Abstract
The mutation responsible for Hutchinson Gilford Progeria Syndrome (HGPS) causes abnormal nuclear morphology. Previous studies show that free radicals and reactive oxygen species play major roles in the etiology and/or progression of neurodegenerative diseases and aging. This study compares oxidative stress responses between progeric and normal fibroblasts. Our data revealed higher ROS levels in HGPS cells compared to age-matched controls. In response to oxidative challenge, progeric cells showed increased mRNA levels for mitochondrial superoxide dismutase (SOD) and SOD protein content. However, this did not prevent a drop in the ATP content of progeria fibroblasts. Previous studies have shown that declines in human fibroblast ATP levels interfere with programmed cell death and promote necrotic inflammation. Notably, in our investigations the ATP content of progeria fibroblasts was only ~50% of that found in healthy controls. Furthermore, HGPS fibroblast analysis revealed a decrease in total caspase-like proteasome activity and in the levels of two active proteolytic complex subunits (β5 and β7). A number of studies indicate that the molecular mechanisms causing accelerated aging in progeric patients also occur in healthy cells of older individuals. Thus, the results of this study may also help explain some of the cellular changes that accompany normal aging.
Keywords: Progeria, aging, proteasome, oxidized proteins, ATP
Introduction
Children with HGPS, a rare genetically-encoded disorder, appear normal at birth but develop symptoms of accelerated aging, including loss of subcutaneous fat, alopecia, bone density reduction, and poor muscle development during their first year of life (Stewart et al., 2007). They usually die before reaching their teens, most often of myocardial infarction or stroke (Capell et al., 2005).
The disease is caused by a single point mutation in the LMNA gene (1824 →T) that encodes the lamin A protein and results in deletion of 50 amino acids near the C-terminus of prelamin (Liu et al., 2006). The underlying mechanism is thought to be loss of an endoprotease cleavage site required for the lamin A protein’s proteolytic maturation (Eriksson et al., 2003). The final mutant product is a long dysfunctional farnesylated protein known as progerin; this corrupted version of filamentous lamin A is unable to form a proper scaffolding network beneath the eukaryotic cell’s inner nuclear membrane which is critical for both nuclear support and chromatin organization (Gerace and Burke, 1988; Goldman et al., 2002).
Progeria and normal aging have a great many pathologies in common; for example, increasing evidence suggests that post-translational modifications in specific proteins are involved in both conditions (Schoneich, 2006). Importantly, the sustained damage inflicted by endogenously produced oxidants has been strongly implicated as the cause of age-related deficits and may also underlie at least some, if not, most of the pathologies seen in patients with HGPS (Shigenaga et al., 1994).
Cell components suffer a constant bombardment of macromolecule-damaging reactive oxygen species (ROS) and are equipped with an array of defenses designed to protect DNA, proteins, and lipids, but the system is far from perfect (Wang et al., 2006). As a consequence, most cells have mechanisms to rapidly dispose of damaged proteins, including those performed by the well-characterized lysosomes and proteasomes (Ciechanover, 2005; Grune et al., 1997; Hipkiss, 2006). When all defense and repair systems fail, the apoptosis pathway normally takes over, eliminating cells beyond rescue and allowing new, healthy ones to take their place. Without this final selection mechanism, damaged cells accumulate, contributing to the aging process and its associated diseases (Ames et al., 1993).
Mitochondrial electron transport systems consume ~85% of the oxygen used by an average cell and in return generate almost all its energy in the form of ATP. Several studies have shown that mitochondrial integrity declines with age, most likely through accumulated mutations, impairing ATP generation (Miyoshi et al., 2006). This failing energy supply has a negative impact on a number of cell processes, including apoptosis. In order to determine if the same decline in mitochondrial capacity plays a role in HGPS, we examined the differences in mRNA levels and expression of several anti-oxidant enzymes in cultured normal and progeria fibroblasts. ATP concentrations and proteasome activity were also measured.
Our results showed higher amounts of ROS in progeric versus normal fibroblasts. In response to this oxidative stress, the mRNA for mitochondrial SOD increased as did SOD protein levels. However, this response was not sufficient to prevent a drop in the ATP content of the progeric cells, which raises questions about other metabolic activities taking place in HGPS cells and the possibility that many functions are at risk.
