Abstract
Spinal cord injury produces prominent disruption of the blood-spinal cord barrier. We have defined the blood-spinal cord barrier breakdown to the protein luciferase (61 kDa) in the acutely injured murine spinal cord and during revascularization. We show that newly formed and regenerating blood vessels that have abnormal permeability exhibit differential expression of the glucose-1 transporter (Glut-1), and that its expression is dependent on astrocytes. There was overt extravasation of luciferase within the first hour after injury, a period that coincided with marked tissue disruption within the epicenter of the lesion. Although there was a significant reduction in the number of blood vessels relative to controls by 24 hr after injury, abnormal barrier permeability remained significantly elevated. A second peak of abnormal barrier permeability at 3–7 days postinjury coincided with prominent revascularization of the epicenter. The barrier to luciferase was restored by 21 days postinjury and vascularity was similar to that of controls. During wound-healing process, the cord was reorganized into distinct domains. Between 14 and 21 days postinjury, each domain consisted primarily of nonneuronal cells, including macrophages. Astrocytes were limited characteristically to the perimeter of each domain. Only blood vessels affiliated closely with astrocytes in the perimeter expressed Glut-1, whereas blood vessels within each domain of the repairing cord did not express it. Together, these data demonstrate that both injured and regenerating vessels exhibit abnormal permeability and suggest that Glut-1 expression during revascularization is dependent on the presence of astrocytes.
Keywords: spinal cord contusion injury, luciferase, astrocyte, glucose-1 transporter
Spinal cord injury results in direct vascular damage and initiates a cascade of events that alter the permeability of the blood-spinal cord barrier. Little attention has been directed at evaluating the integrity of the blood spinal cord barrier in injured and regenerating vessels. We have hypothesized that increased permeability after spinal cord injury occurs in both injured and regenerating vessels that likewise exhibit altered transport properties. The morphologic basis of the blood-brain/spinal cord barrier resides in the presence of tight junctions between endothelial cells and the paucity of transcellular transport via membrane-bound vesicles (Reese and Karnovsky, 1967). Spinal cord injury produces prominent disruption of the barrier and it has been postulated that this abnormal permeability is attributed to a significant increase in transendothelial vesicular transport of proteins (Mautes et al., 2000).
Recent studies have demonstrated distinct differences in wound healing in a murine model of spinal cord injury (Fujiki et al., 1996; Guth et al., 1999). Unlike other species, the injured murine spinal cord does not undergo a characteristic pattern of cystic cavitation. Rather, the irreversibly injured tissue is replaced gradually by nonneuronal cells. What remains unclear is the extent to which this pattern of wound healing influences integrity of the blood-spinal cord barrier in the acute stage as well as during revascularization.
The blood-brain/spinal cord barrier maintains an almost uniform, intimate contact with astrocytes. Astrocytic foot processes form a relatively complete layer that abuts the basal lamina of endothelial cells (Peters et al., 1991; Mautes et al., 2000). There is evidence that astrocytes influence structural/functional barrier properties including polarity (Beck et al., 1984) and activity of enzymes and transporters such as alkaline phosphatase and Glut-1 (Beck et al., 1986; Cancilla and Berliner, 1993; Hayashi et al., 1997). Somewhat controversial evidence exists (Tio et al., 1990; Rubin et al., 1991) that astrocytes and endothelial cells require contact for expression of barrier characteristics (DeBault, 1981; Hayashi et al., 1997). Interactions between astrocytes and the vasculature may be impaired after spinal cord injury, particularly during wound healing when angiogenesis and reactive gliosis predominate.
The vasculature in the central nervous system (CNS) expresses specific transport systems that collectively regulate intracellular movement of molecules (Lee et al., 2001). There is recent evidence that endothelial transport systems may be altered after spinal cord injury. Pan and Kastin (2001) reported upregulation of the specific transporter for tumor necrosis factor α (TNF-α). Such an observation supports the view that the altered microenvironment of injured cord is a consequence of not only barrier disruption but of modifications in transporter system functionality. Glut-1 accounts for more than 90% of glucose transport across the barrier (Pardridge, 1993). It is highly expressed in brain-derived endothelial cells but absent in other organs, such as liver (Pardridge, 1993; Rahner-Welsch et al., 1995). We have focused on endothelial Glut-1 expression in the injured cord. The objectives of this study were to determine the extent to which injured and regenerating blood vessels exhibit increased permeability to proteins after spinal cord injury, and the extent to which changes in permeability during wound healing correlate with altered Glut-1 expression and astrocyte distribution.
MATERIALS AND METHODS
Experimental Model
All procedures were carried out following the UCSF Committee on Animal Research guidelines. Adult male (25–31 g) C57BL6 mice (n = 72) were anesthetized intraperitoneally with 2.5% Avertin (0.02 ml/g body weight) and prepared for surgery. An incision was made along the dorsal midline, the dorsal extensors were reflected laterally, and a laminectomy was carried out at the T8 level. The vertebral column was stabilized by forceps attached to the adjacent spinous processes, and then the spinal cord was injured by a 3-g weight dropped 5 cm onto an impactor tip (diameter 1.5 mm). The overlying skin was then closed with wound clips. Surgical controls consisted of sham animals (n = 5) that received a laminectomy but were not subjected to injury.
Permeability Studies
Quantitative Assessment
Barrier permeability to luciferase (Promega, Madison, WI) was evaluated in naïve (n = 3) and sham-operated (n = 5) animals, at 25 min (n = 4), 35 min (n = 5), 1.5 hr (n = 5), 4.5 hr (n = 5), 1 day (n = 4), and 3 (n = 4), 7 (n = 5), 14 (n = 4), and 21 days (n = 4) after injury. Luciferase (1 mg/ml in Luciferase Storage Buffer; Promega) was diluted 1:1 with 0.05 M phosphate-buffered saline (PBS) in 0.001% bovine serum albumin (BSA). At 30 min before euthanasia at a given time point, each animal was anesthetized with Avertin, the jugular vein exposed, and injected with luciferase (3.33 µl/g body weight). The luciferase was delivered using a leur-lock syringe attached to a modified 27G Surflo Winged Infusion Set (Terumo Medical Corporation, Somerset, NJ). After injection, bleeding was controlled with sterile cotton swabs and Gelfoam (Upjohn, Kalamazoo, MI) as required. Upon Avertin-induced euthanasia, a blood sample was taken from the right ventricle and diluted immediately 1/50 in PBS, and the spinal cord was removed, rinsed with PBS, and quickly frozen.
The frozen cord was divided into six 3-mm segments, one centered on the injury site, and the two adjacent sections both rostral and caudal. A sixth section prepared from the cervical cord served as an internal control. Each segment was thawed, homogenized, and diluted 1:50 by weight in 1× cell culture lysis reagent (Promega). Homogenates were centrifuged (12,000 rpm) for 8 min to remove cellular debris and 1.5 µl of each supernatant was taken and diluted individually 1:100 in 1× cell culture lysis reagent. A 10-µl aliquot of the final dilution was added to 100 µl of Luciferase Assay System solution (Promega), briefly mixed, and quantitated in a luminometer (0 sec delay, integrated over 10 sec; TD-2020, Turner Designs, Sunnyvale, CA).
