Abstract
The dorsal (DR) and median (MR) raphe nuclei contain 5-hydroxytryptamine (5-HT) cell bodies that give rise to the majority of the ascending 5-HT projections to the forebrain. The DR and MR have differential roles in mediating stress, anxiety and depression. Glutamate and GABA activity sculpt putative 5-HT neuronal firing and 5-HT release in a seemingly differential manner in the MR and DR, yet isolated glutamate and GABA activity within the DR and MR has not been systematically characterized. Visualized whole-cell voltage-clamp techniques were used to record excitatory and inhibitory postsynaptic currents (EPSC and IPSC) in 5-HT-containing neurons. There was a regional variation in action potential-dependent (spontaneous) and basal [miniature (m)] glutamate and GABAergic activity. mEPSC activity was greater than mIPSC activity in the DR, whereas in the MR the mIPSC activity was greater. These differences in EPSC and IPSC frequency indicate that glutamatergic and GABAergic input have distinct cytoarchitectures in the DR and MR. 5-HT1B receptor activation decreased mEPSC frequency in the DR and the MR, but selectively inhibited mIPSC activity only in the MR. This finding, in concert with its previously described function as an autoreceptor, suggests that 5-HT1B receptors influence the ascending 5-HT system through multiple mechanisms. The disparity in organization and integration of glutamatergic and GABAergic input to DR and MR neurons and their regulation by 5-HT1B receptors may contribute to the distinction in MR and DR regulation of forebrain regions and their differential function in the aetiology and pharmacological treatment of psychiatric disease states.
Keywords: 5-HT1B receptor, AMPA/kainate receptor, EPSC, GABAA receptor, IPSC, serotonin
Introduction
The dorsal (DR) and median (MR) raphe nuclei are the principal sites of the 5-hydroxytryptamine (5-HT)-containing cell bodies that provide 5-HT innervation to the forebrain including limbic areas responsible for controlling emotional behaviour (Azmitia & Segal, 1978; Kohler & Steinbusch, 1982; Molliver et al., 1990). The raphe and the 5-HT neurotransmitter system have long been implicated in the neurocircuitry mediating stress and in the aetiology of psychiatric disorders such as anxiety and depression. Many investigators have proposed that the DR and MR have differential roles in terms of mediating stress, anxiety and depression (Andrews et al., 1994; File et al., 1996; Graeff et al., 1996; Adell et al., 1997; Andrews et al., 1997; Gonzalez & File, 1997; Gonzalez et al., 1998; Andrade et al., 1999; Andrade & Graeff, 2001; Lowry, 2002). Previously, regional deviations between the MR and DR and within DR subfields have been identified in terms of their cellular characteristics (Kirby et al., 2003; Beck et al., 2004; Marinelli et al., 2004), efficacy of 5-HT1A receptor activation (Blier et al., 1990; Kirby et al., 2003; Beck et al., 2004) and forebrain projections (Azmitia & Segal, 1978; Vertes, 1991; Vertes et al., 1999). At several different levels within the raphe neural circuitry these already-defined regional deviations, and others yet to be identified, could in turn be the cellular mechanisms that underlie the different roles of the DR and MR in stress regulation and in the aetiology and efficacy of various therapeutics used to treat affective psychiatric disorders. Therefore, it is important to continue to tease out the distinctions in the neural circuitry of the DR and the MR.
Raphe neural activity fluctuates throughout the day as demonstrated by microdialysis and extracellular single-unit recording. The primary excitatory and inhibitory neurotransmitter systems, glutamate and GABA, respectively, have been shown to influence raphe activity, and this regulation is also dependent upon the time of day (Nitz & Siegel, 1997; Portas et al., 1998; Gervasoni et al., 2000; Sakai & Crochet, 2001; Adell et al., 2002). It is difficult to determine the basal tone of glutamate and GABA in the raphe in vivo due to these circadian variations in activity and the interaction of the two afferent neurotransmitter systems. Electrophysiology studies conducted in vitro have demonstrated that there are AMPA/kainate receptor-mediated glutamatergic evoked postsynaptic potentials (Pan & Williams, 1989) and excitatory postsynaptic currents (EPSCs) in the DR (Haj-Dahmane, 2001; Liu et al., 2002; Haj-Dahmane & Shen, 2005). Likewise, evoked GABAA receptor-mediated postsynaptic potentials (Pan et al., 1989; Pan & Williams, 1989) and inhibitory postsynaptic currents (IPSC) have also been recorded in the DR (Jolas & Aghajanian, 1997). While glutamatergic and GABAergic activity has been recorded in the DR no studies have focused on a description of the glutamatergic and GABAergic basal activity and its relative distribution in the DR and MR. One of the goals of this study was to describe the relative influence of glutamate and GABA within the DR and the MR in the brain slice preparation.
The 5-HT1B receptor in the raphe has also gained a lot of interest because of its role as an autoreceptor. 5-HT1B mRNA and binding sites are located throughout the raphe (Doucet et al., 1995; Neumaier et al., 1996b; Bonaventure et al., 1998; Sari, 2004; Boschert et al., 1994) and appear to be located on axon terminals. 5-HT1B receptor activation in the raphe increases the firing rate of both DR and MR neurons (Evrard et al., 1999; Adell et al., 2001), decreases evoked 5-HT-mediated IPSPs (Morikawa et al., 2000) and decreases the release of 5-HT in the raphe (Davidson & Stamford, 1995a; Davidson & Stamford, 1995b; Hertel et al., 2001). Unlike the 5-HT1A receptor, the 5-HT1B receptor appears to be more efficacious in the MR than the DR (Roberts et al., 1998). Clearly the 5-HT1B receptor is influential as an autoreceptor. However, the 5-HT1B receptor may also act as a heteroreceptor in the raphe (Adell et al., 2001); 5-HT1B receptor agonists inhibit GABA release in raphe slices (Bagdy et al., 2000) and inhibit glutamate synaptic activity in caudal raphe (Li & Bayliss, 1998). The effects of 5-HT1B activation on different elements of raphe activity may be explained in part by intricate 5-HT1B receptor modulation of 5-HT, GABA and glutamate activity. As the effect of 5-HT1B hetereoreceptor activation has not been defined at the cellular level, the second goal of the work described in this study was to characterize 5-HT1B receptor modulation of glutamate and GABA activity in the DR and MR.
Materials and methods
Slice preparation
Male Sprague–Dawley rats (100–150 g) from Taconic Farms were used (Taconic, Germantown, NY, USA). All animals were used in accordance with US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and approved by the institutional IACUC committee.
The slice preparation was similar to that described previously (Beck et al., 2004). Rats were decapitated and the head rapidly placed in preoxygenated ice-cold artificial cerebrospinal fluid (ACSF) in which sucrose (248 mm) was substituted for NaCl. The brain was rapidly removed and trimmed to isolate the mesencephalon. The blocked brain was affixed to a stage of a Leica microslicer (Leica, Allendale, NJ, USA) with cyanoacrylate glue and submerged with preoxygenated ice-cold sucrose–ACSF. Coronal slices, 200 µm thick, containing the DR and MR, were cut and placed in a holding vial containing ACSF at 37 °C bubbled with 95% O2 and 5% CO2 for 1 h. After 1 h, the slices were kept in room-temperature ACSF bubbled with 95% O2 and 5% CO2. The composition of the ACSF was (in mm), NaCl, 124; KCl, 2.5; NaH2PO4, 1.25; MgSO4, 2.0; CaCl2, 2.5; dextrose, 10; and NaHCO3, 26.
Electrophysiological recordings
A single slice was placed in a recording chamber (Warner Instruments, Hamden, CT, USA) and continuously superfused with oxygenated ACSF at 1.5–2 mL/min at 32 °C maintained by an in-line solution heater (TC-324; Warner Instruments). Neurons were visualized using a Nikon E600 (Optical Apparatus, Ardmore, PA, USA) or Zeiss Axioskop (Zeiss, Thornwood, NY, USA) upright microscope fitted with a 40× water-immersion objective, differential interference contrast and infrared filter. The image from the microscope was enhanced using a CCD camera and displayed on a monitor. Recording pipettes were fashioned on a P-97 micropipette puller (Sutter Instruments, Novato, CA, USA) using borosilicate glass capillary tubing (1.2 mm OD, 0.69 mm ID; Warner Instruments). For recording EPSCs, the resistance of the electrodes was 5–10 MΩ when filled with an intracellular solution of (in mm) K-gluconate, 130; NaCl, 5; Na phosphocreatine, 10; MgCl2, 1; EGTA, 0.02; HEPES, 10; MgATP, 2; and Na2GTP, 0.5; with biocytin, 0.1%; pH 7.3. For measuring IPSCs, the resistance of the electrodes was 4–8 MΩ when filled with an intracellular solution of (in mm) K-gluconate, 70; KCl, 70; NaCl, 2; Na phosphocreatine, 10; EGTA, 4; HEPES, 10; MgATP, 2; and Na2GTP, 0.3; with biocytin, 0.1%; pH 7.3.
