Abstract
Background
Even though there are multiple studies documenting the outcome of the Charnley low-friction arthroplasty as well as abundant studies on uncemented arthroplasties, there is a dearth of comparative studies of the uncemented acetabular component and a cemented component. In this study we aimed to document the long-term clinical and radiographic outcome as well as component survival in a randomized controlled trial.
Materials and methods
Two hundred fifteen patients (240 hips) were randomly allocated to receive a cemented Charnley cup or uncemented Duraloc 1200 cup. All patients received cemented Charnley stems and were evaluated clinically and radiographically after 6 months, and 2, 5, and 10 years.
Results
Harris Hip Scores improved from 48.3 [95% confidence interval (CI) 45.0–51.6] to 90.2 [95% CI 87.9–92.6] in the Charnley group and from 49.3 [95% CI 86.9–91.3] in the Duraloc group at 6 months. After 10 years, the Charnley group’s Harris Hip Score was 89.8 [95% confidence interval (CI) 87.0–92.6], and the Duraloc group’s score was 87.3 (95% CI 84.1–90.6). In the radiographic analysis after 10 years, there was no statistical difference in the prevalence of radiographic signs of loosening. Nine cups were revised in the Charnley group, and five cups were removed in the Duraloc group. The difference was not statistically significant. There was no statistical difference between the cups when aseptic loosening was the end-point, nor in survival analyses.
Conclusions
There is no statistically significant difference in clinical or radiological outcome between the Charnley cup and the Duraloc after 10 years, and no difference in implant survival after 12–14 years. The uncemented Duraloc cup is as good as the cemented Charnley cup after 10 years.
Keywords: Hip, Acetabulum, Cemented, Uncemented, Randomized
Introduction
Hip arthroplasty is a highly successful procedure for alleviating pain and improving overall hip function in arthritis and other destructive hip joint conditions [1]. However, the method of fixation for hip replacement components has remained a matter of controversy [2, 3].
The cemented all-polyethylene acetabular component has been regarded as a gold standard, and multiple reports confirm survival of 85–92% after 16–25 years [4–6] and revision rates of 2–17% after 17–30 years [7–10] when aseptic loosening is the end-point. However, results are worse for younger patients, and rates of revision increase with longer follow-up [5].
The uncemented acetabular component has been regarded as a viable alternative to the cemented cup [2], and the hemispheric porous-coated cup inserted with press-fit technique has emerged as the most commonly used component [11]. Multiple series have demonstrated low rates of revision when aseptic loosening is the end-point, but revision due to osteolysis and polyethylene wear remains a problem [12–15]. Survival of the shell after 8–12 years is reported to be 100% in several studies when aseptic loosening is the end-point [12, 16–19], whereas survival of the acetabular component may be 64–80% when liner exchange, osteolysis, and wear are end-points [12, 14, 16, 20]. In a study of a first-generation porous-coated cup (PCA) with 15–17 years follow-up, 17% of the cups had been revised due to loosening with or without osteolysis [21], whereas a recent 20-year study found 96% survival of shell and 17% liner revision rate [15].
As the acetabular component of the Charnley arthroplasty has remained virtually unchanged for close to 40 years, there is an abundance of clinical studies documenting the results of the cemented acetabular component. A recent PubMed search yielded more than 400 studies on the Charnley arthroplasty, but only 8 were comparative studies [22–29], and only 1 compared the Charnley with a modern hemispheric porous-coated press-fit cup [30]. This was a radiostereometric study in 21 patients which found no difference between the Charnley cup and the Harris Galante cup in terms of fixation.
Thus there is a lack of good evidence with regards to the comparative outcome of the modern porous-coated cup and the traditional cemented all-polyethylene cup. Randomized controlled studies provide the best evidence, and in this report we convey the results of a randomized controlled trial comparing the Duraloc cup with a conventional Charnley cup with 10–14 year follow-up to help resolve the lack of evidence from direct comparisons of these two hip arthroplasty techniques.
Patients and methods
Between April 1994 and June 1997, 215 patients treated at one clinic consented to take part in the study, which was conducted at a county hospital with an annual case load of 300 total hip replacements. According to the inclusion criteria, patients were eligible for participation in the study if they suffered from noninflammatory degenerative disease of the hip including osteoarthritis, posttraumatic arthritis, psoriatic arthritis, and gout. They were also eligible if they suffered from joint diseases of inflammatory origin such as rheumatoid arthritis and juvenile rheumatoid arthritis as well as systemic lupus erythematosus. The upper age limit was 75 years, but there was no lower age limit. Previous prosthetic replacement was a contraindication to participation, but osteotomies and internal fixations were not. Twenty-five patients consented for both of their hips, resulting in a total of 240 hips enrolled. Patients were given sequential enrollment numbers, but the assignment of patients to treatment groups was randomly chosen using a table of random numbers. The randomization was concealed until after surgery had been initiated. In order to reduce potential bias, patients were not told which acetabular implant they received until their 2-year follow-up visit, which was covered in the preoperative consent form.