Luminosity values for the cervical region of each animal were used as internal controls. Cervical controls were chosen after demonstrating that luciferase values were unchanged from baseline values over the entire 21-day experiment. Cervical control sections were similar to naïve tissue in luminosity. Relative luminosity was determined by taking the raw luminosity values for each segment and dividing them by the cervical value. A log transformation was then carried out on all data points. Values at a given segment and time point are shown as the mean ± SEM. Statistical comparisons were made using analysis of variance followed by the Scheffe and Dunnett tests. Significance was defined at the P < 0.05 level.
Anatomic Assessment
The blood-spinal cord barrier to Evans blue albumin (EBA) was evaluated in naïve animals (n = 5) and injured animals at 1 (n = 8), 3 (n = 5), 7 (n = 5), 14 (n = 8), and 21 days (n = 5). The tracer (1.2% in 0.9% NaCl) was given intravenously 30 min before euthanasia. Each animal was perfused transcardially with fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4). The perfused spinal cord was removed, post-fixed for 4 hr, and cryoprotected in 20% sucrose for 4 days. The cryoprotected cord was then blocked in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA), frozen, and sectioned (14–20 µm thick) on a cryostat. All sections were mounted on slides, dried at 37°C for 30 min, and frozen. A nonspecific fluorescence similar to that of EBA fluorescence was apparent in animals that were euthanized at later time points (either 14 or 21 days after injury). We therefore limit our interpretation of EBA localization to earlier time points. We carried out further experiments to determine if there was similar nonspecific luminescence, but found no evidence of intrinsic nonspecific luminescence at the 1- or 21-day points.
Immunolocalization of Glial Fibrillary Acidic Protein, Glut-1, Platelet-Endothelial Cell Adhesion Molecule-1, and CD11b
We examined glial and vascular response during wound healing. The presence of astrocytes and blood vessels was defined by positive staining for glial fibrillary acidic protein (GFAP) and platelet-endothelial cell adhesion molecule-1 (PECAM-1), respectively. Sections were thawed at room temperature, dried at 37°C, rehydrated in 0.05 M PBS (pH 7.4) for 15 min, and incubated in methanol for 15 min. To localize GFAP or PECAM-1, slides were incubated at room temperature as follows: (1) PBS, 5 min; (2) 2% horse serum/0.1% BSA (HS/BSA), 5 min; (3) 10% HS/BSA, 20 min; (4) goat anti-GFAP polyclonal antibody (1:1,000 in 2% HS/BSA; Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-PECAM-1 polyclonal antibody (1:1,000 in 2% HS/BSA, Santa Cruz Biotechnology), 24 hr; (5) PBS, 3× 5 min; and (6) affinity-purified biotinylated horse anti-goat Ig (1:200 in 2% HS/BSA, Vector Laboratories, Burlingame, CA), 1 hr. Slides were then rinsed in PBS 3× 5 min, incubated in either fluorescein-avidin D (1:100 in PBS; Vector Laboratories), Texas red-avidin D (1:100 in PBS; Vector Laboratories), or Alexa Fluor 350-NeutroAvidin (1:100 in PBS; Molecular Probes, Eugene, OR) for 1 hr, and then rinsed three more times in PBS.
Glut-1 localization began immediately after the last wash of the previous labeling, and was conducted in relative darkness to avoid photo-bleaching. Slides were incubated as follows: (1) 2% goat serum/0.1% BSA (GS/BSA), 5 min; (2) 10% GS/BSA, 20 min; (3) rabbit anti-Glut1 polyclonal antibody (1:2,000 in 2% GS/BSA; Chemicon International, Temecula, CA), 24 hr; (4) PBS 3× 5 min; and (5) affinity-purified biotinylated goat anti-rabbit IgG (1:200 in 2% GS/BSA; Vector Laboratories), 1 hr. Sections were then rinsed in PBS 3× 5 min and incubated in either fluorescein avidin D (1:100 in PBS) or Alexa Fluor 350-NeutroAvidin (1:100 in PBS) for 1 hr. Slides were rinsed in PBS 3× 5 min, treated with Vectashield mounting medium (Vector Laboratories) and coverslipped.
To localize CD11b, slides were incubated at room temperature as follows: (1) PBS, 15 min; (2) 2% BSA, 1 hr; (3) rat CD11b antibody (1:1,000 in 2% BSA; Serotec, Raleigh NC), 24 hr; (4) PBS 3× 5 min; and (5) affinity-purified biotinylated rabbit anti-rat Ig (1:200 in 2% BSA, Vector Laboratories), 1 hr. Slides were then rinsed in PBS 3× 5 min, incubated in fluorescein-avidin D (1:100 in PBS) for 1 hr, and rinsed again three times in PBS.
Quantification of Revascularization
Glut-1-labeled PECAM-1 confirmed vessels were quantified in the uninjured control cord. In the injured cord, blood vessels labeled consistently with PECAM-1; however, there was a population of blood vessels that were PECAM-1 positive and Glut-1 negative. Therefore, to quantify vessels in the injured segment, we relied on PECAM-1 labeling. We used methods similar to those described by Loy et al. (2002) to quantify revascularization. In each injured cord, serial epicenter sections were examined and the area of maximal damage was selected for density analysis. The central gray matter region of the cord (751 µm × 590 µm) was captured with a CCD camera mounted to a Nikon Optiphot microscope. Images of injured sections were analyzed in random order by a time point-blinded observer. A grid (divided into 20 µm × 20 µm squares) was then placed over the entire cross section using Adobe Photoshop 6.0. Within these areas, per-area blood vessel density was collected using NIH Image 1.62. Statistical comparisons of multiple groups were made using analysis of variance (ANOVA) followed by the Scheffe and Dunnett tests. Significance was defined at the P < 0.05 level.
RESULTS
Barrier Disruption to Evans Blue Albumin Exhibits Both a Diffuse and Cellular Distribution
As expected, there was no leakage to EBA in cords prepared from naïve animals (Fig. 1). There was pronounced leakage to EBA within the epicenter by 1 day after injury. Both punctate staining, presumably reflecting EBA within cells, and diffuse staining, suggestive of extracellular localization, were apparent at 24 hr. A similar pattern of intense staining was observed at 3 and 7 days after injury and coincided with marked infiltration of macrophages (Fig. 1).