The DR recordings were confined to the ventromedial DR subdivision, which contains the densest cluster of 5-HT neurons. A visualized cell was approached with the electrode, a gigaohm seal established and the cell membrane ruptured to obtain a whole-cell recording using an Axoclamp 200B, Multiclamp 700B amplifier (Axon Instruments, Foster City, CA, USA) or a HEKA EPC 10 amplifier (HEKA Electronik, Germany). Once the whole-cell recording was obtained, the postsynaptic currents (PSCs) were recorded at a holding potential of −70 mV. Signals were filtered at 1 kHz, digitized at 10 Hz and stored on-line.
The access resistance and the input resistance were continuously monitored throughout the recording period. Recordings were abandoned if the access resistance was more than four times the initial electrode resistance or changed by > 15%. After recording spontaneous EPSCs in the DR or MR for at least 6 min, 1 µm tetrodotoxin (TTX) was added into the ACSF and the resulting miniature EPSCs (mEPSCs) were recorded for two 3-min periods. Inhibitory spontaneous (action potential-dependent) PSCs (sIPSCs) were also initially recorded for 6 min; this was followed by the addition of TTX (1 µm) and CNQX or DNQX (20 µm). Experiments were conducted to determine whether there was a difference between sIPSC and mIPSC activity in the DR and MR. Once we determined whether there was a significant difference between sIPSC and mIPSC activity in the MR and DR, sIPSC activity was not recorded in an effort to shorten the protocol to obtain data regarding the 5-HT1B regulation of mIPSC activity.
To examine the effect of 5-HT receptor activation, the 5-HT1, 7 receptor agonist 5-carboxyamodotryptamine maleate (5-CT) or the selective 5-HT1B receptor agonist CP 93,129 were tested on mPSCs in the DR and MR neurons. A concentration of 100 nm 5-CT or 1–10 µm CP 93,129 was applied for 4–6 min after recording the mPSC activity. The selective 5-HT1A receptor antagonist WAY 100,635 (100 nm) and the selective 5-HT1B receptor antagonist SB 216,641 (200 nm) were tested by adding them to the ACSF for 8–10 min followed by superfusion with 100 nm 5-CT plus the antagonist.
Drugs were added to the ACSF in known concentrations. A stock solution was made and diluted on the day of the experiment to obtain the desired concentration in the ACSF. All chemicals for making the ACSF, electrolyte solution, bicuculline methiodide, TTX and 5-CT were purchased from Sigma-Aldrich (St Louis, MO, USA); CP 93,129, SB 216,641, DNQX and CNQX were obtained from Tocris (Ellisville, MO). WAY 100,635 was generously donated by Wyeth-Ayerst (Princeton, NJ, USA) and purchased from Sigma-Aldrich.
Data analysis
PSCs were analysed using the Minianalysis program by Synaptosoft (Decatur, GA, USA). The parameters for synaptic event analysis were optimized for each cell. The threshold for PSC detection was normally set ~ 5 pA. Data were visually analysed and double peaks were highlighted for appropriate analysis by the Minianalysis program. The Minianalysis program provided a summary table containing values for mean and median frequency, amplitude and rise time (10–90%). Frequency, amplitude and rise time values were plotted in cumulative probability plots and/or histograms for each cell. Cumulative probability plots were analysed with the Kolmogorov–Smirnov (K-S) test. The decay time value reported is the tau obtained from an exponential fit of the 10–90% decay of the averaged PSC from 200 randomly chosen events.
Paired and unpaired Student’s t-test or anova were used to test for statistical significance. The follow-up t-test used if the anova was significant was the Student–Neuman–Keuls. A probability of P < 0.05 was considered significant. All data are reported as mean ± SEM.
Tryptophan hydroxylase (TPH) immunohistochemistry staining and intracellular labelling
To identify whether the recorded cells were 5-HT-containing cells we used an antibody for the 5-HT-synthesizing enzyme TPH. The neurons that stained positive for TPH are referred to as 5-HT-containing. Standard immunofluorescence procedures were used to visualize the recorded cell and 5-HT content. After recording, slices were fixed by submersion in 4% paraformaldehyde prepared in 0.1 m phosphate buffer (pH 7.4). Sections were incubated with mouse anti-TPH (1 : 500, Sigma) for 24 h at room temperature. Subsequently, immunohistochemical labelling was visualized using a AlexaFluor 488 (1 : 200; Invitrogen, Carlsbad, CA, USA)-conjugated donkey antimouse secondary antibody for 60 min at room temperature. The biocytin was visualized using streptavidin-conjugated AlexaFluor 633 (1 : 100; Invitrogen) for 90 min at room temperature. Between incubations slices were rinsed with phosphate-buffered saline solutions (3 × 10 min) and all incubations were carried out with mild agitation on a shaker. The sections were mounted on superfrost slides and coverslipped with Prolong Gold antifade reagent kit (Invitrogen). Immunofluorescence label was visualized using a Leica DMR fluorescence microscope and a Leica confocal DMIRE2 microscope (Leica, Allendale, NJ, USA). Images were captured using a digital camera and Openlab 3.09 software (Improvision, Inc., Lexington, MA, USA) under the fluorescence microscope, and digital camera and Leica Confocal software (Version 2.5, Leica) under the confocal microscope. When using the confocal microscope sequential collection of images 0.6 µm in thickness were acquired at the level of the cell body of the biocytin-labelled neuron. Images were adjusted to optimal colour balance and contrast using Adobe Photoshop 6.0 software (Adobe, San Jose, CA, USA).
Results
The data from DR and MR neurons that were neurochemically identified as 5-HT-containing through immunohistochemical identification of TPH content were used for data analysis. Figure 1 contains photomicrographs demonstrating the immunohistochemical characterization of 5-HT-containing neurons in the DR (A) and MR (B). The largest panel shows the merged 20× images from the biocytin-filled TPH-stained neuron. The right top panel shows the biocytin-filled neuron that was recorded, the middle right panel is the TPH staining and the bottom right panel shows the merged panels at a higher magnification.
FIG. 1.
Immunohistochemical identification of recorded neurons in DR and MR. Fluorescent photomicrographs were taken on a confocal microscope at a thickness of 0.6 µm. (A) 5-HT-containing neuron in DR. (B) 5-HT-containing neuron in MR. The large centre panel shows a low-power merged image of the biocytin-stained neuron and TPH immunohistochemistry. The boxed area is shown at a higher magnification in the panels on the right. The top panel contains the biocytin-filled neuron, the middle panel contains the photomicrograph of 5-HT immunohistochemistry in the same focal plane as the recorded neuron and the bottom right panel shows the merged panels.
The spontaneous (action potential-dependent) EPSC (sEPSC), but not miniature EPSC (mEPSC), activity was greater in DR 5-HT-containing neurons than in MR neurons (Fig. 2). Examples of raw traces are provided in Fig. 2, A1 and A2, from 5-HT-containing neurons in the DR and MR. The left hand traces in each panel are sEPSCs. The mEPSCs are traces recorded with the addition of 1 µm TTX; therefore the mEPSCs are not action potential-dependent. Below the traces are the cumulative probability plots (Fig. 2, B1 and B2). There was a significant shift in the cumulative probability plot for the cell recorded in the DR (K-S test, P < 0.0001), but not for the cell in the MR. In panel D is a summary graph of the frequency of the sEPSC and mEPSCs recorded in the DR and MR. There was a significant decrease in frequency with the addition of TTX in DR 5-HT neurons (repeated-measures t-test, P < 0.00007, n = 40). There was no change in frequency with the addition of TTX in MR 5-HT-containing neurons (n = 23). The frequency of the sEPSCs, but not the mEPSCs, recorded in the MR 5-HT neurons was significantly lower than that in the DR (t = 2.03, P < 0.05). In all cases (n = 16) where CNQX (20 µm) was tested it abolished the mEPSCs, confirming that the traces were glutamatergic AMPA/kainate receptor-mediated mEPSCs (data not shown).