Patients were grouped in accordance with the Charnley classification (Table 1) to allow stratification according to presence of comorbidities and condition of other joints [31]. Surgery was performed using a direct lateral approach [32] by five orthopedic surgeons. The femoral component was cemented using third-generation cementing techniques with vacuum mixing, retrograde filling of the canal, and pressurization prior to insertion of the femoral component [33]. Cement containing gentamycin and a Charnley stem (DePuy, Leeds, UK) with 22.225 mm head diameter was used in all cases.
Table 1.
Charnley classification including modification of group B
| A | Single-joint arthropathy and no significant medical comorbidity |
| B | One other joint in need of an arthroplasty, or an unsuccessful or failing arthroplasty in another joint |
| B1 | Contralateral hip in need of arthroplasty, but untreated |
| B2 | Contralateral hip has been successfully treated with an arthroplasty |
| C | Multiple joints in need of arthroplasty, multiple failing arthroplasties or significant medical or psychological impairment |
For the uncemented group, the Duraloc 1200 cup (DePuy, Leeds, UK), a hemispherical modular cup consisting of a titanium shell with a porous-coated surface, was used. The surface has a mean pore size of 250 μm. The Duraloc 1200 cup was considered a second-generation cup because it had a minimum polyethylene thickness of 6 mm, dome-loading of the polyethylene, and an improved locking mechanism designed not to interfere with liner–shell conformity [34]. The shell had a central hole for the insertion device and 12 holes for screw fixation. An ultrahigh-molecular-weight polyethylene (UHMWPE) liner (Enduron; DePuy, Leeds, UK) with a 10° posterior lip was used in all cases.
The Charnley cup (DePuy, Leeds, UK) used for the other group was an all-polyethylene cup with a flange. The Ogee cup was used in 101 cases and the Low Posterior Wall cup was used in 19 cases. The surgeon cut the flange to fit the rim of the acetabulum, which provided increased pressure to the cement, augmenting cement penetration into the bone of the acetabulum. Surgery was performed under laminar air flow.
For prophylaxis against thromboembolic events, dalteparin (Fragmin®), a low-molecular-weight heparin, 5000 IE was given subcutaneously on the night before surgery, 4–8 h postoperatively, and daily for the length of the stay. Cefuroxim (Zinacef®) was given routinely in the study period as prophylaxis for infection. Patients were screened for urinary-tract infection prior to surgery and treated appropriately if bacteriuria was detected. Postoperatively, patients were allowed restricted weight bearing on the day after surgery. All patients were encouraged to use two crutches for at least 6 weeks.
The objectives of the study were to assess the safety and efficacy of the implants by means of clinical evaluation by means of Harris Hip Score (HHS), and radiological evaluation after 6 months, and 2, 5, and 10 years, as well as adverse event reporting. Though not part of the original study protocol, we conducted an implant survival analysis as well. No subgroup analysis was performed.
Patients were seen by their surgeon 6 weeks after surgery and by a physiotherapist 6 months, and 2, 5, and 10 years after surgery. The physiotherapist was specifically trained to evaluate hip replacement patients. The patients, but not the physiotherapist, were blinded as to which implant had been used in order not to bias the subjective part of the evaluation. The physiotherapist obtained a Harris Hip Score [35] at each visit. Radiographs were obtained at all visits and analyzed by a radiologist not directly involved in the study but very competent in this field. Radiographic changes that were noted included radiolucencies, bone resorption, cortical hypertrophy, cement fracture, and migration of components in the femoral zones of Gruen [36] and acetabular zones of DeLee [37]. No measure of polyethylene wear and no formal quantification of osteolysis was performed as this was not a part of the original study protocol.
All patient charts were examined during the summer of 2008, and censoring dates were set to July 31, 2008 for patient and implant survival. Thus, the follow-up was 12–14 years in the survival analysis. During the chart review we collected information that was not included in the protocol, including duration of surgery, bleeding, and any secondary use of antibiotics that might indicate complications not routinely recorded in the research protocol.
Statistical analysis
Two-sample t-tests were used for comparing continuous data. Chi-square and Fisher exact test were used to compare categorical variables. Survival data were analyzed using Kaplan–Meier plots and log-rank test. Logistic regression analysis was employed to explore possible risk factors for prosthetic infection. Results are considered statistically significant when p-values are below 0.05 or when the 95% confidence intervals do not overlap. In 25 cases two arthroplasties were included in the study, and these were analyzed as independent cases for reasons outlined in the “Discussion.”
Ethics
This study was initiated prior to the institution of a Institutional Review Board at our hospital. However, the procedures were conducted in accordance with the Declaration of Helsinki and the study has been evaluated by the present research ethics committee, which did not have any objections. All patients provided informed consent prior to surgery.
Results
There were 58 men and 157 women enrolled in the study, with mean body mass index (BMI) of 26.50 kg/m2 (SD 3.4 kg/m2) and 26.87 kg/m2 (SD 4.5 kg/m2), respectively. There was a statistically significant difference between the Charnley and Duraloc group in the distribution of hips between class A and B in the Charnley classification (Table 2; p = 0.049), with the Charnley group having more B1 patients and the Duraloc group more A patients. There was no statistically significant difference in other preoperative characteristics or baseline demographics (Table 3) between the groups. Operative time was significantly longer for Charnley (71 min) than for Duraloc (66 min) (p = 0.033), but there was no significant difference in bleeding (636 versus 602 ml) (p = 0.295).