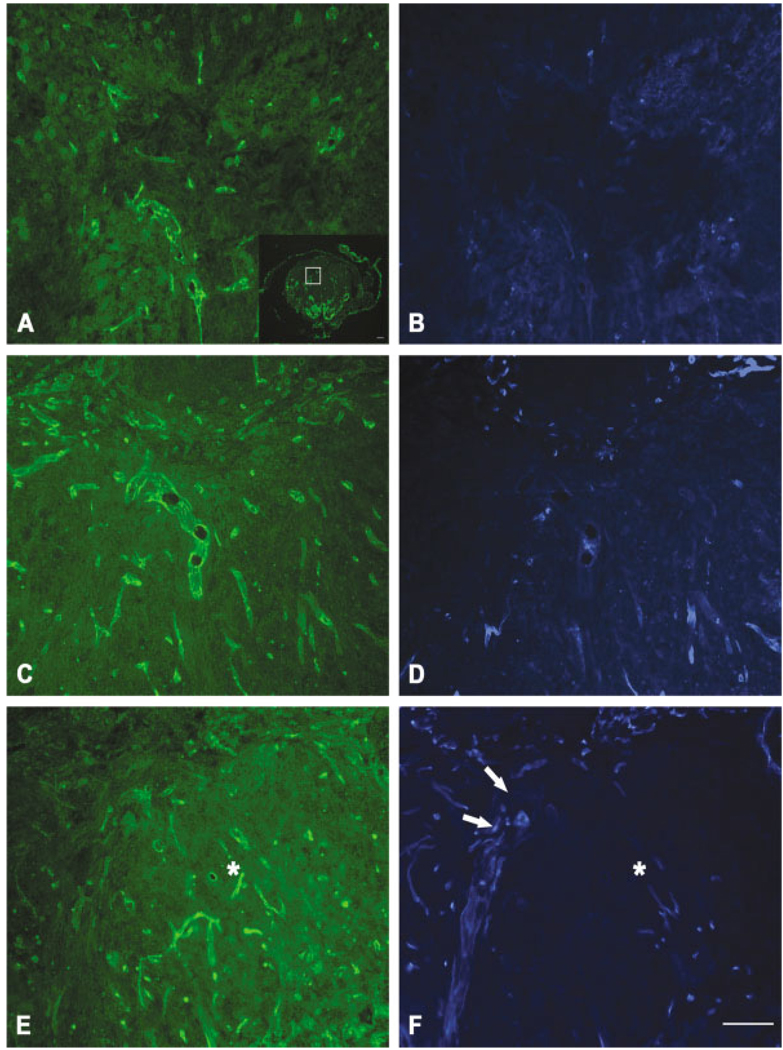
Fig. 1.
Distribution of Evans blue albumin (EBA, A–E) and immuno-localization of CD11b (F) after spinal cord injury. Dark-field image of a control (injured) spinal cord (A). Enclosed area denotes pericentral gray matter used for assessment of EBA extravasation in uninjured cord (B) and at 1 (C), 3 (D), and 7 (E) days after spinal cord injury. No extravasation is noted in control, uninjured spinal cord (B). There is both a diffuse and a cellular pattern of EBA fluorescence within the epicenter at 1 (C), 3 (D), and 7 (E) days postinjury. Inset (E) is shown at higher magnification in F, which has been immunolabeled for macrophages. Scale bars = 100 µm.
Spinal Cord Injury Results in Biphasic, Temporal Pattern of Blood-Spinal Cord Barrier Leakage to Luciferase
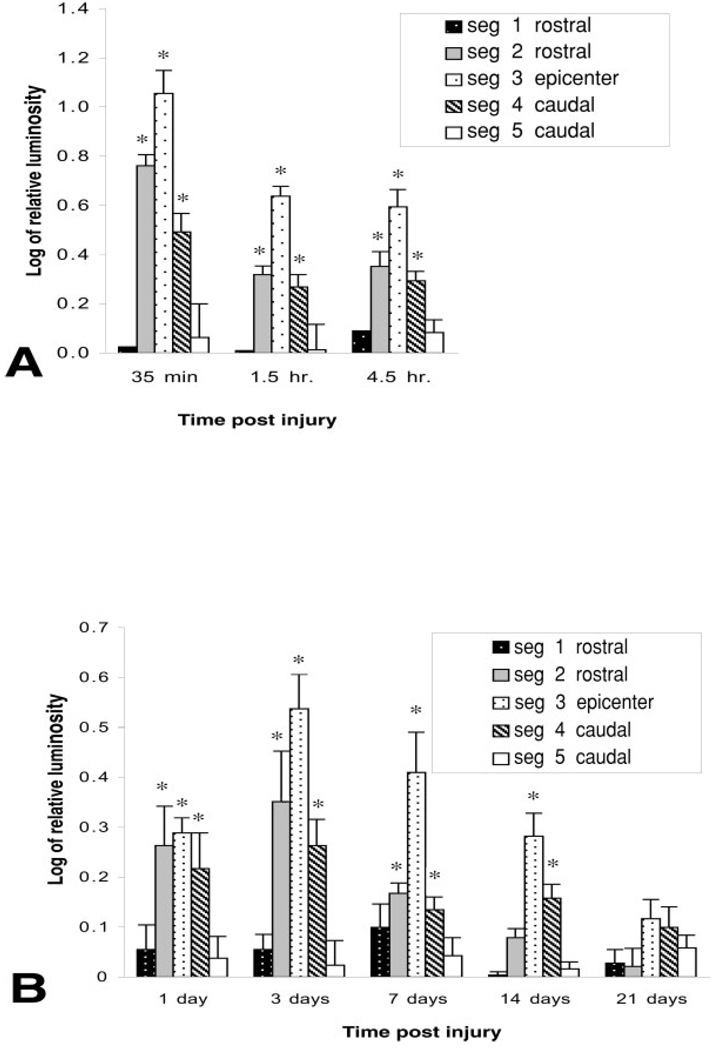
Luminosity was compared in samples prepared from the cervical regions of injured animals with samples prepared from naïve, uninjured cords. There were no significant differences between these groups. Cervical samples were therefore chosen as an internal control for these studies. The most pronounced leakage to luciferase occurred within the first 35 min after injury followed by a significant reduction, relative to this value, thereafter (Fig. 2). Although there was a gradual decline in leakage to luciferase within the first 24 hr after injury, values remained significantly elevated relative to controls. A second peak of abnormal barrier permeability occurred at 3 days after injury and significantly exceeded that observed at 24 hr. Although values declined thereafter, they remained significantly elevated relative to controls up to 14 days postinjury. No significant leakage was noted by 21 days after injury.
Fig. 2.
Time course of blood-spinal cord barrier leakage to luciferase. A 15-mm length of cord, centered over impact site, was divided into five segments of equal lengths. Luciferase activity was determined in rostral segments (seg 1 and seg 2), epicenter (epi), and caudal segments (seg 4 and seg 5). Epicenter of injury demonstrates biphasic permeability. The most overt leakage occurs in the first 35 min after injury. Although there is a decline in leakage by 24 hr postinjury, a significant increase in permeability, relative to the 24-hr point, occurs by 3 days after injury**. Values decline thereafter but remain significantly elevated, relative to controls, up to 14 days after injury*. Significant leakage to luciferase occurs in both rostral and caudal segments, adjacent to the epicenter, within the first 3 days after injury. Whereas the barrier to luciferase is restored in rostral segments between 7 and 14 days postinjury, the caudal segment, adjacent to the epicenter, remains significantly permeable until 21 days after injury. Values represent mean ± SEM. *P < 0.05 compared to controls. F-value, 9.64; df = 5. **P < 0.05 as compared to 24-hr point.