Materials and Methods
Reagents
2′, 7′-Dichlorofluorescein diacetate (DCF-DA), Z-Leu-Leu-Glu-7-amido-4-methylcoumarin (LLE-AMC), and lactacystin were purchased from Sigma Aldrich.
Cell Culture
Primary human dermal fibroblasts were cultured in minimum essential medium (MEM) (Invitrogen/GIBCO, Carlsbad, CA) and supplemented with 15% fetal bovine serum (FBS) (Invitrogen), 2 mM L-glutamine, and 1.2% of a penicillin-streptomycin solution (Invitrogen). The primary fibroblast cell lines used for this study were AG08470A (normal young) ordered from Coriell Cell Repositories (Camden, NJ); and HGADFN167 (HGPS), HGADFN003 (HGPS), HGFDFN168 (normal, Father of HGADFN167), and HGMDFN090 (normal, Mother of HGADFN167) from the Progeria Research Foundation (Peabody, MA). These cells are labeled throughout this paper as Control, Patient1, Patient 2, Father, and Mother, respectively
Mouse embryonic fibroblasts (MEFs) were cultured in MEM (Invitrogen/GIBCO) and supplemented with 15% FBS (Invitrogen). These cells were explanted from the transgenic mouse model of the disease that carries the G608G mutated human LMNA gene on a 164-kb bacterial artificial chromosome (BAC) (Varga et al., 2006). Wild type MEFs (465K and 330B) served as controls for MEFs containing either one (465G and 465B) or two (465F and 330D) copies of the G608G mutation. These cells are labeled throughout this paper as control, one-copy transgene, and two-copy transgene , respectively. Cell numbers were counted using a NucleoCounter (New Brunswick Scientific, Edison, NJ).
Carbonyl Detection
The presence of oxidized proteins was determined using an Oxyblot Oxidized Protein Detection Kit (Millipore, previously Chemicon; Billerica, MA). DNPH (2, 4-dinitrophenyl hydrazine) derivatization was carried out for 15 minutes following the manufacturer’s instructions on 10 µg of protein. One-dimensional electrophoresis of the derivatized samples and controls was carried out on a 4–12% NuPage gel (Invitrogen). Samples were then transferred to PVDF membranes (Invitrogen) and blocked in blocking buffer (LI-COR Biotechnology, Lincoln, NE). The membrane was incubated with anti-DNP antibody overnight followed by washing and incubation with an antimouse IRdye 680 (1/20,000) (LI-COR Biotechnology). The relative abundance of oxidized protein was quantified using the Odyssey Infrared Imaging System.
DCFDA Experiment
Intracellular ROS production was detected using the nonfluorescent compound DCFDA (2′,7′-Dichlorofluorescin diacetate). As it crosses the membrane, the compound undergoes deacetylation by intracellular esterases producing the nonfluorescent DCF that transforms into a highly fluorescent compound during oxidation (Royall and Ischiropoulos, 1993).
For the DCF assay, cells were detached from the culture flask using trypsin (Invitrogen) and 1×106 cells were transferred to a test tube. The volume of the sample was brought to 1 mL using media lacking FBS, and 500 µL of 75 µM DCF solution was added. The mixture was incubated for 10 minutes at room temperature and then centrifuged at 900 rpm for 5 minutes at 4 °C. The supernatant was removed and the pellet washed twice with Hank's balanced salts solution (HBSS) (Invitrogen). Cells were re-suspended in 1mL of HBSS and their fluorescent spectra determined using a PTI fluorometer (Photon Technology International, Trenton, NJ).
Real-time PCR Analysis
Total RNA was extracted from fibroblasts derived from an age-matched control and a patient using TRIzol (Invitrogen). First-strand cDNAs were synthesized using ThermoScript RT-PCR system (Invitrogen), following the manufacturer’s instructions. Quantitative real-time PCR was performed using the 7900H sequence detection system (Applied Biosystems, Foster City, CA). The primers and TaqMan probes used to detect the indicated human genes were purchased from Applied Biosystems: Trx, Hs00828652_m1; Cat, Hs00156308_m1; GPx1, Hs00829989_gH; SOD1, Hs00533490_m1; SOD2, Hs00167309_m1; MsrA, Hs00737165_m1; Actin, 4333762T.
