model and were interested in exploring further was alternating fixation.4 Recently Economides et al.6 have shown that monkeys with surgically induced exotropia also develop alternating fixation behavior. Alternating fixation is when a strabismic individual or monkey has the capability to fixate a target of interest with either eye and can spontaneously alternate (or switch) the fixating eye. A saccadic eye movement in which the fixating eye is switched may therefore be called an alternating-fixation saccade or more simply, an alternating saccade. Fixation switches tends to occur in patients who have relatively little preference for one eye over the other (i.e., no dominant eye). In exotropia, the ability to alternate the fixating eye is likely to depend on visual suppression mechanisms that suppress parts of the retina of fixating and nonfixating eyes.6–8 Horton et al.7 showed, via metabolic studies in two animals with exotropia and poor fixation preference (i.e., animals who showed frequent fixation switches), that the temporal retina of each eye (at eccentricity beyond 10° for the fixating eye) is suppressed. Other animals, described in the same study, which showed significant preference for one eye, appeared to suppress nasal and temporal retinas of the nonfixating eye. Studies in humans with exotropic strabismus also indicate that there is suppression of the temporal retinas.9–11 Given this pattern of suppression, some predictions can be made regarding a fixation switch during a saccadic task in alternating exotropia. A large target step that places the target image onto the unsuppressed nasal retina of the previously nonfixating eye and therefore the suppressed temporal retina of the previously fixating eye (i.e., large leftward target jumps when the right eye is initially fixating the target or large rightward target jumps when the left eye is initially fixating the target) would evoke a fixation switch. Note that according to this hypothesis, for a fixation switch to occur, the target step must be large enough (greater than the strabismus angle) to appear in the nasal retina of the previously nonfixating eye.
In perhaps the only study in the literature that examined properties of alternating saccades in humans with exotropia, Van Leeuwen et al.8 showed that some metrics of alternating and nonalternating saccades are similar. However, their experimental paradigm was limited to a predictable self-generated horizontal saccade task and also they analyzed only the amplitude– peak velocity relationship in their subjects. Thus, there were three main goals of this study. The first was to establish the AMO animal model as effective in producing alternating fixation and alternating saccade behavior. Establishing a monkey model opens the avenue for identification of neural substrates using conventional neurophysiological methods. A potential advantage of studying alternating saccade behavior in a sensory model of strabismus is that the animals have not undergone strabismus surgery, unlike in the monkeys with surgical exotropia6 or human patients who have usually undergone corrective strabismus surgery.8 A second goal was to map out the spatial pattern of alternating fixation behavior and compare these to patterns of visual suppression that might be expected in exotropia. The third goal was to compare metrics of alternating and nonalternating saccades over a large range of horizontal and vertical amplitudes and orbital positions. Some of these results have appeared before in abstract form and in conference proceedings.12,13
Methods
Subjects and Rearing Paradigms
Behavioral data were collected from two strabismic (S1 and S2) juvenile rhesus monkeys (Macaca mulatta) weighing 8 to 11 kg. Monkeys with strabismus were reared at the Yerkes National Primate Research Center in an AMO paradigm.4,14 In the AMO rearing procedure, soon after birth (within the first 24 hours), an occluding patch (either opaque goggles or dark contact lenses) is placed in front of one eye for a period of 24 hours and thereafter switched to the fellow eye for the next 24 hours. The patch is alternated daily for a period of 4 to 6 months. Therefore, during AMO rearing, the monkey's binocular vision is severely disrupted during the first few months of life, the critical period during which proper eye alignment, stereovision, and binocular sensitivity normally develop in the brain.15–17 During rearing, the animals are checked every 2 to 3 hours to verify that the occluding lens is in place. The compliance to occlusion lens rearing is usually greater than 90%.
Surgical Procedures and Eye Movement Measurements
After special rearing, the animals were allowed to grow normally until they were approximately 3 years of age before starting experiments. Sterile surgical procedures performed under aseptic conditions using isoflurane anesthesia (1.25%–2.5%) were used to stereotaxically implant a head stabilization post. In the same surgery a scleral search coil was also implanted in one eye by using the technique of Judge et al.18 Later in a second surgery, a second scleral search coil was implanted in the other eye. All procedures were performed in strict compliance with NIH guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University.
Binocular eye position was measured by the magnetic search coil method (Primelec Industries, Regensdorf, Switzerland).19,20 Calibration of the eye coil signal was achieved by rewarding the monkey with a small amount of juice or other reward when the animal looked within a small region (±2° window) surrounding a 0.25° target dot that was rear projected on a tangent screen 60 cm away from the animal. Calibration of each eye was performed independently during monocular viewing.