Barrier Leakage to Luciferase Extends Along the Cord Axis
Barrier leakage was not restricted to the epicenter, but rather extended into segments that were within 6 mm rostral and caudal to the epicenter (Fig. 2). This axial leakage was most apparent within the first 4.5 hr after injury and remained significantly elevated by 3 days postinjury. The barrier was re-established between Days 7 and 14 in rostral segments. In contrast, there was a delay in restoration of the barrier, caudal to the spinal cord injury, with the caudal segment remaining significantly permeable, and returning to control values by 21 days, as reported by others (Noble and Wrathall, 1987).
Revascularization in Injured Cord Occurs by 7 Days After Injury
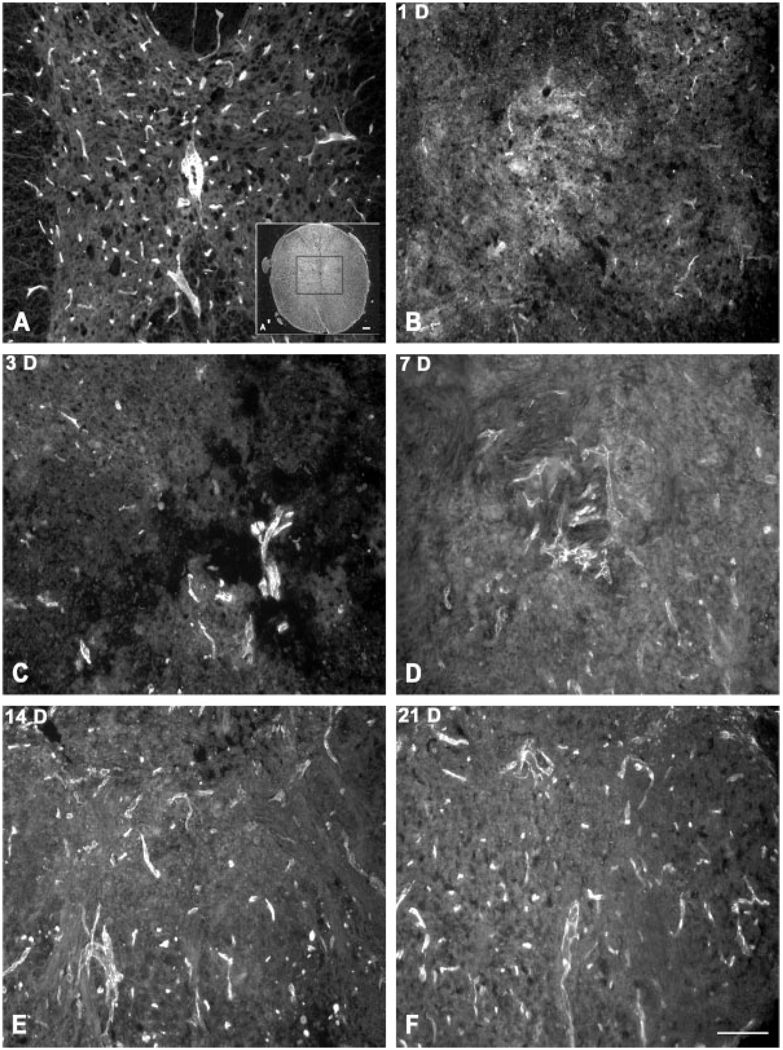
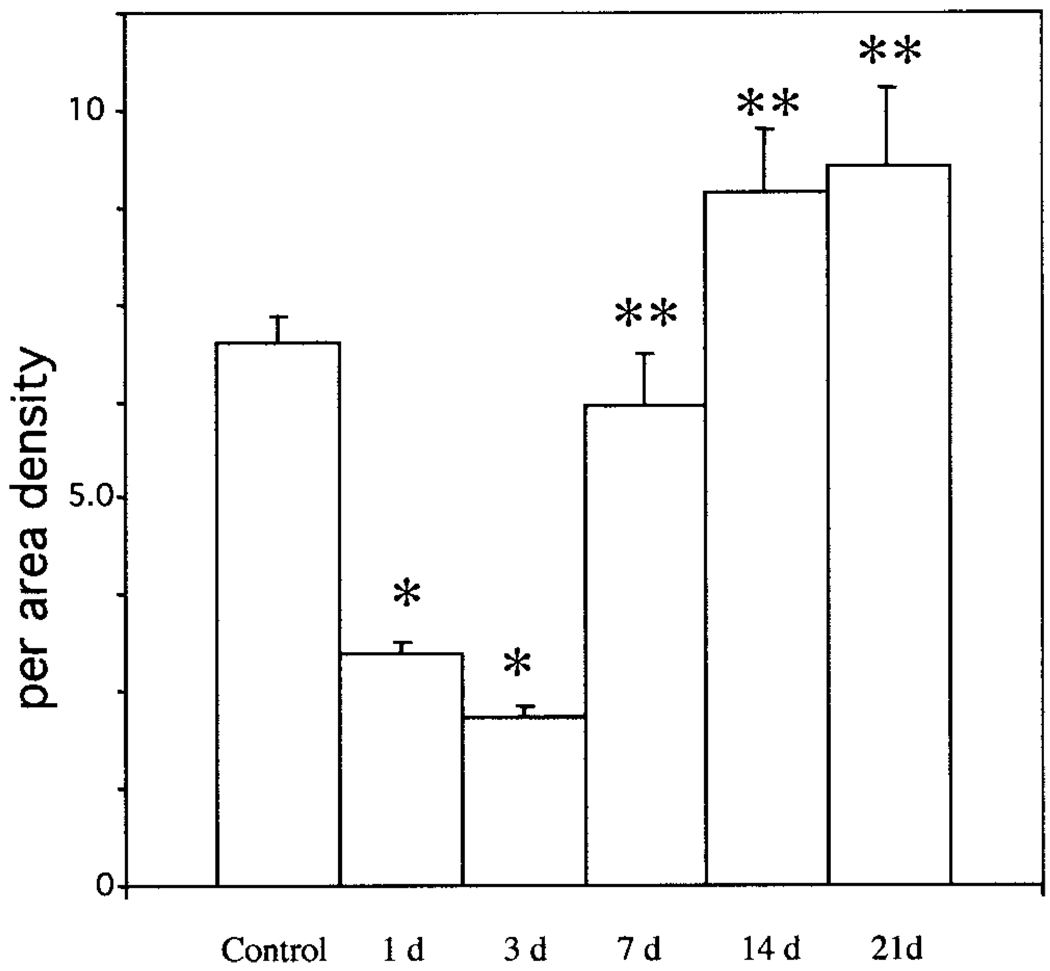
There was a significant loss of PECAM-1-labeled blood vessels by 1 and 3 days postinjury (Fig. 3, Fig. 4) whereas a significant increase in the numbers of PECAM-1-labeled vessels was observed between 3 days and 7 days after injury returning to control values by 7 days after injury (Fig. 4). Thereafter, the number of PECAM-1-labeled vessels remained similar to that observed in the controls.