Proteasome Activity Measurements in Fibroblasts
Cells were harvested using trypsin and the pellet was washed twice with cold PBS and frozen at −80°C. To process the samples, pellets were thawed with 200 µL of 50 mM HEPES buffer pH 7.8, vortexed, and disrupted using a Kontes pellet pestle mortar keeping the samples in ice during disruption. After a 20 minute centrifugation at 14000 rpm, the protein concentration of the supernatant was measured by the bicinchoninic acid method (BCA) using BSA as a standard. To perform the proteasome activity measurements, 10 µg of soluble protein were mixed with a 100 µM concentration of the fluorogenic substrates LLE-AMC for the caspase-like activity in a total volume of 200 µL. Separate samples were incubated with 1mM lactacystin, a proteasome inhibitor. The mixtures were incubated in the dark at 37°C for 1 hour. After the incubation period, the samples were read with excitation at 380 nm and emission at 460 nm. The measurements were performed in triplicate. Proteasome activity was calculated as the difference between samples with and without lactacystin.
ATP Measurements
Intracellular ATP content was measured using an ATP Bioluminescence Assay kit HS II (Roche Applied Science, Indianapolis, IN). Cells were harvested, counted, and then lysed with the lysis buffer provided in the kit. Fifty microliters from each diluted sample or standard were transferred into a disposable cuvette, and 50 µL of luciferase reagent was added. The light emitted was measured immediately and integrated for 10 s using a TD-20_20 Turner Designs luminometer (Sunnyvale, CA). The blank value (no ATP) was subtracted from each sample’s raw data, and ATP concentrations were calculated from a standard curve.
Cell Proliferation Assay
Water soluble tetrazolium salts were used to measure cell proliferation and viability (Iwaki et al., 1995). This assay detects living cells whose mitochondrial dehydrogenases cleave the tetrazolium compound to formazan. We used the Reagent WST-1 kit from Roche and analyzed the same number of cells as were used for ATP content determination.
SDS PAGE and Western Blotting
Cell pellets of fibroblasts derived from father, mother, an age-matched control, and patients were lysed in the 2× Laemmli sample buffer with 5%β-mercaptoethanol and boiled for 5 min. Protein concentrations were measured using the Bio-Rad RcDc protein assay method. Samples were separated on 10–20% SDS-PAGE gel and transferred to PVDF membrane and probed with antibodies.
Antibodies
The polyclonal anti-manganese superoxide dismutase (MnSOD ) and anti-catalase antibodies were purchased from Fitzgerald (Concord, MA), monoclonal mouse anti-human lamin A+C from Chemicon International, monoclonal anti-beta subunit of 20 S proteasome and polyclonal anti-beta 5 subunit from Biomol International (Plymouth Meeting, PA), monoclonal anti-mitochondrial Hsp70 antibodies (mtHsp70) from ABR-Affinity BioReagents (Golden, CO), and anti-actin antibodies from Sigma (St. Louis, MO).
Results
ROS and Protein Oxidation in Normal vs. Progeria Fibroblasts
The accumulation of ROS-mediated oxidative damage is a key characteristic of aging (Soskic et al., 2008). It is thought to occur because of a shift in the pro-oxidant/anti-oxidant balance in favor of the former, resulting in macromolecular-level impairments, including DNA strand breakage as well as damage to membrane ion transport systems, enzymes, and other proteins, and lipid peroxidation (Cimen, 2008). The accumulation of oxidatively modified proteins is a complex function of several factors, including the 1) rates of ROS formation, 2) levels of antioxidant defenses against ROS, 3) sensitivity of the proteins to oxidation, and 4) repair or elimination of damaged proteins (Stadtman, 2001).