Experimental Paradigms and Data Analysis
Eye movement data were collected as the strabismic animals performed a saccade task where the target appeared at random horizontal or vertical locations within a ±15° grid (5° increments). Data were collected during both monocular and binocular viewing in separate experimental sessions. Binocular eye and target position feedback signals were digitized at 1 kHz with 16-bit precision (Labview software and DAQ boards from National Instruments, Austin, TX). The analyses of the saccade data were partially automated and performed with custom software (MatLab; The MathWorks, Natick, MA).21 The computer displayed the target position, binocular eye position, eye velocity, and eye acceleration traces of a single-saccade trial on the screen. Velocity and acceleration signals were generated by digital differentiation of the position signal using a central difference algorithm. Position, velocity, and acceleration signals were filtered using software FIR filters (80 points; 0 – 80 Hz band-pass) also designed in the custom software. The investigator viewed the traces and decided whether the saccade trial was to be accepted or rejected. Trials that were rejected were usually those in which the animal was not fixating before the target step, the saccade did not appear to be directed toward the target, or the saccade did not occur within 500 ms of the target step. Once a decision to accept the trial was made, the mean ± SD control eye acceleration before the saccade was calculated over a 100-ms fixation period selected by the user. Saccade onset was automatically determined by the software as the first time point at which eye acceleration was greater than 3 SD away from the control eye acceleration and saccade offset was determined as the last time point at which eye deceleration was less than 3 SD away from the same mean eye acceleration. Although detection of saccade onset and offset was automated, the investigator visually examined the velocity and acceleration traces of every saccade and had the option of either accepting or changing the computer's selection. Typically, only a small percentage of the computer's choices were changed by the investigator. For the binocular viewing data, the investigator also made the determination if the saccade was of the alternating/nonalternating variety, and this information was recorded in the computer along with the saccade parameters.
After data collection and initial analysis of saccade onset and offset, the data were parsed into the following six bins depending on viewing condition and saccade type: (1) saccades during monocular right eye viewing (MR), (2) saccades during monocular left eye viewing (ML), (3) binocular viewing nonalternating saccades with the right eye fixating (BR), (4) binocular viewing nonalternating saccades with the left eye fixating (BL), (5) binocular viewing alternating saccades where the fixating eye was switched from right eye to left eye (BRL), and (6) binocular viewing alternating saccades where the fixating eye was switched from left eye to right eye (BLR).
Once the data were parsed, saccade metric parameters (amplitude, latency, peak velocity, and duration) for each eye were calculated. Since saccades included both horizontal and vertical components, vectorial values were used for amplitude and peak velocity, duration was the maximum of the duration of the horizontal and vertical components, and latency was the minimum of the latency of the horizontal and vertical components. Amplitude–peak velocity and amplitude– duration relationships were plotted, and data were fit according to the following equations commonly used to describe main-sequence data.6,8,22,23
In these equations, the parameters PVmax, C, D0, and D1 characterize the main-sequence relationships and can therefore be used to identify certain abnormalities in generation of saccadic eye movements. For example, slow saccades would result in a lower PVmax. PVmax, C, D0, and D1 were estimated from the right eye and left eye saccade data separately. Fitting was performed in a commercial program (SigmaPlot, ver. 10.0; Systat Software, San Jose, CA). One-way ANOVA at a significance value of 0.05 (SigmaStat ver. 3.5; SPSS, Chicago, IL) was used to compare each estimated parameter across the six saccade conditions (MR, ML, BR, BL, BRL, and BLR).
For saccade latency data, histograms of the inverse of saccade latency were developed and a Gaussian was fitted to the data. The inverse of latency was used for developing the Gaussian fit because it has been shown that this parameter is more representative of a Gaussian process than is saccade latency directly.24–26 The mean and SD of this Gaussian fit was compared across the six saccade conditions using ANOVA.
Results
Properties of Strabismus in the AMO Animals
The two animals included in the study were both exotropic. During right eye viewing of a straight-ahead target, monkey S1 showed an exotropia of 10° and S2 an exotropia of 11°. During left eye viewing of the same target, S1 showed an exotropia of 15° and S2 an exotropia of 14°. Although visual acuity was not measured in these animals, AMO rearing is expected to induce minimal amblyopia. Other visual function and oculomotor properties of animals reared using AMO methods may be found elsewhere.4,5,21,27–29 The main focus of this study was to investigate alternating fixation and alternating saccade behavior.