Fig. 3.
Immunolocalization of PECAM-1-labeled blood vessels within the lesioned epicenter. PECAM-1-labeled vessels are distributed throughout uninjured spinal cord (A). The central gray matter, as outlined in the inset (A′), was selected for analysis of PECAM-1-labeled vessels after spinal cord injury (B–F). There is an overt loss in PECAM-1-labeled vessels by 1 day after injury. Large, PECAM-1-positive vessels are apparent, although limited in number, by 3 (C) and 7 (D) days postinjury. Both large and small diameter vessels are identified at 14 (E) and 21 (F) days after injury. Scale bar = 100 µm.
Fig. 4.
Revascularization after spinal cord injury. PECAM-1-positive blood vessels were quantified in uninjured and injured spinal cord. There is a significant loss of blood vessels in the first 3 days after injury*. Revascularization is most prominent between 3 and 7 days postinjury, with a significant increase in the number of vessels by 7 days compared to the 3-day point**. Although there is a modest increase in the number of vessels at 14 and 21 days postinjury, these values are not statistically different from that of controls. Values represent mean per-area densities ± SEM. *P < 0.01 as compared to controls; **P < 0.01 as compared to the 3-day point.
Re-Establishment of Glut-1 During Revascularization Coincides With Regional Expression of Reactive Gliosis
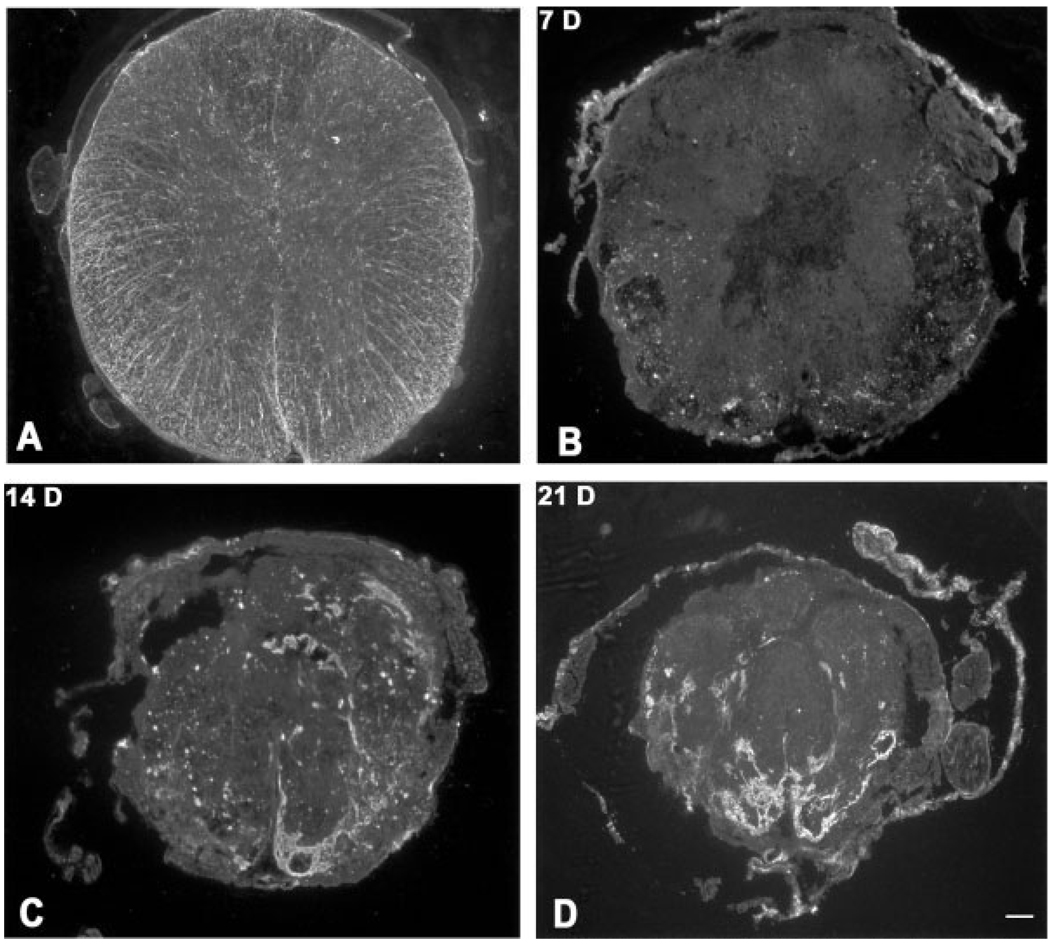
There was marked dissolution of tissue and a blurring of the gray-white matter border at the epicenter within the first 3 days after injury (Fig. 5). Astrocytes were restricted primarily to the outer 2/3 of the white matter at 3 days postinjury, and by 7 days there was a pronounced loss of GFAP-labeled astrocytes (Fig. 6). Between 7 and 14 days postinjury, there was an overt reorganization of the epicenter into domains, which were particularly obvious under dark-field optics (Fig. 5). Each domain appeared as large, bright structures, surrounded by dense bands under dark-field optics. We next examined the temporal expression of Glut-1 (Fig. 7). There was a pronounced loss of Glut-l-labeled vessels within the first 7 days postinjury. At 7 days after injury, the majority of vessels within the healing cord were PECAM-1-positive. Glut-1-labeled vessels were apparent by 14 days after injury. It is notable, however, that there seemed to be more PECAM-1- than Glut-1-labeled vessels. To explore further this finding, sections were double immunolabeled with PECAM-1 and Glut-1 (Fig. 7). Whereas most Glut-1 vessels were PECAM-1-positive, not all PECAM-1-labeled vessels co-expressed Glut-1. By 14 days postinjury, PECAM-1-labeled vessels were noted throughout the epicenter. In contrast, Glut-1-positive vessels seemed restricted primarily to the perimeter of domains. With progressively increasing numbers of vessels during wound healing, this heterogeneity in the vascular expression of Glut-1 became more marked at 21 days after injury.
Fig. 5.
Formation of heterodomains during wound healing after spinal cord injury. Dark-field optics define the unusual reorganization pattern within the lesioned epicenter. Distinct organization of gray and white matter in control cord (A) contrasts dissolution of tissue at 1 (B) and 3 days (C) after injury. Between 3 (C) and 7 (D) days after injury, dark central homogenous zones become evident. Between 14 (E) and 21 (F) days after injury, the cord is reorganized into bright zones that are surrounded by dark bands. Scale bar = 100 µm.
Fig. 6.
Evolution of glial scarring after injury. Immunolocalization of GFAP-positive astrocytes in the control cord (A) and at 7 (B), 14 (C), and 21 (D) days postinjury. There is an overt loss of GFAP-labeled astrocytes in the first week after injury. Thereafter, astrocytes reorganize into distinct dense bands that segregate the cord into distinct domains. Scale bar = 100 µm.