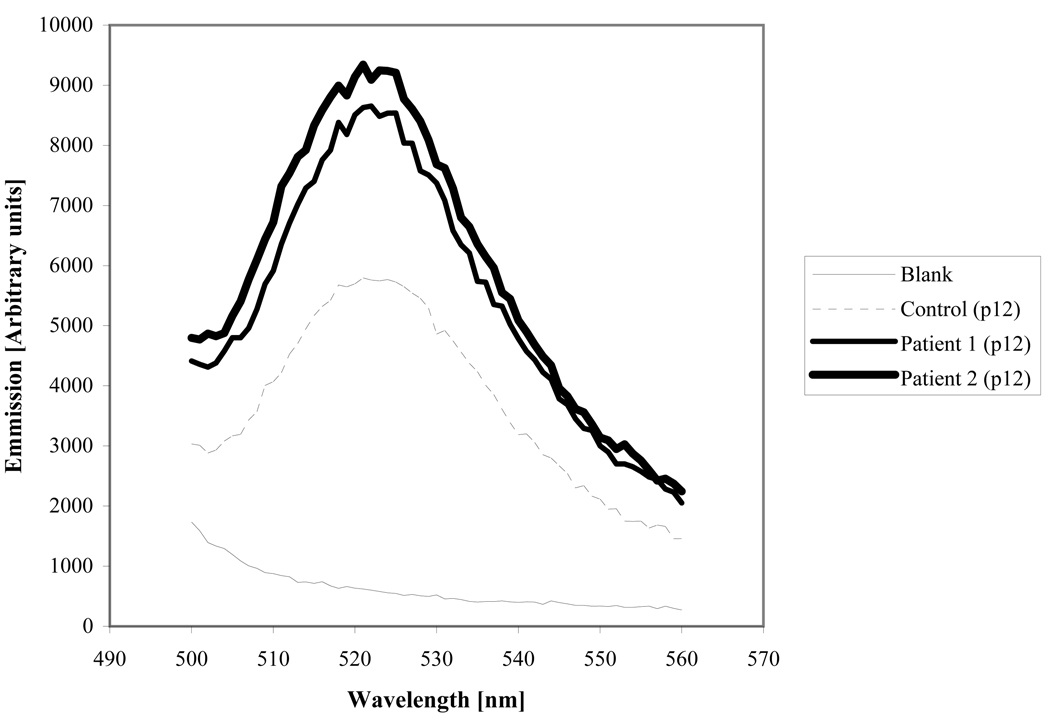
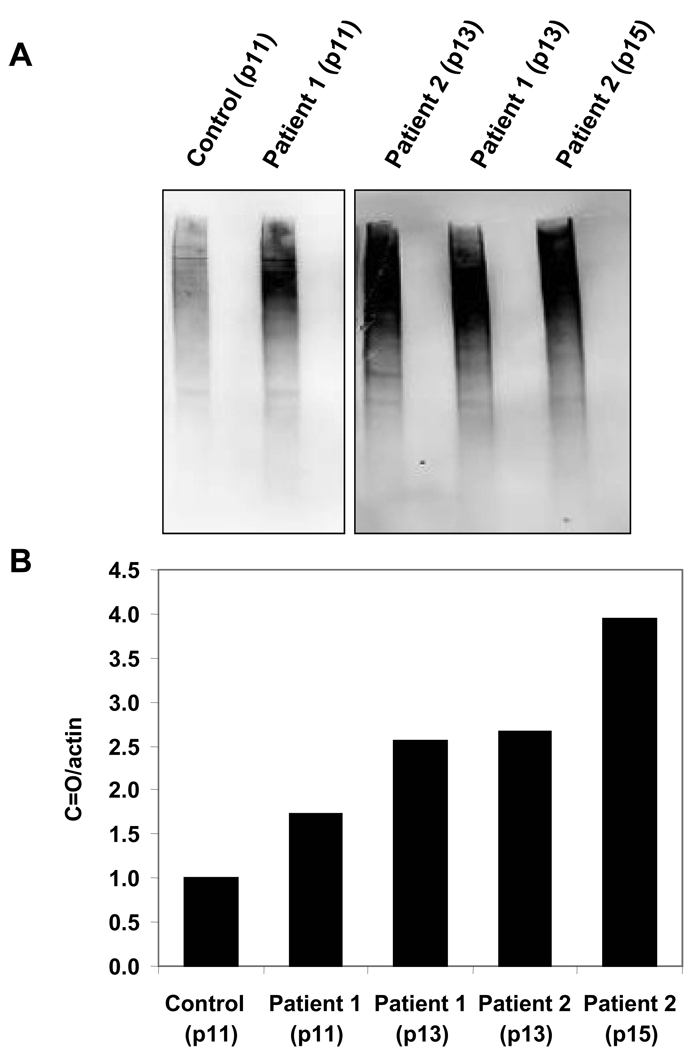
Strikingly, HGPS fibroblasts showed a 1.6-fold increase in DCF fluorescence compared to age-matched controls (Fig. 1). This result proved consistent at various passage numbers. Higher ROS levels caused increased protein oxidation in the progeria samples. Carbonylated protein levels in two different patients’ samples ranged between 1.7 and 4 times that in the age-matched control. The carbonyl content increased with the passage number of the cells, showing an accumulation of protein damage that could not be resolved by cellular repair systems (Fig. 2).