FIG. 2.
sEPSC and mEPSC activity in DR and MR neurons. EPSCs recorded from 5-HT-containing neuron of (A) the DR and (B) the MR. (A1 and A2) Each panel shows tracings from control sEPSCs and following the addition of 1 µm TTX to obtain action potential-independent mEPSCs. Holding potential of −70 mV. The scale bars apply to all raw data traces. (B1 and B2) Below the tracings are cumulative probability plots depicting the interevent interval for the sEPSC and mEPSC traces recorded during a 1-min time period. Note the shift in the probability plot by the addition of TTX for the DR 5-HT (K-S test, P < 0.0001), but no shift in the plot for the 5-HT-containing neuron of the MR. (C1 and C2) Amplitude histograms of mEPSCs from DR and MR for a 1-min trace. (D) Summary bar graph of the frequency of sEPSCs and mEPSCs recorded from 5-HT-containing neurons in the DR (n = 40) and MR (n = 23). There was a significant decrease in the frequency of the sEPSC as compared to the mEPSC in the DR 5-HT (repeated measures t-test, *P < 0.00007), but not in the MR 5-HT neurons. Also, the frequency of the sEPSC from the MR 5-HT-containing neurons was significantly lower than those recorded in the DR (t-test, #P < 0.05). (E) Summary bar graph of the mean amplitude of the mEPSCs recorded from DR and MR 5-HT-containing neurons. Values are mean ± SEM.
The frequency of the mEPSCs and the amplitude, rise time and decay time of the EPSCs were compared between the DR and MR 5-HT neurons and are summarized in Table 1. The amplitude histograms for the 5-HT-containing neurons in the DR and MR were very similar, and examples are shown in Fig. 2, C1 and C2. Also, the mean amplitude of the sEPSC and mEPSCs were not different in the 5-HT-containing neurons of the DR and MR, as seen in the Table 1 and the raw data traces of Fig. 2, A1 and A2, and summarized in Fig. 2E. The rise time was not different between the DR and MR 5-HT neurons. The decay time of the mEPSCs was significantly shorter in the cells recorded from MR 5-HT-containing neurons (t = 3.6, P < 0.00065).
TABLE 1.
Characteristics of mEPSCs recorded from 5-HT-containing neurons of the MR and DR
| n | Frequency (Hz) |
Mean amplitude (pA) |
Rise time (ms) |
Decay time (ms) |
|
|---|---|---|---|---|---|
| 5-HT DR | 40 | 7.0 ± 0.9 | 21.1 ± 0.9 | 1.3 ± 0.04 | 2.9 ± 0.18 |
| 5-HT MR | 23 | 5.2 ± 0.9 | 20.1 ±1.7 | 1.2 ± 0.06 | 2.0 ± 0.16* |
P < 0.0006 vs. DR. DR, dorsal raphe; MR, median raphe.
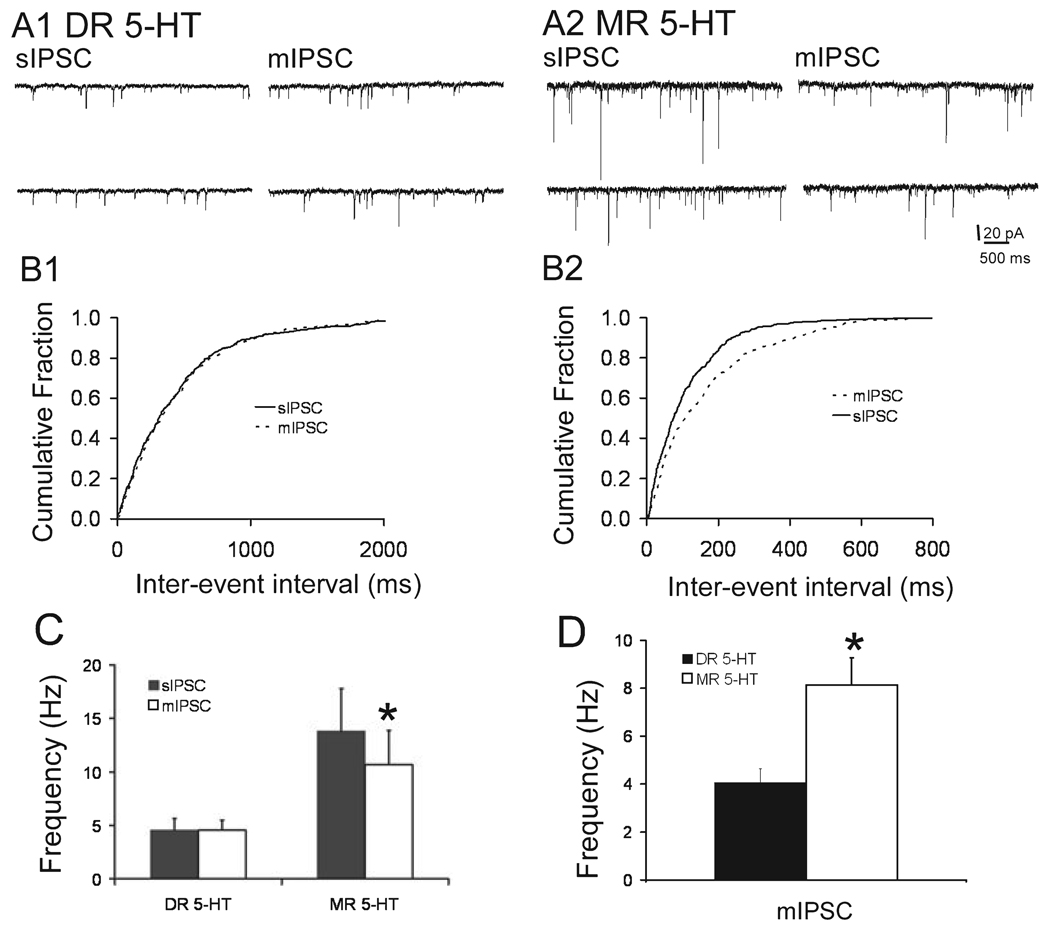
In direct contrast to what was found with the glutamatergic EPSC synaptic activity, action potential dependent GABAergic synaptic activity was found only in the MR and the frequency of the GABAergic mIPSC activity was greater in the MR than the DR. The sIPSC frequency was significantly decreased by the addition of TTX only in the MR. Raw traces showing the sIPSC and mIPSC activity recorded in the MR and DR are shown in the top panels of Fig. 3, A1 and A2. Below the raw data traces are the cumulative frequency graphs (Fig. 3, B1 and B2); the shift in frequency with addition of TTX was significant in the MR (K-S P < 0.00001), whereas there was no shift in the probability plot for the cell recorded from the DR. The bar graph in panel C summarizes the data from 11 cells recorded from the DR and seven cells from the MR (paired t = 3.132, P = 0.028 for MR). In addition, the frequency of the mIPSCs was different between the MR and DR (P = 0.03); to further confirm this finding of differences in the mIPSC frequency, we pooled all the data of the mIPSCs recorded from DR and MR neurons in this study and found the difference to be significant (Fig. 3D; t = 2.95, P < 0.0045, n = 27 for DR and 36 for MR).
FIG. 3.
Frequency of GABAergic activity was greater in MR than DR neurons. (A1 and A2) Raw data traces of the sIPSC and mIPSC activity in a DR and MR 5-HT-containing neuron. Holding potential of −70 mV. Scale bars apply to all traces. (B1 and B2) Cumulative probability graphs, showing the significant shift by the addition of TTX in only the MR neurons (K-S, P < 0.00001). (C and D) Bar graphs summarizing all of the data for all of the recorded neurons. (C) TTX sensitivity of the IPSC activity in the MR 5-HT-containing neurons (paired t = 3.123, *P = 0.0028, n = 7). There was no change in the frequency of the IPSC activity by TTX in the DR (n = 11). (D) Bar graph summarizing the difference in frequency of the mIPSCs in the DR and MR (t = 2.95, *P < 0.0045; n = 27 for DR and 36 for MR).
The amplitude of the mIPSCs was significantly greater in the MR than DR 5-HT-containing neurons (Fig. 4, A1 and A2). For each recorded neuron a frequency histogram of the mIPSC amplitudes was constructed. Figure 4, B1 and B2 contain representative histograms from a DR and MR 5-HT-containing neuron (respectively). Note the different x and y-axis scales. The summary graphs for the mean amplitude in Fig. 4C and maximum amplitude in Fig. 4D depict the fact that the amplitude of the mIPSCs was greater in the MR than the DR (t = 3.79, P < 0.003 and t = 3.29, P < 0.002, n = 27 DR and 36 MR). A summary histogram in Fig. 4E shows the distribution of the mean amplitudes of the mIPSCs for each of the recorded neurons. The shaded areas outline the distribution of the events for the MR and DR. This bar graph shows that the distribution of the mean amplitude events in the MR was centred more to the right compared to the distribution for the cells recorded from the DR. In the MR, there were some neurons that had very large mean amplitude mIPSCs, i.e. ≥40 pA.