Spatial Pattern of Saccade Alternation
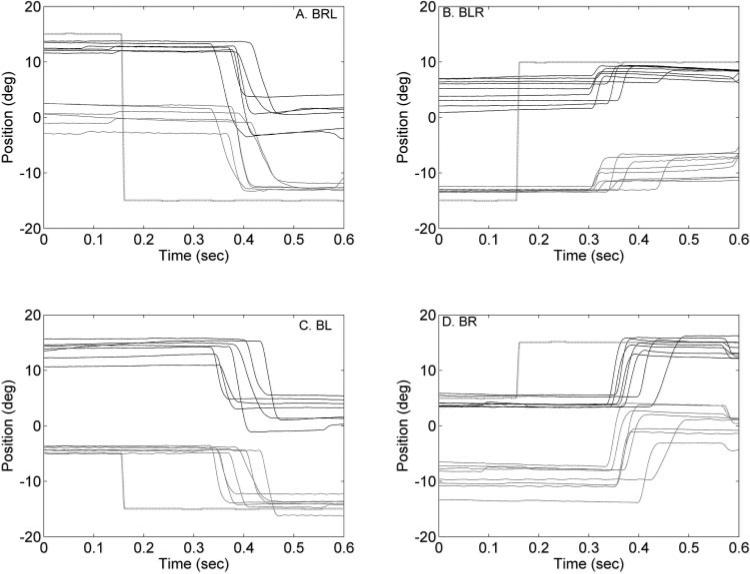
Figure 1 shows typical raw data acquired during binocular viewing illustrating the property of alternating saccades in animal S1. Each panel shows saccades of a specific type and amplitude (BR, BL, BRL, and BLR). Large target steps that placed the target on the nasal retina of the previously nonfixating eye resulted in the generation of an alternating saccade and a fixation switch (Figs. 1A, 1B). Figures 1C, 1D show examples where the target jumped further eccentric on the nasal retina of the fixating eye (temporal retina of the nonfixating eye) and the result was a nonalternating saccade (i.e., no fixation switch). Also, as observed in Figure 1, there was less variability in the starting and ending positions of the fixating eye compared with the starting and ending positions of the nonfixating eye. For the example trials shown in Figure 1A, the starting positions of the right eye (fixating eye) in the different saccade trials were close to the target starting position of +15° (Fig. 1A). The starting positions of the left eye (nonfixating eye) for the different trials were more variable. At the end of the saccade, however, the left eye (fixating eye) positions were tightly locked to the target ending position of –15°, whereas the right eye (nonfixating eye) was much more variable.
Figure 1.
Raw data illustrating alternating (A, B) and nonalternating (C, D) saccades during binocular viewing in animal S1. In each panel, several trials of saccade data, aligned on target onset, are shown. On the x-axis is time and on the y-axis is the horizontal eye position. Rightward eye positions are positive, and leftward eye positions are negative. Black traces: right eye position; gray traces: left eye position and black dotted line: the target position. (A, B) Large target steps (15° R to 15° L in A and 15° L to 10° R in B) into the temporal hemifield (nasal retina) of the previously nonfixating eye (left eye in A and right eye in B) resulted in the generation of alternating saccades and therefore a switch in the fixating eye. (C, D) Examples of nonalternating saccades where there is no switch in the fixating eye (left eye fixation in C and right eye fixation in D).
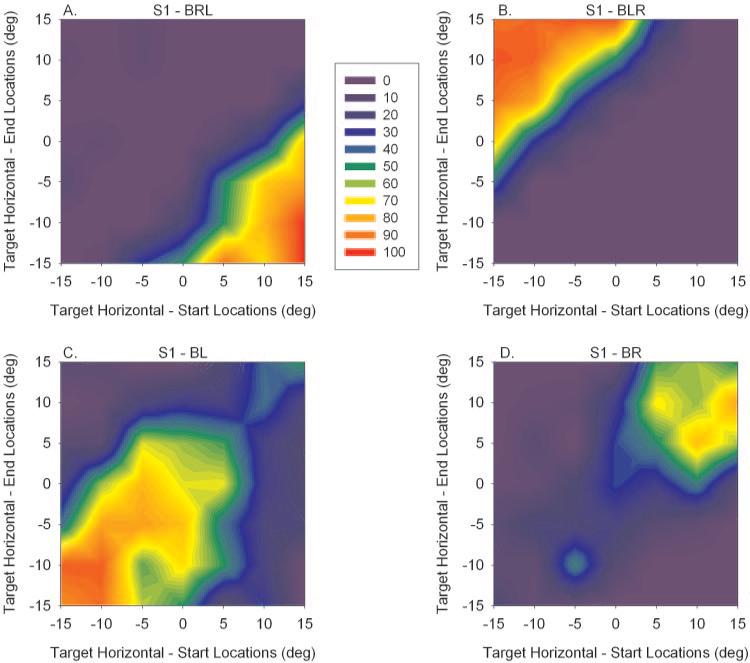
Figure 1 shows data for only a subset of target starting and ending locations. Data were collected for a range of horizontal and vertical target starting and ending points ranging from –15° to +15° (5° increments). For each combination of target starting and ending locations (horizontal component only; 49 in total), the frequency of generation of alternating (BRL and BLR) and nonalternating saccades (BR and BL) were calculated. For example, target steps from a horizontal component target starting location of +15° to a horizontal component target ending location of –15° induced BRL (alternating right eye fixation to left eye fixation) saccades on all (13/13) trials. The data that summarize the incidence of alternating and nonalternating saccades for all combinations of horizontal component of target start and end locations is shown as a filled contour plot in Figure 2 for animal S1. In these plots, the vertical component of target locations were disregarded because the relationship between fixation switch and target location on temporal/nasal retina (and not superior/inferior retina) is of primary interest.
Figure 2.