Figure 1.
Fluorescent emission due to intracellular oxidation of DCF-DA by reactive oxygen species in a suspension of human dermal fibroblasts from progeria patients and an age-matched control.
Figure 2.
Levels of oxidized proteins in human dermal fibroblasts from progeria patients and age matched control. A. Carbonyl-containing proteins determined using Western Blot techniques. All samples were processed in duplicate, using one as a control without DNPH treatment to discard non-specific signals. Samples treated with DNPH were loaded to the left of their respective control. B. Relative carbonyl signal intensity of human dermal fibroblasts from progeria patients at different passage numbers and an age-matched control. The carbonyl level of the control was used as a reference for the patients’ samples. Actin was used to normalize the carbonyl presence to the protein concentration in every sample.
Increased MnSOD Content in Progeria
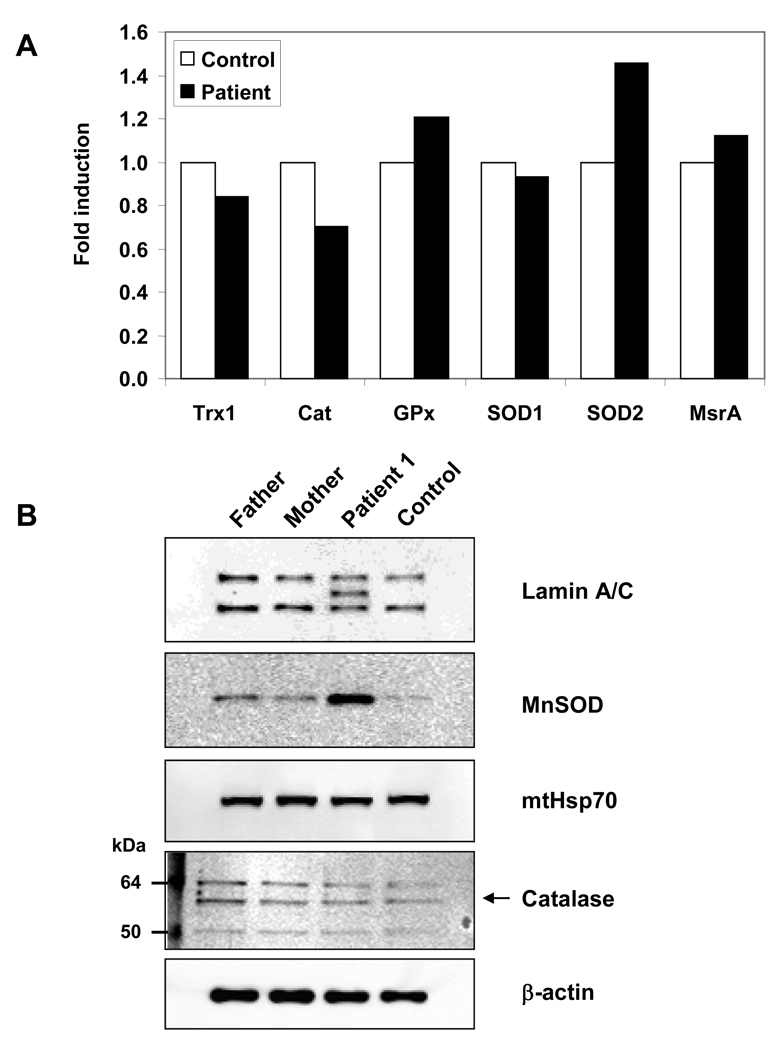
Cells have several antioxidant systems in place to defend themselves against ROS-induced damage and for regulating cellular reduction/oxidation (redox) status. Confronted with high levels of oxidative stress, cells increase both enzymatic and non-enzymatic defense systems designed to reduce ROS concentrations (Maulik and Das, 2008). Because of the slow growth rate of progeria fibroblasts and the reduced cellular protein content, recovering sufficient material for enzyme analysis was not practical and real-time PCR studies were done instead. Results showed an increase in the mRNA levels of MnSOD, but not in the other enzymes screened in our analysis (Fig. 3A). The PCR results were confirmed with Western blots using MnSOD and catalase antibodies. As predicted by real-time PCR, there was a considerable increase in the amount of MnSOD in the HGPS patient’s sample tested and no significant change in catalase. (Fig. 3B).