Fig. 7.
Double immunolabeling for PECAM-1 (A,C,E) and Glut-1 (B,D,F) at 7 (A,B), 14 (C,D), and 21 (E,F) days postinjury. Inset is higher magnification of area in 21-day postinjury cord labeled for GFAP. There seems to be an increase in PECAM-1-labeled vessels between 7 and 14 days after injury. Glut-1-labeled vessels also seem to increase with time but are evident in a more restricted distribution. Only limited numbers of vessels are labeled with both PECAM-1 and Glut-1. By 21 days after injury, PECAM-1-labeled vessels are noted throughout a domain (E, asterisk) whereas Glut-1 labeling is restricted to the perimeter (arrows) surrounding the domain (F, asterisk). Scale bar = 100 µm.
The preferential localization of Glut-1-labeled vessels in the perimeter of the domains was strikingly similar to astrocyte distribution during wound healing. We double immunolabeled sections for both astrocytes and Glut-1 to explore further this relationship (Fig. 8). Astrocytes were confined primarily to the dense bands that surrounded the perimeter of each domain (Fig. 8), with a few exceptions observed within the domain proper. As documented by confocal microscopy, most Glut-1-positive vessels were affiliated closely with astrocyte-rich regions and, with few exceptions, were largely absent in areas devoid of GFAP staining, including the central-most part of each domain (Fig. 8). Taken together, these data suggest that Glut-1 expression in blood vessels during wound healing is dependent on the presence of astrocytes.
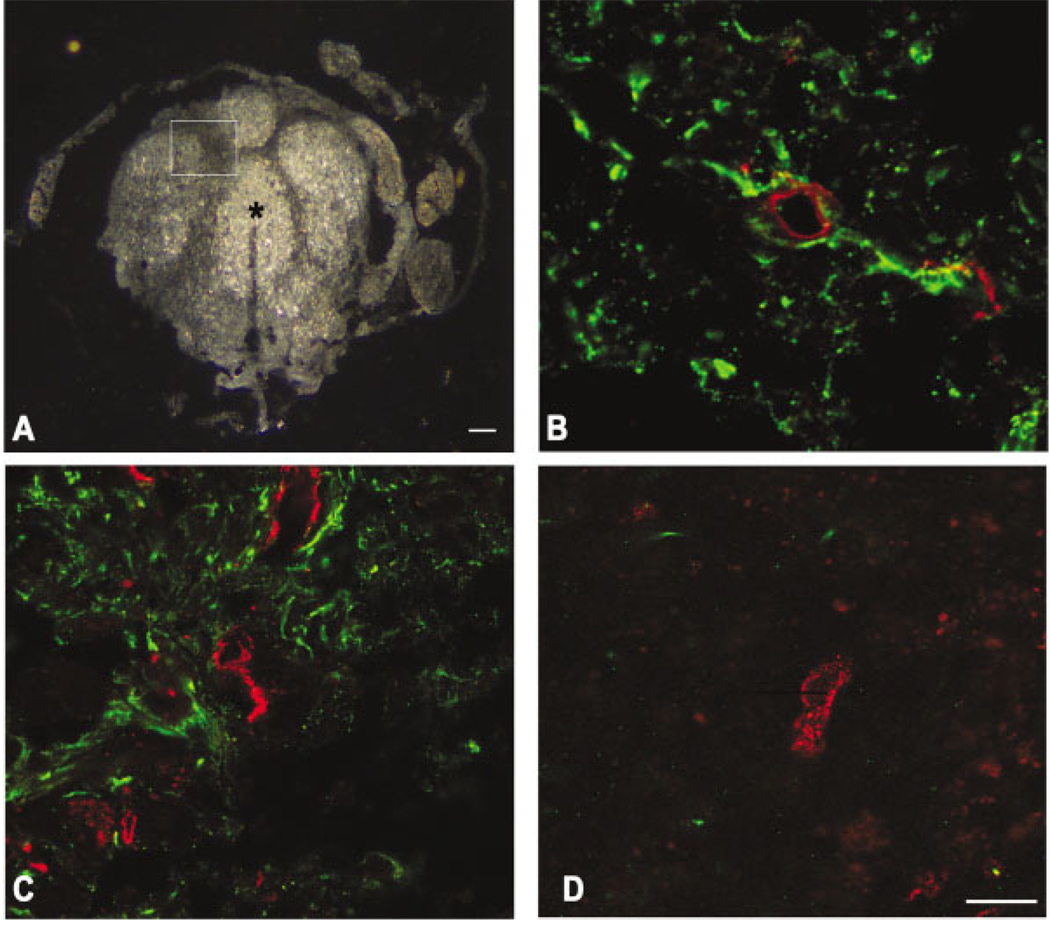
Fig. 8.
Relationship of vessels to astrocytes by confocal microscopy at 21 days postinjury. Heterodomains are evident by dark-field microscopy (A). Enclosed area and the center of a domain (asterisk) are shown at higher magnification in C and D, respectively. In a section rostral to the epicenter, a PECAM-1-labeled vessel (red) is surrounded by astrocytic processes (green, B). Similarly, at the injury epicenter, Glut-1-labeled vessels (red) are in close proximity to astrocytes (C) within the perimeter of the domain (dark band of inset, A). These findings contrast the center of a domain (asterisk in A) mostly void of astrocytes and is composed of vessels that are predominately PECAM-1 positive (red, D). Scale bars = 100 µm (A); 10 µm (B–D).
DISCUSSION
We report that spinal cord injury produces a biphasic opening of the barrier. The first peak of abnormal leakage occurs within the first several hours after injury, whereas a second peak is evident between 3 and 7 days postinjury, a period corresponding to significant revascularization of the injured cord. We show further a heterogeneous expression of barrier properties in regenerating vessels. Those vessels that are in close proximity to astrocytes express Glut-1 whereas vessels lacking this glial proximity do not express this transporter. Together, these data suggest that during wound healing after spinal cord injury, regenerating blood vessels do not exhibit typical barrier properties, and in fact the expression of these properties may be dictated by molecular cues generated by neighboring cells, including astrocytes. This is the first report that defines and characterizes barrier permeability in injured and regenerating blood vessels after spinal cord injury in the mouse.
Luciferase as a Marker of Barrier Integrity
We offer the first quantitative description of the temporal profile of barrier disruption to luciferase, an enzyme similar in molecular dimensions to albumin, in both injured and regenerating vessels of contused murine spinal cord. Luciferase has been shown recently to be a quantifiable and sensitive indicator of barrier function in both brain (Deng et al., 1998) and contused murine spinal cord (Noble et al., 2002). Tissue levels of luciferase can be quantified via enzymatic oxidation of its substrate luciferin in a light-emitting reaction. This assay is advantageous over other methods, in that it can be detected at very low levels. In addition, its measurement is not obscured by tissue factors such as hemorrhage, which can confound the interpretation of colorimetric protein detection methods (Noble et al., 2002).