Table 2.
Preoperative characteristics of the patients according to group
| Diagnosis | Charnley | Duraloc |
|---|---|---|
| Osteoarthritis | 93 | 94 |
| Congenital hip dysplasiaa | 24 | 18 |
| Posttraumatic arthritis | 2 | 4 |
| Rheumatoid arthritis | 1 | 3 |
| Avascular necrosis | 0 | 1 |
| Class | Charnley | Duraloc |
|---|---|---|
| A | 46 | 66 |
| B1 | 40 | 24 |
| B2 | 29 | 25 |
| C | 5 | 5 |
aMild dysplasia not necessitating advanced acetabular procedures
Table 3.
Baseline values of patient demographics
| Charnley | Duraloc | |||||
|---|---|---|---|---|---|---|
| Mean | 95% Confidence interval | Mean | 95% Confidence interval | |||
| Age (years) | 65 | 64 | 66 | 66 | 65 | 67 |
| Gendera (%) | 76 | 68 | 84 | 71 | 63 | 79 |
| Harris Hip Score | 47 | 45 | 50 | 49 | 47 | 52 |
| Body mass index (kg/m2) | 27 | 27 | 28 | 27 | 26 | 27 |
aProportion female
Follow-up
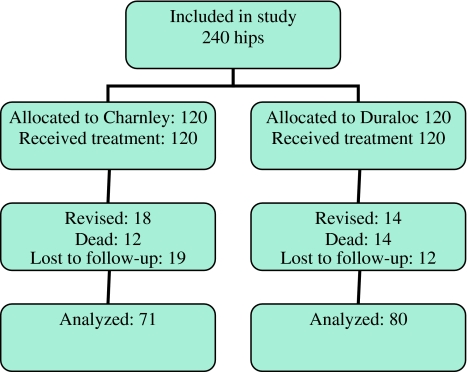
During the entire study period, 53 hips were lost due to the death of the patient, 24 in the Charnley group and 29 in the Duraloc group. However, only 25 patients died before their 10-year appointment, representing 12 cases in the Charnley group and 14 cases in the Duraloc group (Fig. 1). We were able to locate all patients in the study, but 31 patients were not able to attend their 10-year appointment, mostly because of ill health. Furthermore, 31 femoral revisions were performed, 17 in the Charnley group and 14 in the Duraloc group. For this reason, 71 patients in the Charnley group and 80 patients in the Duraloc group were available for 10-year Harris Hip Score and radiographic analysis.
Fig. 1.
Flow diagram illustrating the flow of hips through the study. Numbers for revision include femoral revisions
Bilateral cases
The preoperative characteristics of the bilateral cases are shown in Table 4. The patients who were included in the study with two hips had a statistically significant lower BMI than the unilateral patients in the Duraloc group, but not in the Charnley group.
Table 4.
Baseline characteristics of the unilaterally and bilaterally operated cases in the Charnley (91 and 29) and Duraloc (99 and 21) groups
| Acetabulum | Mean | 95% Confidence interval for mean | |
|---|---|---|---|
| Lower bound | Upper bound | ||
| Charnley | |||
| Baseline HHS | |||
| Unilateral | 46.7 | 43.8 | 49.5 |
| Bilateral | 49.8 | 43.3 | 56.3 |
| Total | 47.4 | 44.8 | 50.0 |
| Age (years) | |||
| Unilateral | 65.5 | 64.0 | 67.0 |
| Bilateral | 63.9 | 60.8 | 67.0 |
| Total | 65.1 | 63.7 | 66.4 |
| BMI (kg/m2) | |||
| Unilateral | 27.4 | 26.4 | 28.3 |
| Bilateral | 25.3 | 24.1 | 26.5 |
| Total | 26.9 | 26.1 | 27.7 |
| Duraloc | |||
| Baseline HHS | |||
| Unilateral | 48.2 | 45.3 | 51.1 |
| Bilateral | 55.2 | 50.0 | 60.3 |
| Total | 49.4 | 46.9 | 52.0 |
| Age (years) | |||
| Unilateral | 66.2 | 64.8 | 67.7 |
| Bilateral | 64.4 | 60.8 | 68.1 |
| Total | 65.9 | 64.6 | 67.3 |
| BMI (kg/m2)a | |||
| Unilateral | 27.1 | 26.3 | 27.9 |
| Bilateral | 24.5 | 23.0 | 26.0 |
| Total | 26.7 | 25.9 | 27.4 |
aSignificant difference as evidenced by nonoverlapping confidence intervals
Harris Hip Score
There was a significant difference between preoperative and postoperative scores for both groups (p < 0.0005). The Harris Hip Score improved from a baseline score of 47.7 to 87.7 at 6 months in the Charnley group, and from 49.4 to 88.2 in the Duraloc group. The difference between the intervention groups was not statistically significant at any time point (Table 5). There was a clear but not statistically significant decline in Harris Hip Score after 5 years in both groups, with the decline starting earlier for the Duraloc hips. Based on the function part of the Harris Hip Score, there was a reduction in function for both groups starting at 2 years of follow-up (Fig. 2). The pain component of the Harris Hip Score remained stable for both groups.