Filled contour plots developed from saccade data in animal S1 showing frequency of fixation switch (A, B) or lack thereof (C, D) for saccades starting and ending at various spatial locations. On the x-axis is the horizontal component of the target starting location, whereas on the y-axis is the horizontal component of the target ending location. Vertical components of target movement are not shown. The color scale is the percentage of saccades that were of a particular variety (BRL, BLR, BR, or BL). Actual data are located at 5° increments on the plot. An average of 20 trials was collected for each combination of target starting and ending location. The filled contour plot was obtained by interpolating for in-between locations. (A, B) Fixation-switch behavior was observed when the target stepped into the nasal retina of the previously nonfixating eye. (C, D) Spatial locations of target starting and ending combinations in which no switch in fixation was observed. These were predominantly for target steps into the temporal retina of the previously nonfixating eye. Also observed in (C, D) is that animal S1 slightly favored fixation with his left eye.
A major result is that the target steps most likely to result in the generation of an alternating saccade (hottest colors in Figs. 2A, 2B) were those that fall onto the nasal retina of the previously nonfixating eye. Thus, in Figure 2A (BRL), large (>15° amplitude) leftward target steps resulted in a fixation switch from the right to the left eye. Note that since the nonfixating eye deviates in strabismus, both the starting location and the target amplitude are important factors in determining whether the target ending location falls on the nasal retina of the previously nonfixating eye. For example, for a target starting location of +15°, leftward target steps of 15° (target ending location of 0°), 20° (target ending location of –5°), 25° (target ending location of –10°), and 30° (target ending location of –15°) show high incidence of fixation switch. Since the angle of exotropia is approximately 15°, smaller (<15° amplitude) leftward target steps and all rightward target steps did not place the target onto the nasal retina of the previously nonfixating eye (still on the temporal retina) and no alternating saccade was generated. For example, for a starting location of +10°, leftward target jumps of 5° (to target ending location of +5°) or 10° (to target ending location of 0°) did not show incidence of a fixation switch (Fig. 2A). Similarly, in BLR, large (>15° amplitude) rightward target steps resulted in a fixation switch from the left to the right eye (Fig. 2B). Smaller (<15° amplitude) rightward target steps and all leftward target steps did not induce a fixation switch.
Figures 2C (BL) and 2D (BR) show the target locations where the animal was most likely to fixate with the same eye before and after the saccade (i.e., no fixation switch). These are target steps that place the target further eccentric on the temporal retina of the nonfixating eye (rightward target steps with the right eye fixating in Fig. 2D and left ward target steps with the left eye fixating Fig. 2C). Also included are the smaller target steps alluded to earlier that were in the correct direction (i.e., rightward target steps with the left eye fixating in Fig. 2C or leftward target steps with the right eye fixating in Fig. 2D), but they were not large enough to place the target onto the nasal retina of the previously nonfixating eye. Also resulting from this analysis is the indication that animal S1 had a slight preference for fixating with his left eye than his right eye (larger hot area for BL than for BR).
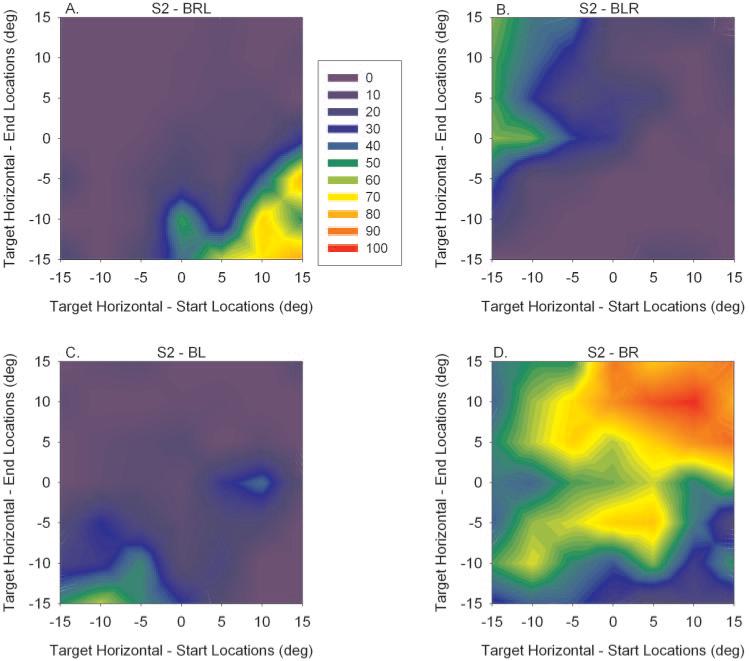
Data from animal S2 are similarly plotted in Figure 3. Although the data were not as apparent as in animal S1, the results were qualitatively similar. The fixation switch from the right to the left eye is shown in the bottom right of Figure 3A, and the fixation switch from the left to the right eye is shown in the top left of Figure 3B. The primary difference seems to be that the animal S2 showed significant preference for right eye fixation (Fig. 3D, large, hot area for BR). Supporting this observation, this analysis showed that 56% of the binocular viewing trials analyzed in animal S2 were of the BR variety.
Figure 3.