Figure 3.
mRNA and protein content of antioxidant enzymes in human dermal fibroblasts of a progeria patient and controls A. mRNA expression levels, normalized to that of actin, of thioredoxin1, catalase, glutathione peroxidase, copper-zinc superoxide dismutase, manganese superoxide dismutase, and methionine sulfoxide reductase present in the cell extracts of an age-matched control and a progeria sample. B. Lamin A/C and antioxidant enzymes content in human dermal fibroblasts of progeria patient 1 and three healthy samples including an age-matched control. The arrow points to the catalase band recognized by the antibody.
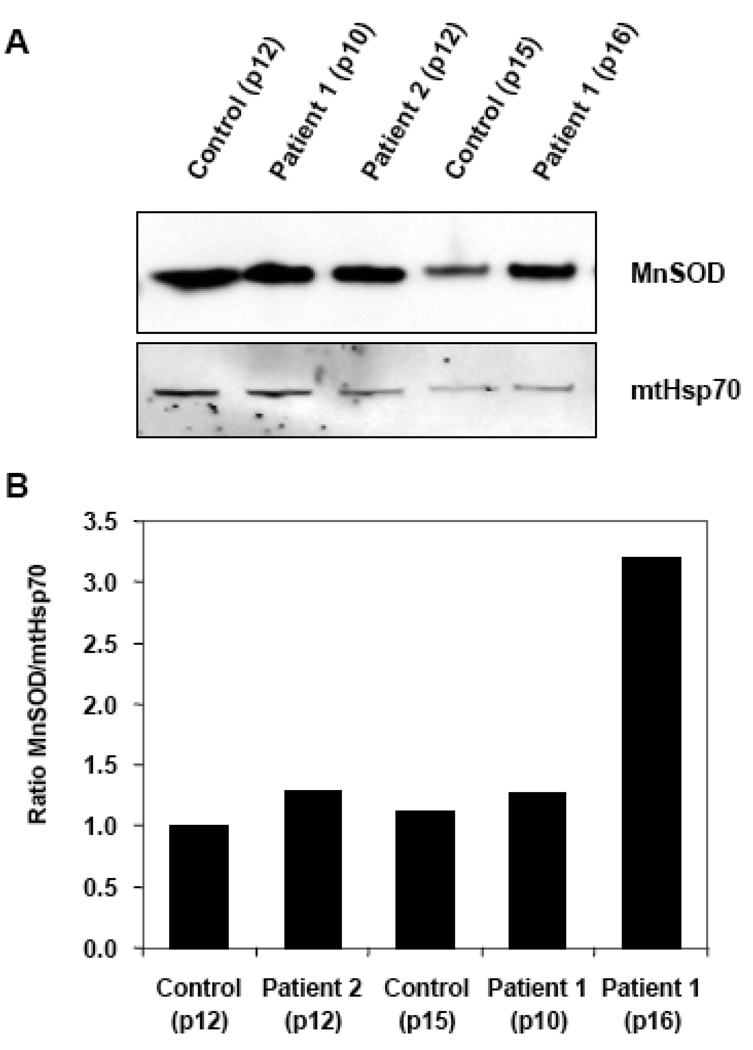
Further Western Blot analysis showed an increase in the relative content of MnSOD in the progeria samples compared to healthy control tissue. The increase became even more robust when the cell passage number was taken into account. For example, the relative MnSOD content of progeria cells from passage 10 to 16 increased 2.5-fold, whereas the control cells were unchanged from passage 12 to 15 (Fig. 4). MnSOD effects results could be more accurately accessed by factoring in the amount of mitochondrial heat shock protein (mtHsp70) to control any differences in cellular mitochondria.