Table 5.
Mean Harris Hip Score including confidence intervals (95%) for both interventions
| Charnley | Duraloc | |||||
|---|---|---|---|---|---|---|
| CI | CI | |||||
| Mean | Lower | Upper | Mean | Lower | Upper | |
| Preoperative | 48.3 | 45.0 | 51.6 | 49.3 | 46.3 | 52.4 |
| 6 months | 90.2 | 87.9 | 92.6 | 89.1 | 86.9 | 91.3 |
| 2 years | 92.7 | 89.6 | 95.8 | 94.0 | 92.4 | 95.7 |
| 5 years | 93.9 | 91.6 | 96.2 | 91.4 | 89.3 | 93.5 |
| 10 years | 89.8 | 87.0 | 92.6 | 87.3 | 84.1 | 90.6 |
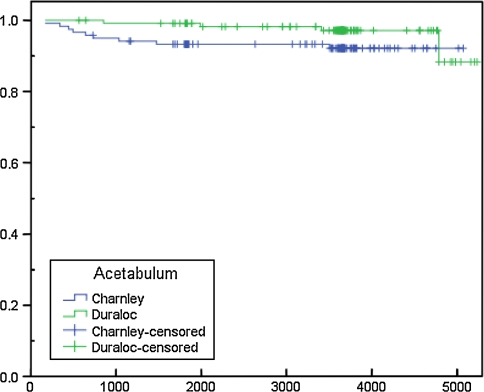
Fig. 2.
Survival in days of Charnley and Duraloc acetabular components with revision for any reason as end-point
Revisions
A total of 13 acetabular components were revised during the study (5.4%), 9 in the Charnley group and 4 in the Duraloc group, which was not statistically significant (p = 0.12; chi-square). In the Charnley group, three cups were revised due to aseptic loosening, one due to dislocation, and five due to prosthetic infection.
The five hips that became infected were treated with two-stage revision after 5, 11, 14, 24, and 48 months. While the difference in the rate of prosthetic infection between the groups was not quite statistically significant (p = 0.06; Fisher’s exact test), further exploration of the reasons for the disproportionately high rate of infection in the Charnley group revealed that the mean operating time was longer in the infected group (83 versus 68 min; p = 0.065) and the patients that became infected were significantly older (71.2 versus 65.4 years, p = 0.035) than the patients who did not become infected. There was a significant association between secondary use of antibiotics and later prosthetic infection (p = 0.001). Only 1 of the 188 cases who did not have a urinary infection developed a hip infection, whereas 4 of the 41 cases with urinary infection later sustained a prosthetic infection. In logistic regression analysis, secondary use of antibiotics for any reason significantly increased risk of having a later prosthetic infection by 12.5 (CI 95% 1.2–133), after correction for age, gender, comorbidities (Charnley class), surgeon, and study group (Charnley versus Duraloc).
Of the four revised cups in the Duraloc group, no cups were revised due to aseptic loosening. Three cups were removed in conjunction with revision of a loose stem, and one cup that did not show signs of being loose was removed during revision for instability. There were no isolated exchanges of liner, but the liner was changed en passant in conjunction with femoral revision in nine cases in the Duraloc group. If these liner exchanges were included among the revisions, 13 Duraloc cups were revised (11%) versus 9 Charnley cups (8%), a difference that was still not statistically significant (p = 0.37; chi-square).
Implant survival
Survival of the implants was determined using Kaplan–Meier survival analysis (Fig. 2) using revision for any reason as end-point, except liner exchange en passant. The curves (Fig. 2) indicate a slightly better survival for the Duraloc cup for the first 12 years, but the log-rank test between the implants was not significant (Mantel-Cox; p = 0.09).
Dislocation and other complications
In the Charnley group, four patients had dislocations which were treated by closed reduction. One patient was later revised due to recurring instability from loosening of the femur. In the Duraloc group, ten patients had dislocations, one of which was later revised because of recurring instability from loosening of the femur. Another patient in the Charnley group and two in the Duraloc group reported instability, but they did not have documented dislocation necessitating reduction. Thus a total of 17 patients reported instability, 5/120 in the Charnley group (3.3%) and 12/120 (10.0%) in the Duraloc group (p = 0.098).
There were 33 complications that were not treated surgically, 15 in the Charnley group and 18 in the Duraloc group (p = 0.32; Table 6). In the retrospective chart review, 52 cases (24 in the Charnley group and 28 in the Duraloc group) were identified in which a second course of antibiotics was given, of which 41 cases were given antibiotics indicating a urinary infection and 11 cases were other antibiotics indicating a range of infection types.
Table 6.
Complications reported in the study that were not treated surgically
| Charnley | Duraloc | |
|---|---|---|
| Cardiovascular | 0 | 1 |
| CNS (stroke) | 0 | 2 |
| Pulmonary embolism | 1 | 3 |
| Hematemesis | 1 | 0 |
| Respiratory | 1 | 0 |
| Weakness of muscles | 3 | 5 |
| Wound problems | 6 | 5 |
| Other | 3 | 2 |
CNS, central nervous system
Radiographic results
For the acetabular component, 71 radiographs in the Charnley group and 80 in the Duraloc group were obtained. In the Charnley group, three patients had radiolucencies of 1 mm in zone A. One patient had changes in zones A and C, while three patients had changes in all three zones. In the Duraloc group, one patient had radiolucencies in zone A. There was no migration of the cup in any of the groups. Thus, 7/71 patients had some evidence of loosening of the cup in one or more zones in the Charnley group, while only 1/80 in the Duraloc group had any evidence of loosening (p = 0.024).