Similar data as shown in Figure 2 obtained from animal S2. Data are qualitatively similar to those in S1 except that animal S2 showed much greater preference for viewing with his right eye (D).
Saccade Main-Sequence Relationships
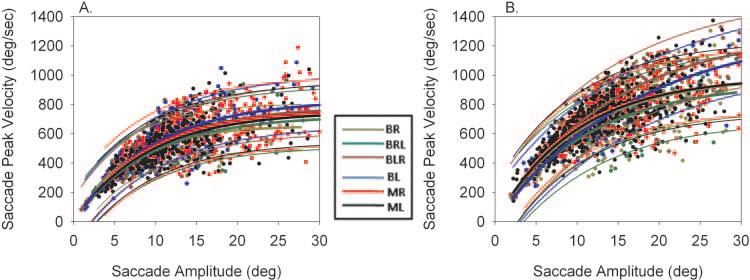
The next step was to analyze the metrics of alternating saccades and compare it to nonalternating saccades and monocular viewing saccades. Approximately 1000 binocular viewing saccades (BR, BL, BRL, and BLR) and 1000 monocular viewing saccades (MR and ML) were collected in each animal. Of the binocular viewing saccades, 39% in monkey S1 and 31% in monkey S2 were of the alternating variety (BLR and BRL). Figure 4 shows the amplitude–peak velocity main sequences from animals S1 and S2 during right eye viewing for the six different saccade types. The figure illustrates that there was considerable overlap in the data for the different saccade types. An exponential rise-to-maximum curve was used to fit each set of data. Figure 4 also shows a plot of the estimated curves along with the 95% prediction intervals. There was significant overlap in the prediction intervals (all the regression lines lie within the 95% prediction intervals of the fellow fits) suggesting that there was no difference among the different saccade types.
Figure 4.
Amplitude–peak velocity main sequence relationships in animals S1 (A) and S2 (B) for the different saccade types. Individual data points are from right eye movement data. Also plotted are the exponential curve fits and 95% prediction intervals. There is considerable overlap in the prediction intervals among the different samples of saccades.
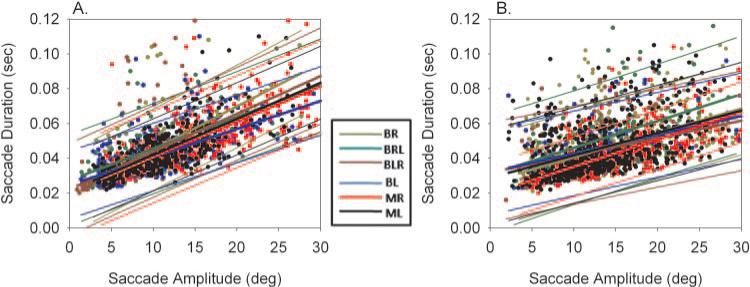
The amplitude– duration relationship in the different types of saccades in two animals was also examined. Figure 5 shows these relationships in the two animals. A presentation of data similar to that in Figures 4 is used in this figure. A linear fit was applied to the amplitude– duration data and the regression line along with 95% prediction intervals plotted. Once again there was significant overlap in the data and the prediction intervals among the different saccade types, suggesting that the saccade data in the various categories all came from the same population.
Figure 5.
Amplitude–duration main sequence relationships in animals S1 (A) and S2 (B) for the different saccade types. Individual data points are from right eye movement data. Also plotted are the linear regressions and 95% prediction intervals. Once again, there was considerable overlap in the prediction intervals among the different samples of saccades.
The regressions shown in Figures 4 and 5 yielded estimates for the parameters PVmax, C, D0, and D1 developed from either the right eye saccadic movement or the left eye movement. A one-way ANOVA comparison of the estimated parameters in each monkey yielded only idiosyncratic differences among the six saccade conditions that were analyzed. In monkey S1, parameter PVmax estimated during BL saccades by using right eye movement data was significantly different from all other conditions. However, significant differences from all other conditions were not observed when estimates developed from left eye movement data were used for the comparisons. The parameter C estimated during BR saccades with the left eye movement data showed a significant difference from other saccade conditions, but the same was not true when right eye movement data were used for developing estimates. Neither parameter D0 nor D1 showed differences across all saccade conditions in monkey S1. In monkey S2, parameters PVmax and C estimated during BL and BLR conditions were significantly different from other saccade conditions. In monkey S2, D0 estimated during BR saccades was also significantly different from other saccade conditions. In summary, statistical analysis of estimated parameters did not reveal any consistent differences in the saccade main-sequence relationships among the different saccade conditions tested.