Blood-Spinal Cord Barrier After Injury
Tracer studies
Abnormal barrier leakage to luciferase occurred throughout the first 14 days after injury with re-establishment of the barrier to this protein by 21 days. Others have evaluated blood-spinal cord barrier permeability after contusion in the rat using gadopentetate-dimeglumine Gd for magnetic resonance imaging (MRI) (Bilgen et al., 2001), relatively small molecular tracers such as C14 aminoisobutyric acid (Popovich et al., 1996), and proteins including horseradish peroxidase (Noble and Wrathall, 1989) that are similar in molecular dimensions to albumin and luciferase. There is evidence that the time course for barrier leakage to these tracers may be size dependent. For example, abnormal permeability to C14-labeled aminoisobutyric acid is maintained for at least 28 days after injury whereas permeability to horseradish peroxidase is restricted to the first 14 days postinjury. Our findings parallel that of others (Noble and Wrathall, 1987, 1989) and suggest that the time course of barrier disruption to large proteins after contusion injury is similar in both rats and mice.
Biphasic barrier permeability
Leakage of the barrier to luciferase after spinal cord injury exhibits a biphasic temporal pattern. The most robust leakage occurs within the first several hours after injury and likely reflects direct damage to blood vessels. By 24 hr postinjury, there is a significant reduction in leakage relative to the earlier time points. At 3 days after injury, however, there is a significant increase in leakage relative to that measured at 24 hr. This more delayed time period has been shown by others to coincide with loss of expression of endothelial barrier antigen, an indicator of barrier dysfunction (Perdiki et al., 1998).
Inflammation may contribute to the more delayed second phase of barrier breakdown to proteins after spinal cord injury. In support of this possibility is the observation that macrophage recruitment and infiltration increase markedly at 3 days after spinal cord injury in the rat (Schwab et al., 2001). Activated macrophages produce a variety of factors that influence barrier function, including cytokines such as interferon α, TNF α, interleukins-1, -4, and-13, transforming growth factor α, and β, and vascular endothelial growth factor. These cytokines can alter the integrity of tight junctional proteins or influence cytoskeletal proteins (Hunt et al., 1984; Nathan, 1987; Fitch and Silver, 1997; Gloor et al., 2001). In addition, delayed barrier permeability 14–28 days postinjury has been demonstrated to colocalize with zones of immunohistochemically defined microglial clusters (Popovich et al., 1996; Jensen et al., 1999).
The delayed abnormal barrier permeability observed from (Jensen et al., 1999) 3 to 14 days after injury might reflect unique biologic properties of vessels during revascularization. We show that this abnormal permeability occurs in the face of significant revascularization after spinal cord injury. Similarly, 7 days after contusion injury in the rat, an angiogenic response occurs within gray matter, evolves into remaining white matter, diminishes, and disappears between Days 28 and 60 concurrent with cystic cavity formation (Casella et al., 2002; Loy et al., 2002).
It is not surprising that permeability is increased to luciferase during revascularization. Microvascular hyperpermeability accompanies angiogenesis (Griffiths et al., 1978; Nag et al., 2002). This microvascular permeability plays a critical role in promoting angiogenesis by generating a matrix, formed from the deposition of plasma proteins in the extravascular space, that supports ingrowth of new blood vessels (Dvorak et al., 1995). Thus, the prolonged permeability noted after spinal cord injury might simply be inherent to normal wound-healing/revascularization response.
Luciferase Permeability Versus Glut-1 Expression
Our findings demonstrate an early loss of Glut-1 immunoexpression when the vasculature is hyperpermeable to luciferase. Re-establishment of the barrier to proteins such as luciferase, however, does not coincide with restoration of Glut-1 expression in the revascularized spinal cord. This suggests that the functionality of the vasculature is impaired or altered to reflect the local environment. Glut-1 is critical in maintaining adequate glucose levels for normal neuronal function. Thus, absence or reduction of this transporter may impair neuronal function; however, the most overt absence of Glut-1 occurs in regions of the cord dominated by nonneuronal elements, including macrophages (Ma et al., 2001). Altered expression of the transporter may therefore reflect changing metabolic requirements of nonneuronal cells involved in wound healing. Glucose is a critical metabolic substrate for macrophages and there is evidence that macrophages increase glucose uptake in response to thermal injury or sepsis (Gamelli et al., 1996). The functional consequences of reduced vascular Glut-1 expression on macrophage function in injured spinal cord remain unclear.
Astrocytes and Revascularization of Injured Spinal Cord
Histopathologic changes that accompany spinal injury in a murine model have been described recently (Kuhn and Wrathall, 1998; Ma et al., 2001). Contusion injury generates a central lesion dominated by nonneuronal elements including macrophages and the extracellular matrix proteins laminin, fibronectin, and collagen (Ma et al., 2001). We examined further the wound-healing response in injured spinal cord using dark-field microscopy. During wound healing, the cord is reorganized into bright zones surrounded by dark bands. Each of these bright zones, termed a domain, consists primarily of a large cluster of macrophages. The perimeter of each domain is surrounded by a rim of reactive astrocytes, which appears by dark-field optics as a dense band. This relative absence of astrocytes within regions dominated by macrophages has likewise been reported after contusion and crush injuries to the murine spinal cord (Fujiki et al., 1996; Ma et al., 2001). We found that each domain, including the astrocyte-rich perimeter, was well vascularized. Most blood vessels within each domain did not exhibit Glut-1, however, whereas vessels in the perimeter did express this transporter.
A central question is why vessels within each domain where nonneuronal cells are dominant do not express Glut-1. The answer may lie in the reorganization of the cord in response to wound healing. In particular, astrocytes are confined to the perimeter of each domain whereas revascularization occurs throughout the injured segment. Astrocytes may play a critical role in influencing properties of regrowing vessels. There exists in vitro and in vivo evidence that the blood-brain/spinal cord barrier is dependent at least in part on the influence of perivascular cells, including astrocytes. In vitro studies have demonstrated that high transmembrane electrical resistance (Raub, 1996) and glucose transport (Hurwitz et al., 1993) can be induced in non-CNS endothelial cells. For example, aortic endothelial cells develop high transmembrane electrical resistance and express Glut-1 when co-cultured with astrocytes. (Hayashi et al., 1997; Stanness et al., 1997). Cultured endothelial cells derived from brain express low transmembrane electrical resistance, a feature attributed to their inability to maintain typical tight junctions when exposed to an environment that does not faithfully mimic in vivo conditions (Rubin et al., 1991). There is evidence that astrocytes influence the relative “tightness” of these junctions. If brain-derived endothelial cells are grown in culture media conditioned with astroglial-derived factors, macromolecular movement across the monolayer is reduced by 70%. This restricted movement likely reflects increased effectiveness of interendothelial junctions in limiting transport between adjacent endothelial cells (Prat et al., 2001).