Discussion
Both groups improved their Harris Hip Score significantly after surgery, and the magnitude of improvement compared well with what is usually seen after total hip arthroplasty [18, 38]. The difference in Harris Hip Score between the implants was 2.4 points after 5 years and 2.5 points after 10 years, in favor of the Charnley cup. The study probably did not have sufficient power to detect a difference of this magnitude as statistically significant. Harris Hip Scores between 90 and 100 are regarded as excellent, and we feel that a clinically relevant difference between treatment groups would have to be 5 points. In the study by Kalairajah [38], the mean HHS was 89 and the standard deviation was 13.3. In a study designed to detect a 5% effect size with 80% power and 95% certainty and a standard deviation of 13.3, one would need 87 subjects in each treatment group. In our study, loss of patients due to revision, death, and ill health was underestimated, resulting in somewhat small samples.
The observed decline in HHS from 5 years in the Charnley group and from 2 years in the Duraloc group is in accordance with what is usually seen. When splitting the Harris Hip Score into a pain component and a functional component, it can be seen that the arthroplasties remain pain free even though function declines. For this reason, we feel that the decline in HHS corresponds to a decline in general health due to aging of the patients, which has been reported in some [23] but not all [39] earlier studies. This supports the previous findings that call for a separate instrument to assess activity level of the arthroplasty patient beyond what is measured by the Harris Hip Score [40].
There was a large discrepancy in the frequency of infection which warrants more investigation. Five of the 240 arthroplasties became infected (2.1%), but all occurred in the Charnley group. There was a significant association between urinary infection and later deep infection of the hip, which is consistent with findings in previous reports [41–43]. However, since we do not have information on the infecting agent, it is not possible to suggest a causal relationship between urinary-tract infection and subsequent prosthetic infection. However, the finding is interesting and may suggest an underlying predisposition for infection. In any case, the finding certainly represents a cautionary reminder concerning perioperative instrumentation of the urinary tract. On a slightly different note, it may be argued that the patients who became infected should be removed from the survival analysis, but we have elected to keep them, since infection is an important aspect of implant survival in the clinical setting.
The radiographic analysis indicated that 9.9% of the Charnley cups and 1.2% of the Duraloc cups had some signs of loosening. However, since none of them had changed position, they were not deemed to be definitely loose. In our study, we included any sign of loosening larger than 1 mm noted by the radiologist in the analysis, and many of these signs were probably very subtle. This may have exaggerated the number of cups with radiographic signs of loosening, but the relationship between radiographic signs and loosening is complicated, as radiographically loose cups may function well clinically whereas painful, loose cups do not always display definite signs of loosening radiographically. Furthermore, we have not studied wear and osteolysis, which are known to affect predominantly uncemented cups. For this reason, our findings may underestimate problems with uncemented cups.
There are limitations to any long-term study of this nature. Because of death and deterioration in general health, only 59% in the Charnley group and 67% in the Duraloc group were available for clinical and radiographic evaluation at the 10-year mark. While it is has been shown that the results in patients lost to follow-up are worse than patients who stay in clinical studies [44], we were able to determine reason for loss to follow-up for almost all of our patients, with the vast majority of those who declined a follow-up visit doing so because of advanced comorbid diseases and not because of poor function of the hip. In addition, our overall follow-up rate was similar to other long-term studies of hip function [18, 19, 41], even though our patient population was significantly older. The generalizability of the study is felt to be good as the study was conducted at a nonacademic center, included most patients under 75 years of age, and the surgery was performed by general orthopedic surgeons.
The lack of precise recording of comorbidities is also a limiting factor. Indices of comorbidities have previously been shown to predict functional outcome as well as complications after total hip arthroplasty [45–51]. The Charnley score is not a dedicated comorbidity instrument and might not be sensitive enough to record subtle nuances in patient health status, which could have contributed to a better understanding of the large discrepancy in infection rates between the two study groups. Another limitation is the lack of a formal account for patient activity level [40, 52]. Level of activity is important as it is of primary interest to the patients for performing recreational activities [53] as well as for improving physical fitness, although increased level of activity correlates with wear and potential failure of an implant [54–56]. The Harris Hip Score contains assessment of physical function, but it does not quantify what the patient actually does, only what he or she is capable of doing. Dedicated scales have been developed for the sole purpose of estimating level of activity before and after total hip arthroplasty (THA), but these scales were not available for use in this study [56–59].