FIG. 4.
Amplitude of mIPSCs was greater in MR than DR 5-HT-containing neurons. (A1 and A2) Raw data traces of mIPSC activity. Holding potential of −70 mV. Scale bars apply to all traces. (B1 and B2) Frequency histograms of the amplitude of each IPSC event recorded during a 1-min trace. Note the scales are different for both the x and y axes. The mean and maximum amplitude of the IPSCs were significantly different as shown in Panel C (t = 3.79, *P < 0.0003; n = 27 DR and 36 MR) and Panel D (t = 3.29, *P < 0.002), respectively. (E) The distribution of the mean IPSC amplitude was shifted to the right for neurons recorded from the MR as compared to the distribution for neurons from the DR.
All of the characteristics of the mIPSCs that were measured are summarized in Table 2. There was no significant difference in the rise time or decay times between the neurons recorded in DR and MR. The mIPSC was totally blocked by bicuculline (n = 10, data not shown).
TABLE 2.
Characteristics of mIPSCs recorded from 5-HT-containing neurons of the MR and DR
| n | Frequency (Hz) |
Mean amplitude (pA) |
Maximum amplitude (pA) |
Rise time (ms) |
Decay time (ms) |
|
|---|---|---|---|---|---|---|
| 5-HT DR | 27 | 4.1 ± 0.6* | 15.9 ± 0.9** | 67.3 ± 8.37*** | 1.4 ± 0.08 | 4.06 ± 0.31 |
| 5-HT MR | 36 | 8.1 ± 1.1 | 23.1 ± 1.5 | 114.32 ± 11.0 | 1.3 ± 0.08 | 4.69 ± 0.32 |
P < 0.0045,
P < 0.003 5,
P < 0.002 vs. 5-HT MR. DR, dorsal raphe; MR, median raphe.
Interestingly the relative contribution of glutamatergic to GABAergic synaptic activity was different between the DR and MR. In the DR, the frequency of the basal mEPSC activity was almost twice as high as the frequency for the GABAergic mIPSC activity (7 Hz compared to 4 Hz, t = 2.58, P = 0.01). The converse was true in the MR where the frequency of the glutamatergic mEPSC activity was half that of the GABAergic mIPSC activity (4 compared to 8 Hz, respectively; t = −1.88, P = 0.03).
Characterization of 5-HT receptor mediating inhibition of mEPSC
The 5-HT1,7 agonist 5-CT inhibited the frequency of mEPSCs (Fig. 5). The raw traces (Fig. 5, A1 and A2) show the mEPSC before and during the addition of 100 nm 5-CT. The cumulative probability graphs are located below the raw data traces. The addition of 5-CT shifted the curves, reducing the frequency and increasing the interevent interval in both the neuron recorded from the DR and from the MR (K-S tests, P < 0.001). There was no effect of 5-CT administration on the other characteristics of the mEPSCs in either the DR (n = 21) or MR (n = 9), i.e. amplitude rise time or decay time (Table 3).
FIG. 5.
5-CT and CP 93,129 decreased the frequency of mEPSC activity in DR and MR. (A1 and A2) Traces of mEPSC activity in a DR and an MR 5-HT-containing neuron prior to and following the administration of 100 nm 5-CT to the superfusion fluid. Traces were obtained 6 min after the start of the superfusion. Holding potential −70 mV. Scale bars apply to all traces. (B1 and B2) Cumulative probability plots for each of the neurons are presented below the raw data tracings. 5-CT shifted the cumulative probability plots to the right (K-S tests, P < 0.001). (C1 and C2) CP 93,129 (1–10 µm) inhibited mEPSC activity in representative traces from a DR and an MR 5-HT-containing neuron. Holding potential −70 mV and scale bars apply to all traces. (D1 and D2) Cumulative probability plots are shifted by CP 93,129 (K-S tests, P < 0.0001). (E) Bar graph summarizing the change in frequency in the DR and MR neurons by 5-CT and CP 93,129. The change in frequency was significant in both the DR (paired t-test, *P < 0.009 and 0.001; n = 21 and 19, respectively) and MR (paired t-test, *P < 0.002 and 0.001; n = 9 and 12, respectively). (F) Magnitude of outward current elicited by 5-CT (paired t-test, t = −4.04, P = 0.0006, DR; t = −3.4, P = 0.009, MR) but not by CP 93,129 in DR and MR 5-HT-containing neurons.
TABLE 3.
5-HT1B agonists did not alter amplitude, rise time or decay time of mEPSCs in DR or MR
| Analysis of mEPSCs |
|||
|---|---|---|---|
| Amplitude (pA) |
Rise time (ms) |
Decay time (ms) |
|
| 5-CT experiment in DR (n = 21) | |||
| mEPSC, control | 22.0 ± 1.1 | 0.89 ± 0.05 | 3.4 ± 0.3 |
| mEPSC with 5-CT | 19.5 ± 1.1 | 0.94 ± 0.05 | 3.1 ± 0.2 |
| CP93,129 experiment in DR (n = 19) | |||
| mEPSC, control | 20.0 ± 1.3 | 0.91 ± 0.06 | 2.4 ± 0.2 |
| mEPSC with CP 93,129 | 18.0 ± 1.1 | 1.04 ± 0.09 | 3.0 ± 0.3 |
| 5-CT experiment in MR (n = 9) | |||
| mEPSC, control | 18.5 ± 1.5 | 0.78 ± 0.03 | 2.5 ± 0.1 |
| mEPSC with 5-CT | 19.2 ± 1.7 | 0.83 ± 0.05 | 2.6 ± 0.2 |
| CP93,129 experiment in MR (n = 12) | |||
| mEPSC, control | 20.0 ± 1.9 | 0.99 ± 0.09 | 2.6 ± 0.2 |
| mEPSC with CP 93,129 | 21.1 ± 2.6 | 0.99 ± 0.08 | 2.7 ± 0.2 |
To identify the nature of the receptor mediating the inhibition in EPSC frequency by 5-CT, selective agonists and antagonists were used. The 5-HT1B agonist CP 93,129 (1–10 µm) was also found to decrease the frequency of the mEPSC in both the MR and DR as can be seen in the raw data traces and cumulative frequency plots shown in Fig. 5C and D (K-S tests, P < 0.001). The data for all of the recorded neurons tested with 5-CT and CP 93,129 are summarized in Fig. 4E. Both drugs produced approximately the same reduction in mEPSC frequency, i.e. 40%. The change in frequency was significant for both 5-CT and CP 93,129 in both the DR (paired t-test, P < 0.009 and < 0.001, n = 21 and 19, respectively) and MR (paired t-test, P < 0.002 and 0.001, n = 9 and 12, respectively).
The agonist 5-CT also produced an increase in outward current due to 5-HT1A receptor activation (Beck et al., 2004) as shown in Fig. 5F (DR, paired t-test, t = −4.04, P = 0.0006; MR, t = −3.4, P = 0.009). The selective 5-HT1B agonist CP 93,129 did not elicit any outward current. As there was a significant change in outward current and membrane conductance with the activation of the 5-HT1A receptor it could be reasoned that this could lead to a change in EPSC amplitude and therefore an apparent decrease in frequency. However, there was no significant change in amplitude of the mEPSC by 5-CT. Also, the selective agonist CP 93,129 produced the same magnitude decrease in frequency with no change in outward current or change in mEPSC amplitude. As summarized in Table 3 there were no significant effects of 5-CT or CP 93,129 on amplitude, rise time or decay time in either the DR or MR (Table 3). Therefore, the decrease in mEPSC frequency cannot be attributed as an artifact to another receptor-mediated response.