Saccade Latency
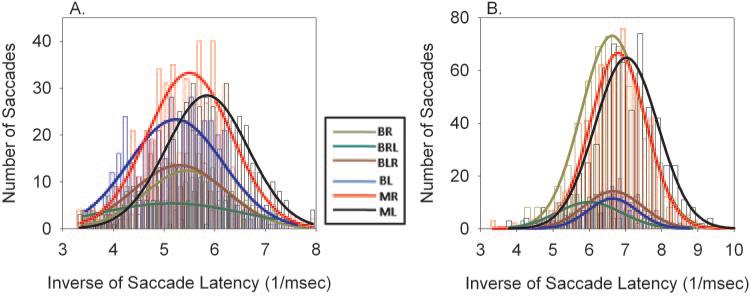
Saccade latency across the different saccade types was also compared. In normal humans and animals, a histogram of saccade latency follows a skewed distribution with a rapid rise and a relatively long tail. Carpenter and Williams26 developed a model for saccadic latency (the LATER model) and showed that the reciprocal of the saccade latency is representative of a Gaussian process.26 Therefore, to make statistical comparisons of latency within each of the saccade types in this study, histograms of the inverse of saccade latency were developed and then a Gaussian was fit to the data. Figure 6 plots histograms of inverse of saccade latency for the different saccade types along with the Gaussian fits for animals S1 and S2.
Figure 6.
Inverse of saccade latency along with Gaussian fits for monkeys S1 (A) and S2 (B). The data show that there is a tendency for monocular viewing saccades to be of slightly shorter latency (right shift in plots for the MR and ML fits) than binocular viewing saccades.
In animal S1, I found that saccades during binocular viewing were of significantly longer latency than saccades during either monocular viewing condition (difference in means, 7–23 ms; one-way ANOVA; P > 0.05) except between MR and BR. In animal S2, saccades during binocular viewing were of significantly longer latency than monocular left eye viewing saccades (difference in means, 8–25 ms; one-way ANOVA; P > 0.05). Monocular right eye viewing (MR) saccades were of shorter latency than BLR and BR saccades but not BL and BRL saccades. Latency of nonalternating saccades was not consistently different from alternating saccades.
Discussion
In this study, alternating saccade behavior in AMO strabismic monkeys was analyzed with the goal of (1) establishing the AMO as a suitable animal model to study alternating fixation behavior, (2) examining spatial patterns of alternating fixation, and (3) comparing metrics of alternating saccade behavior to nonalternating saccades and monocular viewing saccades.
Animal Model for Alternating Fixation Behavior
This study showed that AMO animals with sensory exotropia demonstrated alternating fixation and alternating saccade behavior similar to previous reports in humans8,11 and in monkeys with surgically induced exotropia.6 Thus, the AMO strabismus model is appropriate for examining visual and oculomotor mechanisms that drive alternating saccade behavior. One difference in alternation behavior observed in the sensory strabismic animals (present study) and the surgical strabismic monkeys described by Economides et al.6 is that the orbital position at which the animal switches fixation appears to be much sharper and more consistent in the surgical strabismic animals compared with the sensory strabismic animals (notice the gradual change in colors in Figs. 2 and 3 that occurs in the border between the hot [red] and cool [blue] areas). It may be that in the surgical strabismic animals, the inability to adduct immediately after the medial rectus tenotomy procedure resulted in the development of zones and depths of visual suppression in each eye that is different when compared with the sensory strabismic animals that did not have any significant limitation in ocular motor range at any point in their development. The observed difference may also be simply a byproduct of the slightly different testing methodology used, since Economides et al.6 used a task in which each trial originated from central fixation, whereas the current study used a random target presentation method. Of course, this observation must be viewed with caution since the number of animals examined here and in the study by Economides et al. was small.
Alternating Fixation and Visual Suppression
The spatial pattern of saccade alternation observed in these monkeys appears to be generally consistent with the published reports of hemifield suppression in exotropic monkeys.7 Animal S1 appeared to be most like the two animals that Horton et al.7 described, with exotropia and poor fixation preference (i.e., animals that were judged to have alternating fixation), whose temporal retinas (>10° in the fixating eye) were suppressed. Based on this pattern of suppression, one would expect that a fixation switch would occur if the target fell on the nasal retina of the previously nonfixating eye. Note also that the pattern of lack of fixation switch for smaller target jumps is consistent with temporal retina suppression hypothesis.
Animal S2 seemed to show significant preference for fixation with the right eye and tended to fixate with his left eye only when the target was placed far to the left (beyond 15°). Such a pattern of monocular preference is quite common as, described by Horton et al.7 in two other animals in the same study. In animal S2, based on the patterns of alternation and monocular preference, the prediction would be that visual suppression was more widespread in the left eye. A potential weakness of this study is that visual suppression in these strabismic animals was not directly measured. It would be important to verify that the spatial maps for alternating fixation such as those shown in Figures 2 and 3 indeed correlate directly with the maps of visual suppression developed independently with visual evoked potential (VEP) or psychophysical methods.9,30,31 If it turned out that there was a tight correlation, alternating fixation and alternating saccade behavior may be used as probes to study visual suppression mechanisms in strabismus and how they relate to developing a command signal for generating a saccade that brings one or the other eye onto the target.