The issue of bilateral procedures is controversial since the presence of two procedures in one patient violates the assumption of independent observations on which many statistical tests rely [60]. However, other authors have discussed this and found that inclusion of bilateral procedures did not alter the results [61, 62]. In a recent study from the Finnish Arthroplasty Register, 27% of the cases analyzed were bilateral cases, and inclusion of bilateral cases in the analysis was considered appropriate, even though the statistical technique (Cox regression) formally requires independent observations. In out study, 21% of the cases were bilateral. We did find statistically significant differences in preoperative BMI, which raises the question of whether other unknown confounders might influence the results. However, the issue of bilaterality was not addressed in the study protocol. The presence of an arthroplasty in the contralateral hip was not an exclusion criterion for the study, nor was there any criterion excluding patients with poor function of the contralateral hip. For these reasons, we find it justified to include the patients who had two arthroplasties during the study and treat them as independent cases.
While the Charnley cup has remained unchanged since the inception of this study, uncemented cups have undergone a continuous process of change. As screw-holes are believed to transmit increased stress to the polyethylene, in addition to providing a potential pathway for polyethylene debris, a shell with 12 screw-holes is now rarely used in primary surgery. Furthermore, the polyethylene used in this study has largely been replaced by cross-linked polyethylene (PE) or alternative bearings, and there is an international trend moving toward larger head sizes. Nevertheless, the Charnley low-friction arthroplasty continues to be regarded by many as a gold standard against which new implants are compared [63].
In conclusion, our 10-year results confirm previous reports from noncontrolled studies that survival of an uncemented hemispherical porous-coated cup as well as the cemented all-polyethylene cup is excellent. With no statistically significant differences in outcomes or survival between the two implants, surgeons should choose the system that they are either more familiar with in terms of surgical technique or that would most benefit the individual patient. Further studies might indicate whether one implant will perform better than the other in the long term.
Acknowledgments
The authors wish to express their gratitude to Helen Scmidt, Janne Hensmo, and other physiotherapists as well as to Dr. Asle Bunes, radiologist, for their hard work and dedication. Odd Fellow Norway provided the first author with a grant contributing to the completion of the study.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY (2004) Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg [Am] 86-A:963–974 [DOI] [PubMed]
- 2.Harris WH (2003) Results of uncemented cups: a critical appraisal at 15 years. Clin Orthop Relat Res 121–125 [DOI] [PubMed]
- 3.Illgen R, Rubash HE. The optimal fixation of the cementless acetabular component in primary total hip arthroplasty. J Am Acad Orthop Surg. 2002;10:43–56. doi: 10.5435/00124635-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Garellick G, Herberts P, Stromberg C, Malchau H. Long-term results of Charnley arthroplasty. A 12–16-year follow-up study. J Arthroplast. 1994;9:333–340. doi: 10.1016/0883-5403(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF (2002) Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am 84-A:171–177 [DOI] [PubMed]
- 6.Kobayashi S, Takaoka K, Saito N, Hisa K. Factors affecting aseptic failure of fixation after primary Charnley total hip arthroplasty. Multivariate survival analysis. J Bone Joint Surg Am. 1997;79:1618–1627. doi: 10.2106/00004623-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan JJ et al (2004) Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg Am 86-A:690–695 [DOI] [PubMed]
- 8.Hartofilakidis G, Karachalios T, Karachalios G (2005) The 20-year outcome of the charnley arthroplasty in younger and older patients. Clin Orthop Relat Res 177–182 [DOI] [PubMed]
- 9.Wroblewski BM, Siney PD, Fleming PA. Charnley low-frictional torque arthroplasty in patients under the age of 51 years. Follow-up to 33 years. J Bone Joint Surg Br. 2002;84:540–543. doi: 10.1302/0301-620X.84B4.10293. [DOI] [PubMed] [Google Scholar]
- 10.Smith SW, Estok DM, Harris WH. Total hip arthroplasty with use of second-generation cementing techniques. An eighteen-year-average follow-up study. J Bone Joint Surg Am. 1998;80:1632–1640. doi: 10.2106/00004623-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Peters CL, Miller MD (2007) The cementless acetabular component. 2:946–968
- 12.Gaffey JL et al (2004) Cementless acetabular fixation at fifteen years. A comparison with the same surgeon’s results following acetabular fixation with cement. J Bone Joint Surg Am 86-A:257–261 [PubMed]
- 13.Harris WH (1995) The problem is osteolysis. Clin Orthop Relat Res 46–53 [PubMed]