The selective 5-HT1A (Fletcher et al., 1996) and 5-HT1B (Price et al., 1997; Schlicker et al., 1997) receptor antagonists WAY 100,635 and SB 216,641, respectively, were tested for their ability to block the actions of 5-CT. The results are depicted in Fig. 6. SB 216,641 (200 nm) blocked the inhibition of mEPSC frequency by 100 nm 5-CT (Fig. 6, A1) whereas WAY 100,635 had no effect on the mEPSC inhibition by 5-CT (Fig. 6, A2). The shift in the cumulative frequency plot was not blocked by WAY 100,635 (Fig. 6, B2; K-S test, P < 0.001), but was blocked by SB 216,641 (Fig. 6, B1). These data are summarized in the bar graph in Fig. 6C for all of the cells and statistical analysis revealed that SB 216,641 blocked the effect of 100 nm 5-CT on mEPSC frequency (n = 7 P = 0.25) whereas 5-CT still significantly decreased the frequency of the mEPSC in the presence of WAY 100,635 (n = 8, paired t = 3.00, P = 0.02). The outward current elicited by 5-CT was still present in the presence of the 5-HT1B receptor antagonist SB 216,641 (22.9 ± 4.2 pA) whereas the 5-HT1A-selective antagonist WAY 100,635 blocked the net outward current (0.33 ± 0.33, n = 6). The difference between the magnitude of the outward current in the presence of the two antagonists was significantly different (t = −4.9, P = 0.0004). These results are in agreement with data we have previously published demonstrating that the 5-HT1A receptor mediates the increase in outward current in DR 5-HT-containing neurons (Beck et al., 2004). Based on these results we conclude that the inhibition of mEPSC frequency is mediated by activation of the 5-HT1B receptor and the increase in outward current by the 5-HT1A receptor.
FIG. 6.
The selective 5-HT1B antagonist SB 216,641 blocked the inhibition of EPSC frequency, and the 5-HT1A antagonist WAY 100,635 blocked the outward current elicited by 5-CT. Raw data traces of mEPSCs from a DR 5-HT-containing neuron in the absence and presence of (A1) 100 nm 5-CT plus 200 nm SB 216,641 or (A2) 100 nm 5-CT and 100 nm WAY 100,635. (B1 and B2) Cumulative probability plots demonstrating that the shift in frequency by 5-CT was still apparent in the presence of WAY 100,635 (K-S P < 0.001), but was blocked by SB 216,641. (C) Summary bar graphs of 5-CT-induced decrease in mEPSC frequency was significant in the presence of WAY 100,635 (paired t = 3.00, *P = 0.02, n = 8). (D) Outward current elicited by 5-CT was blocked by WAY 100,635, but was still apparent in the presence of SB 216,641.
Characterization of 5-HT receptor mediating inhibition of mIPSCs
In contrast with the indiscriminate regulation of mEPSC activity in both the DR and MR, 5-CT administration selectively inhibited the frequency of mIPSC activity only in the MR and not the DR. Figure 7 contains raw data traces and cumulative frequency plots for the response to 5-CT (100 nm) and the selective 5-HT1B agonist CP 93,129 (1–10 µm). Neither 5-CT nor CP 93,129 had any effect on the frequency of the mIPSC recorded in the DR 5-HT-containing neurons (Fig. 7, A1, B1, C1 and D1). In contrast, both 5-CT and CP 93,129 decreased the frequency of the mIPSCs in MR 5-HT-containing neurons (Fig. 7, A2, B2, C2 and D2; K-S test, P < 0.0001 for both cumulative probability plots). The data are summarized in the bar graph in Fig. 7E. The decreases in frequency elicited by 5-CT and CP 93,129 were significant in the MR 5-HT-containing neurons (paired t = 4.105, P = 0.022, n = 25 and t = 2.47, P = 0.03, n = 11, respectively) but not significant in the DR 5-HT-containing neurons (n = 18 and 10). In Fig. 7F, the magnitude of the outward current elicited by 5-CT (DR, paired t-test, t = −4.6, P = 0.0003; MR, paired t-test, t = −4.2, P = 0.0003) but not by CP 93,129 is also depicted. There was no effect of either agonist on the amplitude, rise time or decay time of the mIPSCs in either the DR or MR (Table 4). As the decrease in frequency elicited by 5-CT and CP 93,129 in the absence of any effect on mIPSC amplitude, the decrease in frequency elicited by 5-CT and CP 93,129 cannot be attributed to an artifact elicited through the activation of the 5-HT1A receptor.
FIG. 7.
5-CT and CP 93,129 decrease the frequency of mIPSC activity only in MR 5-HT-containing neurons, not in DR neurons. (A1 and A2) Raw data traces showing mIPSC activity in the absence and presence of 100 nm 5-CT. (B1 and B2) Cumulative probability plots showing a significant shift in the MR 5-HT-containing neuron (K-S test, P < 0.0001), but no shift in the DR 5-HT-containing neuron. (C1 and C2) Raw data traces of mIPSC activity in the absence and presence of 1–10 µm CP 93,129. (D1 and D2) Cumulative probability plots demonstrating a significant shift for the MR 5-HT-containing neurons (K-S test, P < 0.0001) but no shift for the DR 5-HT-containing neuron. (E) Bar graph summarizing the inhibition of mIPSC activity by both 5-CT (paired t = 3.906, *P = 0.006, n = 23) and CP 93,129 (paired t = 2.47, *P = 0.03, n = 11) in the MR but not the DR (n = 18 for 5-CT and n = 10 for CP 93,129) 5-HT-containing neurons. (F) Magnitude of outward current elicited by 5-CT (paired t-test, t = −4.6, P = 0.0003, DR; paired t-test, t = −4.2, P = 0.0003, MR) but not by CP 93,129 in DR and MR 5-HT-containing neurons.
TABLE 4.
5-HT1B agonists did not alter amplitude, rise time or decay time of mIPSCs in DR or MR
| Analysis of mIPSCs |
|||
|---|---|---|---|
| Amplitude (pA) |
Rise time (ms) |
Decay time (ms) |
|
| 5-CT experiment in DR (n = 18) | |||
| mIPSC, control | 14.5 ± 1.0 | 1.24 ± 0.08 | 4.2 ± 0.3 |
| mIPSC with 5-CT | 15.1 ± 1.2 | 1.21 ± 0.08 | 5.0 ± 0.8 |
| CP93,129 experiment in DR (n = 10) | |||
| mIPSC, control | 12.6 ± 1.2 | 1.26 ± 0.11 | 3.9 ± 0.5 |
| mIPSC with CP 93,129 | 14.5 ± 1.9 | 1.22 ± 0.16 | 4.3 ± 0.5 |
| 5-CT experiment in MR (n = 23) | |||
| mIPSC, control | 24.8± 2.0 | 1.13 ± 0.08 | 5.0 ± 0.4 |
| mIPSC with 5-CT | 25.9 ± 2.6 | 1.34 ± 0.13 | 5.4 ± 0.5 |
| CP3,129 experiment in MR (n = 11) | |||
| mIPSC, control | 19.2 ± 1.9 | 1.40 ± 0.14 | 3.9 ± 0.3 |
| mIPSC with CP 93, 129 | 20.1 ± 1.8 | 1.52 ± 0.15 | 4.4 ± 0.4 |
The decrease in frequency elicited by 5-CT was blocked in the MR 5-HT-containing neurons by the selective 5-HT1B antagonist SB 216,641 (100 nm), as seen in Fig. 8. Panels A1 and A2 depict the selective inhibition by 5-CT in the MR (paired t = 3.96, P < 0.006, n = 23), but not the DR (n = 17). Note the reversibility of the 5-CT inhibition of mIPSC activity. When 5-CT was tested in the presence of SB 216,641 in the MR there was no change in the frequency of the mIPSCs (Fig. 8B) or shift in the cumulative probability plot (Fig. 8C). The data for all of the neurons recorded in the MR in the presence of SB 216,641 are summarized in Fig. 8D, where it is apparent that the frequency of the mIPSCs does not change. Based on the data obtained with the agonists and antagonists the conclusion is that activation of the 5-HT1B receptor in the MR, but not the DR, inhibits the frequency of mIPSCS.
FIG. 8.
The decrease in frequency elicited by 5-CT was blocked by SB 216,641 in MR 5-HT-containing neurons. (A1 and A2) Bar graphs summarizing the selective inhibition of mIPSC frequency by 5-CT (100 nm) in MR 5-HT-containing neurons (paired t = 3.96, *P < 0.006, n = 23), but not the DR (n = 17). (B) Raw traces showing that SB 216,641 prevented the inhibition of mIPSC activity by 5-CT (100 nm). (C) In the presence of the 5-HT1B inhibitor SB 216,641, 5-CT did not shift the cumulative probability plot. (D) Summary bar chart demonstrating that SB 216,641 blocked the effect of 5-CT on mIPSC frequency.
The primary effect of 5-HT1B receptor activation was an inhibition of synaptic activity. This effect could be attributed to rundown of the response. To demonstrate the stability of the recordings, the bar graph in Fig. 9A summarizes the frequency of both sEPSC and mEPSC activity when recorded over a 30-min time period, the approximate time period for recording receptor-mediated effects (n = 8). Also, Fig. 9B–E shows raw data traces and cumulative probability plots for mEPSCs recorded from a DR 5-HT neuron and mIPSCs recorded from an MR 5-HT neuron in which 5-CT inhibited the frequency of the synaptic activity, which returned to baseline following the removal of 5-CT from the buffer. The cumulative probability plots in Fig. 9D and E demonstrate the significant shift produced by 5-CT (K-S test, P < 0.0001), which returned to control following the removal of the drug.