It also may be true that visual suppression mechanisms are not the only mechanisms involved in the generation of alternating saccades. For example, the data from esotropic primates do not fit easily within this framework.29,32 These reports suggest that esotropic animals (including two with alternating esotropia reported by Wong et al.32) also show suppression of the temporal retinas. However, one might have expected (based on the metabolic results and alternating fixation data from exotropic animals) that metabolic data would show that the nasal hemiretina is suppressed in esotropia to enable alternating cross-fixation. It is not clear whether mechanisms of alternating fixation in esotropia would be fundamentally different from exotropia, but more studies are needed to resolve this question.
Some studies have shown that depth of visual suppression may not be complete.31,33,34 This effect is most likely to occur when the target is high-contrast—that is, above threshold (such as the ones that were used in this study). In such a scenario, certain downstream saccade-related structures in the brain may be encoding multiple internal representations of the target (one corresponding to each eye) and the brain must select between two possible saccadic eye movements that would bring one or the other eye on target. Then the brain probably could implement additional strategies influencing alternating fixation behavior, such as preferring a saccade of the smallest size or of a specific direction.
Metrics of Alternating Saccades
It is perhaps not a big surprise that the main-sequence relationships of alternating and nonalternating saccades (monocular or binocular viewing) were similar. Van Leeuwen et al.8 also showed a similar result, although they compared only horizontal self-generated saccadic eye movements. These investigators also showed that saccade metrics and saccade accuracy were different between exotropic and normal subjects. This last result could not be confirmed, because the current study did not test normal control monkeys in this paradigm. When comparing individual fit parameters of the main-sequence relationships across the different saccade types, some idiosyncratic statistical differences were found. It is unlikely that these statistically significant differences have any physiological basis that teaches us something fundamental about alternating fixation behavior. It is possible that the idiosyncratic differences were due to increased variability in the saccade and fixation behavior in the strabismic animals, possible errors attributed to mechanical factors such as differing mechanical loads on the viewing and nonviewing eyes due to eye eccentricity and different pulse–step mismatches in the fixating and nonfixating eyes.28 None of these factors alter the basic implication of this result, which is that alternating saccades and nonalternating saccades are governed by the same brain stem pulse generation circuit. Any potential differences between alternating and nonalternating saccades must be examined upstream from the brain stem pulse generator. Saccade paradigms that specifically target cortical– cortical or cortical– brain stem connections can probably be used in further study of alternating saccade behavior and can narrow down potential neural circuits that generate this behavior.12
Saccade Latency Differences
There was a tendency for monocular viewing saccades (MR and ML) to be of shorter latency than binocular viewing saccades (BR, BL, BRL, and BLR). This result may be an indication that there are two retinal error representations available to the monkey. Previous studies that have examined target selection in normal animals have shown that saccadic latency is greater when multiple targets are presented to the subject compared with when a single target is presented to the subject.35 In the current experiments, during binocular viewing, the strabismic monkey may be presented with two retinal error representations (one from each eye—equivalent to presenting two targets to a cyclopean eye). Even though the retinal error representation from the partially suppressed eye is much weaker, the brain is still faced with making a decision of which eye to fix on the target and generating an appropriately sized saccade. The superior colliculus is implicated in target selection,36 and examining response properties in the superficial layers of the superior colliculus may help determine whether there are representations of visual error corresponding to each eye.
Acknowledgments
The author thanks Sunal Patel, Tushar Jha, and Michelle Swann for help with data collection and analysis.
Supported by National Institutes of Health Grants R0–1 EY015312, Yerkes Base Grant RR00165, and a grant from the Emory University Research Committee.
Footnotes
Disclosure: V.E. Das, None
References
- 1.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005;112(1):104–108. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg AE, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007;114(1):170–174. doi: 10.1016/j.ophtha.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz B. Genetics of isolated and syndromic strabismus: facts and perspectives. Strabismus. 2002;10:147–156. doi: 10.1076/stra.10.2.147.8133. [DOI] [PubMed] [Google Scholar]
- 4.Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005;13(1):33–41. doi: 10.1080/09273970590910298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tychsen L, Scott C. Maldevelopment of convergence eye movements in macaque monkeys with small- and large-angle infantile esotropia. Invest Ophthalmol Vis Sci. 2003;44(8):3358–3368. doi: 10.1167/iovs.02-0698. [DOI] [PubMed] [Google Scholar]