- 14.Garcia-Rey E, Garcia-Cimbrelo E. Long-term results of uncemented acetabular cups with an ACS polyethylene liner. A 14–16-year follow-up study. Int Orthop. 2007;31:205–210. doi: 10.1007/s00264-006-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valle CJ, et al. Primary total hip arthroplasty with a porous-coated acetabular component. A concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009;91:1130–1135. doi: 10.2106/JBJS.H.00168. [DOI] [PubMed] [Google Scholar]
- 16.Engh CA, Hopper RH, Jr, Engh CA., Jr Long-term porous-coated cup survivorship using spikes, screws, and press-fitting for initial fixation. J Arthroplast. 2004;19:54–60. doi: 10.1016/j.arth.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Reina RJ, Rodriguez JA, Rasquinha VJ, Ranawat CS. Fixation and osteolysis in plasma-sprayed hemispherical cups with hybrid total hip arthroplasty. J Arthroplast. 2007;22:531–534. doi: 10.1016/j.arth.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Clohisy JC, Harris WH. The Harris-Galante porous-coated acetabular component with screw fixation. An average ten-year follow-up study. J Bone Joint Surg Am. 1999;81:66–73. doi: 10.2106/00004623-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Grobler GP, Learmonth ID, Bernstein BP, Dower BJ. Ten-year results of a press-fit, porous-coated acetabular component. J Bone Joint Surg Br. 2005;87:786–789. doi: 10.1302/0301-620X.87B6.15335. [DOI] [PubMed] [Google Scholar]
- 20.Udomkiat P, Dorr LD, Wan Z (2002) Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year follow-up. J Bone Joint Surg Am 84-A:1195–1200 [DOI] [PubMed]
- 21.Bojescul JA, Xenos JS, Callaghan JJ, Savory CG (2003) Results of porous-coated anatomic total hip arthroplasty without cement at fifteen years: a concise follow-up of a previous report. J Bone Joint Surg Am 85-A:1079–1083 [DOI] [PubMed]
- 22.Britton AR, Murray DW, Bulstrode CJ, McPherson K, Denham RA. Long-term comparison of Charnley and Stanmore design total hip replacements. J Bone Joint Surg Br. 1996;78:802–808. [PubMed] [Google Scholar]
- 23.Chandran P, Azzabi M, Miles J, Andrews M, Bradley J (2008) Furlong hydroxyapatite-coated Hip prosthesis vs the Charnley cemented Hip prosthesis. J Arthroplast [DOI] [PubMed]
- 24.Garellick G, Malchau H, Herberts P. The Charnley versus the Spectron hip prosthesis: clinical evaluation of a randomized, prospective study of 2 different hip implants. J Arthroplast. 1999;14:407–413. doi: 10.1016/S0883-5403(99)90095-5. [DOI] [PubMed] [Google Scholar]
- 25.Havelin LI, Espehaug B, Engesaeter LB. The performance of two hydroxyapatite-coated acetabular cups compared with Charnley cups. From the Norwegian Arthroplasty Register. J Bone Joint Surg Br. 2002;84:839–845. doi: 10.1302/0301-620X.84B6.12492. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsson SA, Djerf K, Wahlstrom O (1996) Twenty-year results of McKee-Farrar versus Charnley prosthesis. Clin Orthop Relat Res S60–S68 [DOI] [PubMed]
- 27.Murray MP, Gore DR, Brewer BJ, Zuege RC, Gardner GM (1976) Comparison of functional performance after McKee-Farrar, Charnley, and Muller total hip replacement. A six-month follow-up of one hundred sixty-five cases. Clin Orthop Relat Res 33–43 [PubMed]
- 28.Alho A, Soreide O, Bjersand AJ. Mechanical factors in loosening of Christiansen and Charnley arthroplasties. Acta Orthop Scand. 1984;55:261–266. doi: 10.3109/17453678408992352. [DOI] [PubMed] [Google Scholar]
- 29.Marston RA, Cobb AG, Bentley G. Stanmore compared with Charnley total hip replacement. A prospective study of 413 arthroplasties. J Bone Joint Surg Br. 1996;78:178–184. [PubMed] [Google Scholar]
- 30.Onsten I, Carlsson AS, Ohlin A, Nilsson JA. Migration of acetabular components, inserted with and without cement, in one-stage bilateral hip arthroplasty. A controlled, randomized study using roentgenstereophotogrammetric analysis. J Bone Joint Surg Am. 1994;76:185–194. doi: 10.2106/00004623-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Charnley J, Halley DK. Rate of wear in total hip replacement. Clin Orthop Relat Res. 1975;112:170–179. doi: 10.1097/00003086-197510000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17–19. doi: 10.1302/0301-620X.64B1.7068713. [DOI] [PubMed] [Google Scholar]
- 33.Rasquinha VJ, Dua V, Rodriguez JA, Ranawat CS. Fifteen-year survivorship of a collarless, cemented, normalized femoral stem in primary hybrid total hip arthroplasty with a modified third-generation cement technique. J Arthroplast. 2003;18:86–94. doi: 10.1016/S0883-5403(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 34.Sychterz CJ, Engh CA, Jr, Yang A, Engh CA. Analysis of temporal wear patterns of porous-coated acetabular components: distinguishing between true wear and so-called bedding-in. J Bone Joint Surg Am. 1999;81:821–830. doi: 10.1302/0301-620X.81B5.9383. [DOI] [PubMed] [Google Scholar]