FIG. 9.
Stability of synaptic activity and reversibility of 5-HT1B inhibition of synaptic activity. (A) sEPSC or mEPSC activity was measured over a 30-min time period to demonstrate the stability of the synaptic activity and that it did not run down over time (n = 8). The third column is the average of the 3-min and 30-min recordings. (B and C) Raw data traces of mEPSCs from DR 5-HT and mIPSCs from MR 5-HT-containing neurons before, during and after the removal of 5-CT from the superfusion buffer. The frequency of the mPSC activity returned to baseline levels during the washout. (D and E) 5-CT shifted the cumulative probability plots (K-S test, P < 0.0001) but the frequency returned to baseline levels following the removal of the agonist.
Discussion
The influence of glutamate and GABAergic input to DR and MR neurons has been previously assessed using in vivo measurement techniques. The relative importance of the two neurotransmitter systems was identified, but was influenced by both the behaviour of the animal and whether the neurotransmitter effects were measured in isolation. In this study, the glutamatergic and GABAergic synaptic input in the brain slice preparation was measured in isolation and one of the major findings was that the glutamatergic and GABAergic basal synaptic input to the DR and MR were not the same. The DR is characterized by having greater glutamatergic excitatory input whereas the MR has greater GABAergic synaptic input. The 5-HT1B receptor, previously known to inhibit 5-HT release, was also found to differentially inhibit glutamatergic and GABAergic synaptic activity. Activation of the 5-HT1B receptor reduced the frequency of mEPSC activity in both the DR and the MR, but selectively inhibited GABAergic mIPSC activity only in the MR. These findings extend the understanding of the afferent control of the 5-HT-containing neurons in the DR and MR and the selective effect of the 5-HT1B receptor in inhibiting this afferent input.
Methodological issues
These studies primarily employed whole-cell patch-clamp recordings in the raphe brain slice. The strength of this method is in revealing individual raphe neuron properties and the characteristics of receptor-mediated responses at the cellular level. This preparation is subject to certain methodological limitations. Most notably, afferent input into the raphe is severed. The afferent input from the two primary excitatory and inhibitory neurotransmitters, i.e. glutamate and GABA, is known to originate from many brain areas (Ottersen & Storm-Mathisen, 1984; Peyron et al., 1998; Lee et al., 2003; Gervasoni et al., 2000). However, this input is silenced in the slice preparation, and any glutamatergic or GABAergic activity from sources outside of the slice would not be spontaneously active as part of sEPSC or sIPSC activity. Therefore action potential-dependent glutamatergic and GABAergic neurotransmission recorded in the slice is indicative of the activity of endogenous glutamate and GABA neurons within the network retained in the raphe brain slice. For these reasons, the current report provides important information on synaptic activity retained in the slice and also complements our understanding of synaptic drive of the raphe that has been largely formed by studies using extracellular single-unit recording and microdialysis. The EPSC and IPSC activity were recorded in different sets of neurons, isolated using electrolyte solutions with different chloride concentrations. The IPSC electrolyte was used to obtain a positive shift in the chloride equilibrium potential so the amplitude of the IPSCs would be enhanced and appear as inward currents when the cells were clamped at −70 mV. IPSCs were recorded in the presence of antagonists for glutamate.
Glutamatergic and GABAergic synaptic activity
The presence of action potential-dependent activity indicates that some of the glutamatergic and GABAergic neurons retained in the slice preparation are spontaneously active. There was a significant decrease in the frequency of the EPSCs after TTX administration in the DR, but not MR, 5-HT-containing neurons. In contrast, in the MR there was decrease in the frequency of the IPSCs following TTX that was not present in the DR 5-HT-containing neurons. These findings suggest a difference in the architecture of local glutamatergic and GABAergic innervation of MR and DR. Glutamate-containing cell bodies are found within the DR and MR and their distribution is not uniform (Ottersen & Storm-Mathisen, 1984; Forloni et al., 1987; Commons & Valentino, 2002; Roche et al., 2003; Herzog et al., 2004). Recent studies have reported a distinct topographical localization of GABA cell bodies in the raphe. Very few GABA neurons are found in the rostral DR. In the mid to caudal portions of the DR, GABA cells increase in density and are found primarily in the lateral wings. GABA neurons are located in the ventromedial DR B6 region (Stamp & Semba, 1995; Allers & Sharp, 2003; K. A. Allers, personal communication), and are sparsely distributed throughout the MR (Stamp & Semba, 1995). Even though there is a differential distribution of GABA cell bodies, GABA nerve terminals in the DR are found throughout the DR and are more numerous than 5-HT terminals (Harandi et al., 1987). Synaptic contacts are present between GABA terminals and 5-HT neuron cell bodies and dendrites in the DR (Magoul et al., 1986; Harandi et al., 1987; Wang et al., 1992). Our data provide a physiological correlate to these neuroanatomical studies. Future studies will be designed to determine the exact circuitry of the endogenous glutamate and GABA sources to the 5-HT neurons within the slice preparation; for example, whether GABA neurons in the lateral raphe innervate ventromedial DR and/or MR.
EPSC activity in both the MR and DR was through activation of AMPA/kainate receptors in 5-HT-containing neurons, as the mEPSCs were eliminated by the application of the antagonist CNQX. The frequency of the mEPSC activity was similar in the DR and MR and was similar to that previously reported (Liu et al., 2002; Haj-Dahmane & Shen, 2005). There was no difference in the amplitude or rise time of the mEPSCs between DR and MR. A faster decay time in the mEPSCs may indicate some difference in the AMPA receptor subtypes. Overall, the findings indicate that the frequency of glutamate input, EPSC characteristics and the density of the postsynaptic AMPA receptors is not different between the MR and DR 5-HT neurons. This is in agreement with previous microdialysis experiments demonstrating that the magnitude of release of 5-HT in response to AMPA receptor agonist administration was not different between the DR and MR (Tao et al., 1997).
In contrast, there was a difference in the mIPSC frequency and amplitude, but not the rise time or decay time, in the GABAA receptor-mediated mIPSCs recorded in the MR and DR. This amplitude difference could be a further indication of the higher frequency of GABA mIPSCs in the MR vs. the DR. These differences could be attributable to a greater innervation, increased probability of release and/or quantal release. It is difficult to resolve these possibilities in the brain slice preparation. An amplitude difference may also be due to a difference in the number of GABAA receptors on the neurons. However, evidence against this hypothesis comes from microdialysis studies that demonstrated that the magnitude of release of 5-HT in the MR and DR following the direct application of the GABA agonist muscimol was not different in the MR and DR, indicating no difference in potency (Tao et al., 1996).
The benefit of using the brain slice preparation and whole-cell electrophysiology is that fluctuating afferent influences, as well as the interaction of the GABA and glutamate systems, are eliminated by the use of antagonists to isolate the GABAergic and glutamatergic activity. We found that in the DR the synaptic drive was governed largely by glutamatergic activity whereas in the MR GABAergic input predominated. Using microdialysis in vivo in the freely moving animal to measure the release of 5-HT, the predominant afferent influence as determined by antagonist administration in the DR was found to be GABAergic whereas in the MR it was glutamatergic (Forchetti & Meek, 1981; Tao et al., 1996; Tao & Auerbach, 2003). These authors also found that there was an interaction between the glutamate and GABA neurotransmitter systems and that the activity of these afferent inputs was dependent upon the behavioural state of the animal. The difference in our results and those recorded in vivo can be attributed to tonic afferent input from outside the raphe that drives DR and MR neuronal activity. For example, the primarily inhibitory tone in the DR measured by dialysis may be due to afferent input activating GABAergic neurons in the DR. Recently, for instance, there has been a great deal of attention given to the prefrontal cortex afferent input and influence on DR neural activity. The prefrontal cortex innervates both 5-HT and non-5-HT neurons in the raphe (Varga et al., 2001; Celada et al., 2001; Celada et al., 2002; Jankowski & Sesack, 2004); however, the primarily inhibitory effect of prefrontal cortex stimulation was determined to be through the activation of GABA neurons in the raphe (Varga et al., 2001). Our data from the in vitro slice add additional information regarding the balance between excitatory and inhibitory basal activity recorded from 5-HT-containing neurons in the DR and MR. Comparison of data obtained using electrophysiological methods in the brain slice preparation with data obtained in vivo using extracellular recording techniques and microdialysis will help in understanding how this balance is altered by inputs external to the raphe.