- 6.Economides JR, Adams DL, Jocson CM, Horton JC. Ocular motor behavior in macaques with surgical exotropia. J Neurophysiol. 2007;98(6):3411–3422. doi: 10.1152/jn.00839.2007. [DOI] [PubMed] [Google Scholar]
- 7.Horton JC, Hocking DR, Adams DL. Metabolic mapping of suppression scotomas in striate cortex of macaques with experimental strabismus. J Neurosci. 1999;19(16):7111–7129. doi: 10.1523/JNEUROSCI.19-16-07111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Leeuwen AF, Collewijn H, de Faber JT, van der Steen J. Saccadic binocular coordination in alternating exotropia. Vision Res. 2001;41(25–26):3425–3445. doi: 10.1016/s0042-6989(01)00044-x. [DOI] [PubMed] [Google Scholar]
- 9.Joosse MV, Esme DL, Schimsheimer RJ, et al. Visual evoked potentials during suppression in exotropic and esotropic strabismics: strabismic suppression objectified. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):142–150. doi: 10.1007/s00417-004-0994-8. [DOI] [PubMed] [Google Scholar]
- 10.Joosse MV, Simonsz HJ, van Minderhout EM, Mulder PG, de Jong PT. Quantitative visual fields under binocular viewing conditions in primary and consecutive divergent strabismus. Graefes Arch Clin Exp Ophthalmol. 1999;237(7):535–545. doi: 10.1007/s004170050276. [DOI] [PubMed] [Google Scholar]
- 11.Sireteanu R. Binocular vision in strabismic humans with alternating fixation. Vision Res. 1982;22(8):889–896. doi: 10.1016/0042-6989(82)90025-6. [DOI] [PubMed] [Google Scholar]
- 12.Patel SS, Jha RT, Swann MH, Das VE. Saccade alternation in non-human primates with strabismus. SFN Abstracts. 2006;736.10 [Google Scholar]
- 13.Das VE. Advances in Understanding Mechanisms and Treatment of Infantile Forms of Nystagmus. Oxford University Press; Case Western Reserve University, Cleveland, OH: 2007. Alternating saccades in a primate model of strabismus. [Google Scholar]
- 14.Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann N Y Acad Sci. 2002;956:346–360. doi: 10.1111/j.1749-6632.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 15.Harwerth RS, Smith EL, 3rd, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res. 1990;41(3):179–198. doi: 10.1016/0166-4328(90)90107-p. [DOI] [PubMed] [Google Scholar]
- 16.O'Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997;37(19):2675–2684. doi: 10.1016/s0042-6989(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 17.Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Ann Rev Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- 18.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 20.Hess BJ, Van Opstal AJ, Straumann D, Hepp K. Calibration of three-dimensional eye position using search coil signals in the rhesus monkey. Vision Res. 1992;32(9):1647–1654. doi: 10.1016/0042-6989(92)90157-e. [DOI] [PubMed] [Google Scholar]
- 21.Das VE, Ono S, Tusa RJ, Mustari MJ. Conjugate adaptation of saccadic gain in non-human primates with strabismus. J Neurophysiol. 2004;91(2):1078–1084. doi: 10.1152/jn.00205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker W. The neurobiology of saccadic eye movements. Metr Rev Oculomotor Res. 1989;3:13–67. [PubMed] [Google Scholar]
- 23.Leigh RJ, Zee DS. The Neurology of Eye Movements. Contemporary Neurology Series. 4th ed. Oxford University Press; New York: 2006. [Google Scholar]
- 24.Carpenter RH. Contrast, probability, and saccadic latency: evidence for independence of detection and decision. Curr Biol. 2004;14(17):1576–1580. doi: 10.1016/j.cub.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 25.Reddi BA, Asrress KN, Carpenter RH. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol. 2003;90(5):3538–3546. doi: 10.1152/jn.00689.2002. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377(6544):59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- 27.Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with a-pattern strabismus. Invest Ophthalmol Vis Sci. 2007;48(2):665–674. doi: 10.1167/iovs.06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu L, Tusa RJ, Mustari MJ, Das VE. Horizontal saccade disconjugacy in strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48(7):3107–3114. doi: 10.1167/iovs.06-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tychsen L, Burkhalter A. Nasotemporal asymmetries in V1: ocular dominance columns of infant, adult, and strabismic macaque monkeys. J Comp Neurol. 1997;388(1):32–46. doi: 10.1002/(sici)1096-9861(19971110)388:1<32::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Norcia AM, Hale J, Pettet M, McKee SP, Harrad R. Disparity tuning of binocular facilitation and suppression after normal versus abnormal visual development. Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1168–75. doi: 10.1167/iovs.08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wensveen JM, Harwerth RS, Smith EL., 3rd Clinical suppression in monkeys reared with abnormal binocular visual experience. Vision Res. 2001;41(12):1593–1608. doi: 10.1016/s0042-6989(00)00319-9. [DOI] [PubMed] [Google Scholar]
- 32.Wong AM, Burkhalter A, Tychsen L. Suppression of metabolic activity caused by infantile strabismus and strabismic amblyopia in striate visual cortex of macaque monkeys. J AAPOS. 2005;9(1):37–47. doi: 10.1016/j.jaapos.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.von Noorden GK, Campos EC. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 6th ed Mosby; St. Louis: 2002. [Google Scholar]
- 34.Erkelens CJ, Muijs AJ, van Ee R. Binocular alignment in different depth planes. Vision Res. 1996;36(14):2141–2147. doi: 10.1016/0042-6989(95)00268-5. [DOI] [PubMed] [Google Scholar]
- 35.McPeek RM, Keller EL. Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vision Res. 2001;41(6):785–800. doi: 10.1016/s0042-6989(00)00287-x. [DOI] [PubMed] [Google Scholar]
- 36.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7(7):757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]