- 35.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 36.Gruen TA, McNeice GMP, Amstutz HC. “Modes of Failure” of cemented stem-type femoral components: a radiographic analysis of loosening [Miscellaneous Article] Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 37.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total Hip replacement [Report] Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 38.Kalairajah Y, Azurza K, Hulme C, Molloy S, Drabu KJ. Health outcome measures in the evaluation of total hip arthroplasties–a comparison between the Harris hip score and the Oxford hip score. J Arthroplast. 2005;20:1037–1041. doi: 10.1016/j.arth.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsson SA, Djerf K, Wahlstrom O. A comparative study between McKee-Farrar and Charnley arthroplasty with long-term follow-up periods. J Arthroplast. 1990;5:9–14. doi: 10.1016/S0883-5403(06)80003-3. [DOI] [PubMed] [Google Scholar]
- 40.Beaule PE, Dorey FJ, Hoke R, Leduff M, Amstutz HC. The value of patient activity level in the outcome of total hip arthroplasty. J Arthroplast. 2006;21:547–552. doi: 10.1016/j.arth.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Petersen MB, Poulsen IH, Thomsen J, Solgaard S. The hemispherical Harris-Galante acetabular cup, inserted without cement. The results of an eight to eleven-year follow-up of one hundred and sixty-eight hips. J Bone Joint Surg Am. 1999;81:219–224. doi: 10.2106/00004623-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Sarafis KA, Karatzas GD, Feroussis JC, Yotis CL (1997) Hybrid total hip replacement. A 5-10-year follow-up study of 106 patients. Acta Orthop Scand Suppl 275:21–26 [PubMed]
- 43.Schmalzried TP, Harris WH. Hybrid total hip replacement. A 6.5-year follow-up study. J Bone Joint Surg Br. 1993;75:608–615. doi: 10.1302/0301-620X.75B4.8331118. [DOI] [PubMed] [Google Scholar]
- 44.Murray DW, Britton AR, Bulstrode CJ. Loss to follow-up matters. J Bone Joint Surg Br. 1997;79:254–257. doi: 10.1302/0301-620X.79B2.6975. [DOI] [PubMed] [Google Scholar]
- 45.Imamura K, Black N. Does comorbidity affect the outcome of surgery? Total hip replacement in the UK and Japan. Int J Qual Health Care. 1998;10:113–123. doi: 10.1093/intqhc/10.2.113. [DOI] [PubMed] [Google Scholar]
- 46.Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31:141–154. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Greenfield S. The state of outcomes research: are we on target? N Engl J Med. 1987;320:1142–1145. doi: 10.1056/NEJM198904273201710. [DOI] [PubMed] [Google Scholar]
- 48.Keener JD et al (2003) Long-term function after Charnley total hip arthroplasty. Clin Orthop Relat Res 148–156 [DOI] [PubMed]
- 49.Fortin PR, et al. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 50.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Lubbeke A, Katz JN, Perneger TV, Hoffmeyer P. Primary and revision hip arthroplasty: 5-year outcomes and influence of age and comorbidity. J Rheumatol. 2007;34:394–400. [PubMed] [Google Scholar]
- 52.Dorey FJ, Amstutz HC (2002) The need to account for patient activity when evaluating the results of total hip arthroplasty with survivorship analysis. J Bone Joint Surg Am 84-A:709–710 [DOI] [PubMed]
- 53.Wright JG, Rudicel S, Feinstein AR. Ask patients what they want. Evaluation of individual complaints before total hip replacement. J Bone Joint Surg Br. 1994;76:229–234. [PubMed] [Google Scholar]
- 54.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Meyer HE. The effect of middle-age body weight and physical activity on the risk of early revision hip arthroplasty: a cohort study of 1,535 individuals. Acta Orthop. 2007;78:99–107. doi: 10.1080/17453670610013493. [DOI] [PubMed] [Google Scholar]
- 55.Lavernia CJ, Sierra RJ, Hungerford DS, Krackow K. Activity level and wear in total knee arthroplasty: a study of autopsy retrieved specimens. J Arthroplast. 2001;16:446–453. doi: 10.1054/arth.2001.23509. [DOI] [PubMed] [Google Scholar]
- 56.Saleh KJ, et al. Development and validation of a lower-extremity activity scale. Use for patients treated with revision total knee arthroplasty. J Bone Joint Surg Am. 2005;87:1985–1994. doi: 10.2106/JBJS.D.02564. [DOI] [PubMed] [Google Scholar]
- 57.Naal FD, Impellizzeri FM, Leunig M (2008) Which is the best activity rating scale for patients undergoing total joint arthroplasty? Clin Orthop Relat Res (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 58.Silva M, McClung CD, la Rosa MA, Dorey FJ, Schmalzried TP. Activity sampling in the assessment of patients with total joint arthroplasty. J Arthroplast. 2005;20:487–491. doi: 10.1016/j.arth.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplast. 1998;13:890–895. doi: 10.1016/S0883-5403(98)90195-4. [DOI] [PubMed] [Google Scholar]
- 60.Ranstam J. Problems in orthopedic research: dependent observations. Acta Orthop Scand. 2002;73:447–450. doi: 10.1080/00016470216327. [DOI] [PubMed] [Google Scholar]
- 61.Hulleberg G, Aamodt A, Espehaug B, Benum P. A clinical and radiographic 13-year follow-up study of 138 Charnley hip arthroplasties in patients 50–70 years old: comparison of university hospital data and registry data. Acta Orthop. 2008;79:609–617. doi: 10.1080/17453670810016614. [DOI] [PubMed] [Google Scholar]
- 62.Lie SA, Engesaeter LB, Havelin LI, Gjessing HK, Vollset SE. Dependency issues in survival analyses of 55, 782 primary hip replacements from 47, 355 patients. Stat Med. 2004;23:3227–3240. doi: 10.1002/sim.1905. [DOI] [PubMed] [Google Scholar]
- 63.The Norwegian Arthroplasty Register (2007) Annual Report 2007