5-HT1B inhibition
In the raphe, activation of the 5-HT1B receptor inhibits the release of 5-HT, glutamate and GABA. The 5-HT1B receptors are expressed in both 5-HT and non-5-HT neurons in the raphe (Bruinvels et al., 1994; Doucet et al., 1995; Bonaventure et al., 1998; Riad et al., 2000), where they are thought to function presynaptically to regulate neurotransmitter release. In concert with these anatomical studies, we found that activation of the 5HT1B receptor had selective inhibitory effects on glutamate and GABA synaptic activity in the DR and MR. The characterization of the receptor was conducted using the 5-HT1,7 and selective 5-HT1B agonists 5-CT and CP 93,129 and the selective 5-HT1B and 5-HT1A antagonists SB216,641 and WAY 100,635, respectively. The current finding is consistent with the role of 5HT1B receptors in modulating glutamate and GABA release in other brain regions including the caudal raphe (Johnson et al., 1992; Singer et al., 1996; Li & Bayliss, 1998; Muramatsu et al., 1998; Pickard et al., 1999; Chadha et al., 2000; Yan & Yan, 2001; Golembiowska & Dziubina, 2002; Laurent et al., 2002; Bouryi & Lewis, 2003; Matsuoka et al., 2004; Bramley et al., 2005).
The effect of 5-HT1B receptor activation was not uniform in the DR and MR. Activation of the 5-HT1B receptor resulted in an inhibition of glutamatergic mEPSC activity in both the DR and MR, whereas the inhibitory effect on GABAergic 5-HT1B receptor-mediated mIPSC activity was seen only in the MR. Several studies have reported a differential sensitivity of 5-HT1B receptor inhibition of 5-HT release, with the effect greater in the MR than the DR (Hervas et al., 1998; Adell et al., 2001). This is probably due to a composite effect of 5-HT1B receptor activity on glutamatergic and GABAergic as well as 5-HT axons, and may be influenced by the differing activity states of these synapses. For example, different concentrations of the 5-HT1B receptor agonist did not influence 5-HT release in the DR but did inhibit release at low doses and increase release at high doses in the MR (Adell et al., 2001). This could be explained by the ability of 5-HT1B receptors to inhibit GABA release as well as glutamate and 5-HT in the MR. Numerous studies have demonstrated a change in 5-HT1B receptor number or response following different types of stressors or antidepressant treatment (Bolanos-Jimenez et al., 1995; Neumaier et al., 1996a, 1997; Clark et al., 2002), raising the possibility of plasticity in 5-HT1B receptor regulation of glutamate, GABA and 5-HT neurotransmission in the MR and DR, particularly in response to fluctuating degrees of stress on the animal.
In conclusion, the pacemaker potentials or firing rate of putative 5-HT-containing neurons and extracellular 5-HT levels in the DR change throughout the day and follow alterations in behaviour (Trulson & Jacobs, 1979; Trulson & Jacobs, 1983; Rasmussen et al., 1984; Portas et al., 1998). The MR and DR are known to selectively influence particular behaviours and physiological responses. For example, the MR controls desynchronization of EEG theta rhythm of the hippocampus, and glutamate levels in the MR have been correlated with EEG activity (Kinney et al., 1994; Vertes et al., 1994; Kocsis & Vertes, 1996; Varga et al., 1998; Andrade et al., 1999; Kitchigina et al., 1999; Viana et al., 2002). Selective alterations in either the DR or MR produce discrete effects on different types of anxiety- or stress-related behaviours, modelled by open-field, elevated-plus maze, foot-shock, handling, injection or forced-swim tests (Jacobs & Cohen, 1976; Wirtshafter & McWilliams, 1987; Adell et al., 1997; Andrews et al., 1997; Gonzalez et al., 1998; Andrade et al., 1999; Grahn et al., 2000) as well as on behavioural inhibition (Wirtshafter & McWilliams, 1987; Fletcher, 1993). The current study provides new information regarding differential regulation of DR and MR neural activity by glutamate and GABA. Differences in EPSC and IPSC frequency and action potential-dependent activity point to the distinct architecture of glutamatergic and GABAergic networks in the MR and DR. Moreover, the data suggest that, in addition to its function as an autoreceptor, 5HT1B receptors differentially influence glutamatergic and GABAergic neurotransmission in the MR and DR. The distinction between MR and DR regulation of forebrain regions and their differential function in the aetiology and pharmacological treatment of psychiatric disease states may be explained in part by this disparity in glutamatergic and GABAergic input to DR and MR neurons and 5-HT1B receptor-mediated inhibition.
Acknowledgements
This work was supported by UPHS grants MH60773, MH48125, MH63078 and N00014-03-1-0311 (SGB).
Abbreviations
- 5-CT
5-carboxyamidotryptamine maleate
- 5-HT
5-hydroxytryptamine (or serotonin)
- ACSF
artificial cerebrospinal fluid
- DR
dorsal raphe
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- K-S
Kolmogorov–Smirnov
- mEPSC
miniature EPSC
- mIPSC
miniature IPSC
- MR
median raphe
- PSC
postsynaptic current
- sEPSC
spontaneous EPSC (action potential-dependent)
- sIPSC
spontaneous IPSC (action potential-dependent)
- TPH
tryptophan hydroxylase
- TTX
tetrodotoxin
References
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J. Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Graeff FG. Effect of electrolytic and neurotoxic lesions of the median raphe nucleus on anxiety and stress. Pharmacol. Biochem. Behav. 2001;70:1–14. doi: 10.1016/s0091-3057(01)00512-3. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Silva AM, Silva CL, Graeff FG. Effect of electrolytic lesion of the median raphe nucleus on behavioral and physiological measures of stress. Acta Physiol. Pharmacol. Ther. Latinoam. 1999;49:279–289. [PubMed] [Google Scholar]
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus – dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–234. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur. J. Pharmacol. 1994;264:259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bagdy E, Kiraly I, Harsing LG., Jr Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem. Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Serrano A, Scatton B. Differential responsiveness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and p-chloroamphetamine. Synapse. 1990;5:120–133. doi: 10.1002/syn.890050206. [DOI] [PubMed] [Google Scholar]
- Bolanos-Jimenez F, Manhaes dCR, Seguin L, Cloez-Tayarani I, Monneret V, Drieu K, Fillion G. Effects of stress on the functional properties of pre- and postsynaptic 5-HT1B receptors in the rat brain. Eur. J. Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Langlois X, Leysen JE. Co-localization of 5-HT1B- and 5-HT1D receptor mRNA in serotonergic cell bodies in guinea pig dorsal raphe nucleus: a double labeling in situ hybridization histochemistry study. Neurosci. Lett. 1998;254:113–116. doi: 10.1016/s0304-3940(98)00680-6. [DOI] [PubMed] [Google Scholar]
- Boschert M, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. J. Physiol. (Lond.) 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley JR, Sollars PJ, Pickard GE, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J. Neurophysiol. 2005;93:3157–3164. doi: 10.1152/jn.00770.2004. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafsson JA, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1Dalpha, 5-HT1E, and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA (A), and glutamate receptors. J. Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Martin-Ruiz R, Casanovas JM, Artigas F. Control of the serotonergic system by the medial prefrontal cortex: potential role in the etiology of PTSD and depressive disorders. Neurotox. Res. 2002;4:409–419. doi: 10.1080/10298420290030550. [DOI] [PubMed] [Google Scholar]
- Chadha A, Sur C, Atack J, Duty S. The 5HT (1B) receptor agonist, CP-93129, inhibits [(3) H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br. J. Pharmacol. 2000;130:1927–1932. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J. Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J. Comp. Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br. J. Pharmacol. 1995a;114:1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. The effect of paroxetine on 5-HT efflux in the rat dorsal raphe nucleus is potentiated by both 5-HT1A and 5-HT1B/D receptor antagonists. Neurosci. Lett. 1995b;188:41–44. doi: 10.1016/0304-3940(95)11390-i. [DOI] [PubMed] [Google Scholar]
- Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotoninergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- Evrard A, Laporte AM, Chastanet M, Hen R, Hamon M, Adrien J. 5-HT1A and 5-HT1B receptors control the firing of serotoninergic neurons in the dorsal raphe nucleus of the mouse: studies in 5-HT1B knockout mice. Eur. J. Neurosci. 1999;11:3823–3831. doi: 10.1046/j.1460-9568.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J. Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ. A comparison of the effects of dorsal or median raphe injections of 8- OH-DPAT in three operant tasks measuring response inhibition. Behav. Brain Res. 1993;54:187–197. doi: 10.1016/0166-4328(93)90078-5. [DOI] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middle-fell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav. Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Forchetti CM, Meek JL. Evidence for a tonic GABAergic control of serotonin neurons in the median raphe nucleus. Brain Res. 1981;206:208–212. doi: 10.1016/0006-8993(81)90118-9. [DOI] [PubMed] [Google Scholar]