In vivo experiments support further the role of astrocytes in modulating interendothelial movement of large molecules. Peripheral (non-CNS) endothelial cells, when exposed to astrocytes, become less permeable to macromolecules (Janzer and Raff, 1987). The inductive properties of astrocytes have likewise been demonstrated in the CNS. Bush et al. (1999) evaluated barrier permeability to horseradish peroxidase after selective ablation of astrocytes in transgenic mice sensitive to ganciclovir. Ganciclovir-treated transgenic animals exhibited prolonged abnormal barrier permeability to this tracer for at least 35 days after stab injury. In contrast, the barrier was re-established by 14 days after brain injury in control mice. Cell grafts of astrocytes, implanted into the injured area of ganciclovir-treated transgenic mice at 7 and 14 days postinjury, resulted in re-establishment of the barrier. Together, these data argue strongly in favor of astrocyte-mediated induction of the barrier to circulating proteins. Our findings suggest further that, likewise, astrocytes modulate Glut-1 expression. Within each domain, therefore, the newly formed vessels without Glut-1 expression may possess inadequate barrier function, leading to abnormal permeability at chronic stages after injury.
CONCLUSION
Spinal cord injury produces direct, overt mechanical damage to blood vessels, prolonged disruption of the blood-spinal cord barrier to proteins, and altered Glut-1 expression. It is likely that leakage to protein is integral to wound healing and in fact may reflect ongoing angiogenesis. We found that barrier disruption occurred during revascularization of injured cord. Altered Glut-1 expression coincided with early revascularization of the spinal cord. Re-expression of Glut-1, however, did not parallel restoration of a barrier to luciferase. Rather, Glut-1 re-expression was associated primarily with newly formed blood vessels within astrocyte-rich regions of injured cord. Taken together, our findings demonstrate the complex vascular responses that accompany spinal cord injury and emphasize the dynamic relationship between wound healing and barrier function. These findings establish a framework for defining molecular cues that modulate barrier function.
Acknowledgments
Contract grant sponsor: University of California at San Francisco REAC; Contract grant sponsor: the Dana Foundation; Contract grant sponsor: National Institutes of Health; Contract grant numbers: NS39847, NS39278.
REFERENCES
- Beck DW, Roberts RL, Olson JJ. Glial cells influence membrane-associated enzyme activity at the blood-brain barrier. Brain Res. 1986;381:131–137. doi: 10.1016/0006-8993(86)90700-6. [DOI] [PubMed] [Google Scholar]
- Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood-brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Bilgen M, Abbe R, Narayana PA. Dynamic contrast-enhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn Reson Med. 2001;45:614–622. doi: 10.1002/mrm.1083. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Cancilla PA, Berliner J. Brain endothelial-astrocyte interactions. New York: Raven Press; 1993. [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- DeBault LE. gamma-Glutamyltranspeptidase induction mediated by glial foot process-to endothelium contact in co-culture. Brain Res. 1981;220:432–435. doi: 10.1016/0006-8993(81)91238-5. [DOI] [PubMed] [Google Scholar]
- Deng SX, Panahian N, James H, Gelbard HA, Federoff HJ, Dewhurst S, Epstein LG. Luciferase: a sensitive and quantitative probe for blood-brain barrier disruption. J Neurosci Methods. 1998;83:159–164. doi: 10.1016/s0165-0270(98)00077-6. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Zhang Z, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: activation of macrophages/microglia and astrocytes is delayed in mice carrying a mutation (WldS) that causes delayed Wallerian degeneration. J Comp Neurol. 1996;371:469–484. doi: 10.1002/(SICI)1096-9861(19960729)371:3<469::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gamelli RL, Liu H, He LK, Hofmann CA. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J Leukoc Biol. 1996;59:639–647. doi: 10.1002/jlb.59.5.639. [DOI] [PubMed] [Google Scholar]
- Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 2001;36:258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, McCulloch M, Crawford RA. Ultrastructural appearances of the spinal microvasculature between 12 hours and 5 days after impact injury. Acta Neuropathol (Berl) 1978;43:205–211. doi: 10.1007/BF00691579. [DOI] [PubMed] [Google Scholar]
- Guth L, Zhang Z, Steward O. The unique histopathological responses of the injured spinal cord. Implications for neuroprotective therapy. Ann N Y Acad Sci. 1999;890:366–384. doi: 10.1111/j.1749-6632.1999.tb08017.x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- Hunt TK, Knighton DR, Thakral KK, Goodson WH, 3rd, Andrews WS. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96:48–54. [PubMed] [Google Scholar]
- Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood-brain barrier specific proteins by autologous endothelial cells. Brain Res. 1993;625:238–243. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Hegelund IV, Poulsen FR, Owens T, Zimmer J, Finsen B. Microglial reactivity correlates to the density and the myelination of the anterogradely degenerating axons and terminals following perforant path denervation of the mouse fascia dentata. Neuroscience. 1999;93:507–518. doi: 10.1016/s0306-4522(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Kuhn PL, Wrathall JR. A mouse model of graded contusive spinal cord injury. J Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–596. [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- Nag S. The blood-brain barrier and cerebral angiogenesis: lessons from the cold-injury model. Trends Mol Med. 2002;8:38–44. doi: 10.1016/s1471-4914(01)02221-3. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. The blood-spinal cord barrier after injury: pattern of vascular events proximal and distal to a transection in the rat. Brain Res. 1987;424:177–188. doi: 10.1016/0006-8993(87)91208-x. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Increase in TNFα transport after SCI is specific for time, region, and type of lesion. Exp Neurol. 2001;170:357–363. doi: 10.1006/exnr.2001.7702. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Molecular cloning and regulation of gene expression of blood-brain barrier glucose transporter. New York: Raven Press; 1993. [Google Scholar]
- Perdiki M, Farooque M, Holtz A, Li GL, Olsson Y. Expression of endothelial barrier antigen immunoreactivity in blood vessels following compression trauma to rat spinal cord. Temporal evolution and relation to the degree of the impact. Acta Neuropathol (Berl) 1998;96:8–12. doi: 10.1007/s004010050854. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford University Press; 1991. [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Rahner-Welsch S, Vogel J, Kuschinsky W. Regional congruence and divergence of glucose transporters (GLUT1) and capillaries in rat brains. J Cereb Blood Flow Metab. 1995;15:681–686. doi: 10.1038/jcbfm.1995.84. [DOI] [PubMed] [Google Scholar]
- Raub TJ. Signal transduction and glial cell modulation of cultured brain microvessel endothelial cell tight junctions. Am J Physiol. 1996;271:C495–C503. doi: 10.1152/ajpcell.1996.271.2.C495. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-rain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Frei E, Klusman I, Schnell L, Schwab ME, Schluesener HJ. AIF-1 expression defines a proliferating and alert microglial/macrophage phenotype following spinal cord injury in rats. J Neuroimmunol. 2001;119:214–222. doi: 10.1016/s0165-5728(01)00375-7. [DOI] [PubMed] [Google Scholar]
- Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997;771:329–342. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- Tio S, Deenen M, Marani E. Astrocyte-mediated induction of alkaline phosphatase activity in human umbilical cord vein endothelium: an in vitro model. Eur J Morphol. 1990;28:289–300. [PubMed] [Google Scholar]