- Forloni G, Grzanna R, Blakely RD, Coyle JT. Co-localization of N-acetyl-aspartyl-glutamate in central cholinergic, noradrenergic, and serotonergic neurons. Synapse. 1987;1:455–460. doi: 10.1002/syn.890010509. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembiowska K, Dziubina A. Inhibition of amino acid release by 5-HT1B receptor agonist in the rat prefrontal cortex. Pol. J. Pharmacol. 2002;54:625–631. [PubMed] [Google Scholar]
- Gonzalez LE, File SE. A five minute experience in the elevated plus-maze alters the state of the benzodiazepine receptor in the dorsal raphe nucleus. J. Neurosci. 1997;17:1505–1511. doi: 10.1523/JNEUROSCI.17-04-01505.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LE, Ouagazzal AM, File SE. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median raphe reveals differential GABAergic control in two animal tests of anxiety. Eur. J. Neurosci. 1998;10:3673–3680. doi: 10.1046/j.1460-9568.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Watkins LR, Maier SF. Impaired escape performance and enhanced conditioned fear in rats following exposure to an uncontrollable stressor are mediated by glutamate and nitric oxide in the dorsal raphe nucleus. Behav. Brain Res. 2000;112:33–41. doi: 10.1016/s0166-4328(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S. D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur. J. Neurosci. 2001;14:125–134. doi: 10.1046/j.0953-816x.2001.01616.x. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J. Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi M, Aguera M, Gamrani H, Didier M, Maitre M, Calas A, Belin MF. gamma-Aminobutyric acid and 5-hydroxytryptamine interrelationship in the rat nucleus raphe dorsalis. combination of radio-autographic and immunocytochemical techniques at light and electron microscopy levels. Neuroscience. 1987;21:237–251. doi: 10.1016/0306-4522(87)90336-8. [DOI] [PubMed] [Google Scholar]
- Hertel P, Lindblom N, Nomikos GG, Svensson TH. Receptor-mediated regulation of serotonin output in the rat dorsal raphe nucleus: effects of risperidone. Psychopharmacology (Berl.) 2001;153:307–314. doi: 10.1007/s002130000582. [DOI] [PubMed] [Google Scholar]
- Hervas I, Bel N, Fernandez AG, Palacios JM, Artigas F. In vivo control of 5-hydroxytryptamine release by terminal autoreceptors in rat brain areas differentially innervated by the dorsal and median raphe nuclei. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:315–322. doi: 10.1007/pl00005259. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, el Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Cohen A. Differential behavioral effects of lesions of the median or dorsal raphe nuclei in rats: open field and pain-elicited aggression. J. Comp. Physiol. Psychol. 1976;90:102–108. doi: 10.1037/h0077262. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus. ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J. Comp. Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J. Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP. Injections of excitatory amino acid antagonists into the median raphe nucleus produce hippocampal theta rhythm in the urethane-anesthetized rat. Brain Res. 1994;654:96–104. doi: 10.1016/0006-8993(94)91575-x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus. electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchigina VF, Kudina TA, Kutyreva EV, Vinogradova OS. Neuronal activity of the septal pacemaker of theta rhythm under the influence of stimulation and blockade of the median raphe nucleus in the awake rabbit. Neuroscience. 1999;94:453–463. doi: 10.1016/s0306-4522(99)00258-4. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Midbrain raphe cell firing and hippocampal theta rhythm in urethane- anaesthetized rats. Neuroreport. 1996;7:2867–2872. doi: 10.1097/00001756-199611250-00012. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J. Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Li YW, Bayliss DA. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurones in rat. J. Physiol. (Lond.) 1998;510:121–134. doi: 10.1111/j.1469-7793.1998.121bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Ding Y, Aghajanian GK. Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphe nucleus. Neuropharmacology. 2002;27:329–340. doi: 10.1016/S0893-133X(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J. Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Magoul R, Onteniente B, Oblin A, Calas A. Inter- and intracellular relationship of substance P-containing neurons with serotonin and GABA in the dorsal raphe nucleus: combination of autoradiographic and immunocytochemical techniques. J. Histochem. Cytochem. 1986;34:735–742. doi: 10.1177/34.6.2422252. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J. Neurophysiol. 2004;92:3532–3537. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci. Res. 2004;48:229–238. doi: 10.1016/j.neures.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Berger UV, Mamounas LA, Molliver DC, O’Hearn E, Wilson MA. Neurotoxicity of MDMA and related compounds: anatomic studies. Ann. NY Acad. Sci. 1990;600:649–661. doi: 10.1111/j.1749-6632.1990.tb16916.x. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT (1B) auto- and heteroreceptors. Mol. Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur. J. Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Petty F, Kramer GL, Szot P, Hamblin MW. Learned helplessness increases 5-hydroxytryptamine1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol. Psychiatry. 1997;41:668–674. doi: 10.1016/S0006-3223(96)00114-X. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropharmacology. 1996a;15:515–522. doi: 10.1016/S0893-133X(96)00095-4. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Szot P, Peskind ER, Dorsa DM, Hanson RW. Serotonergic lesioning differentially affects presynaptic and postsynaptic 5-HT1B receptor mRNA levels in rat brain. Brain Res. 1996b;722:50–58. doi: 10.1016/0006-8993(96)00178-3. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am. J. Physiol. 1997;273:R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J. Comp. Neurol. 1984;229:374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Colmers WF, Williams JT. 5-HT-mediated synaptic potentials in the dorsal raphe nucleus: interactions with excitatory amino acid and GABA neurotransmission. J. Neurophysiol. 1989;62:481–486. doi: 10.1152/jn.1989.62.2.481. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol. 1989;61:719–726. doi: 10.1152/jn.1989.61.4.719. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J. Neurosci. 1999;19:4034–4045. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Bjorvatn B, Fagerland S, Gronli J, Mundal V, Sorensen E, Ursin R. On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience. 1998;83:807–814. doi: 10.1016/s0306-4522(97)00438-7. [DOI] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Gothert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. SB-216641 and BRL-15572 - compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Heym J, Jacobs BL. Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Exp. Neurol. 1984;83:302–317. doi: 10.1016/S0014-4886(84)90100-6. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Roberts C, Belenguer A, Middlemiss DN, Routledge C. Differential effects of 5-HT1B/1D receptor antagonists in dorsal and median raphe innervated brain regions. Eur. J. Pharmacol. 1998;346:175–180. doi: 10.1016/s0014-2999(98)00061-2. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J. Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Fink K, Molderings GJ, Price GW, Duckworth M, Gaster L, Middlemiss DN, Zentner J, Likungu J, Gothert M. Effects of selective h5-HT1B (SB-216641) and h5-HT1D (BRL-15572) receptor ligands on guinea-pig and human 5-HT auto- and heteroreceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:321–327. doi: 10.1007/pl00005057. [DOI] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J. Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br. J. Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Influence of AMPA/kainate receptors on extracellular 5-hydroxytryptamine in rat midbrain raphe and forebrain. Br. J. Pharmacol. 1997;121:1707–1715. doi: 10.1038/sj.bjp.0701292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: lack of diurnal variation. Neurosci. Lett. 1983;36:285–290. doi: 10.1016/0304-3940(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Varga V, Kekesi A, Juhasz G, Kocsis B. Reduction of the extracellular level of glutamate in the median raphe nucleus associated with hippocampal theta activity in the anaesthetized rat. Neuroscience. 1998;84:49–57. doi: 10.1016/s0306-4522(97)00489-2. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Kinney GG, Kocsis B, Fortin WJ. Pharmacological suppression of the median raphe nucleus with serotonin1A agonists, 8-OH-DPAT and buspirone, produces hippocampal theta rhythm in the rat. Neuroscience. 1994;60:441–451. doi: 10.1016/0306-4522(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Viana DP, Albo Z, Vertes RP, Kocsis B. Discharge properties of neurons of the median raphe nucleus during hippocampal theta rhythm in the rat. Exp. Brain Res. 2002;145:383–394. doi: 10.1007/s00221-002-1123-8. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res. Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, McWilliams C. Suppression of locomotor activity produced by acute injections of kainic acid into the median raphe nucleus. Brain Res. 1987;408:349–352. doi: 10.1016/0006-8993(87)90403-3. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [(3) H]GABA release from rat ventral tegmental area slices. J. Neurochem. 2001;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]