Figure 4.
MnSOD content in progeria patients and age-matched controls A. Western blots of whole cell extracts of control and progeria samples at different passage numbers probed with anti-MnSOD and anti-Hsp70 antibodies. B. Relative expression of MnSOD in progeria and control fibroblast samples analyzed by Western Blot. The enzyme content in the samples was normalized to the Hsp70 content found in them. Other experiments performed using samples at different passage numbers, agreed with the percentages shown.
Decrease of ATP Content in Human Dermal Fibroblasts from Progeria Patients
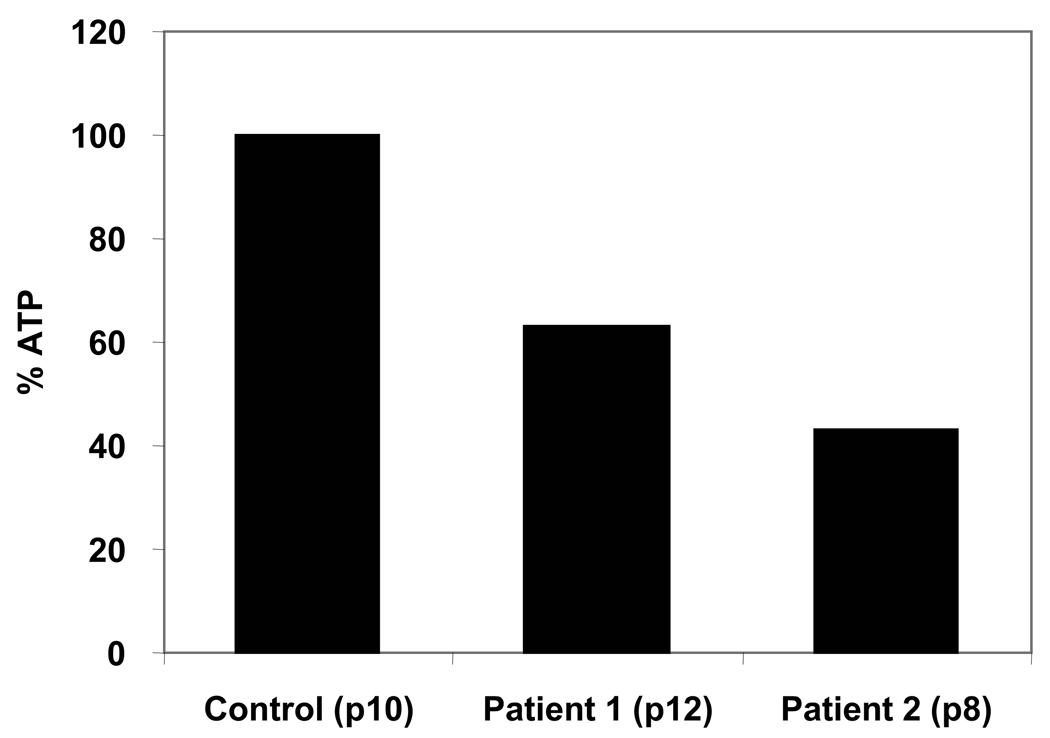
ATP is a major energy source for the majority of cellular processes, including apoptosis. When cells become damaged and nonfunctional, apoptosis provides a mechanism for their elimination, allowing room for their replacement with normal cells (Miyoshi et al., 2006). Impaired apoptotic processes result in the accumulation of damaged cells and contribute significantly to aging (Kurz et al., 2008). Notably, the ATP content of progeria fibroblasts was only ~50% of the levels found in healthy control subjects (Fig. 5). These measurements were repeated several times taking care to confirm the accuracy of mitochondrial function testing (see Materials and Methods section).
Figure 5.
ATP levels of dermal fibroblasts measured as described in Materials and Methods. The measurements were repeated with cells at different passage numbers and the results obtained showed a similar decrease in the ATP content in the cells that agreed within 10% of the ones shown in this figure.
Decrease in Proteasome Activity
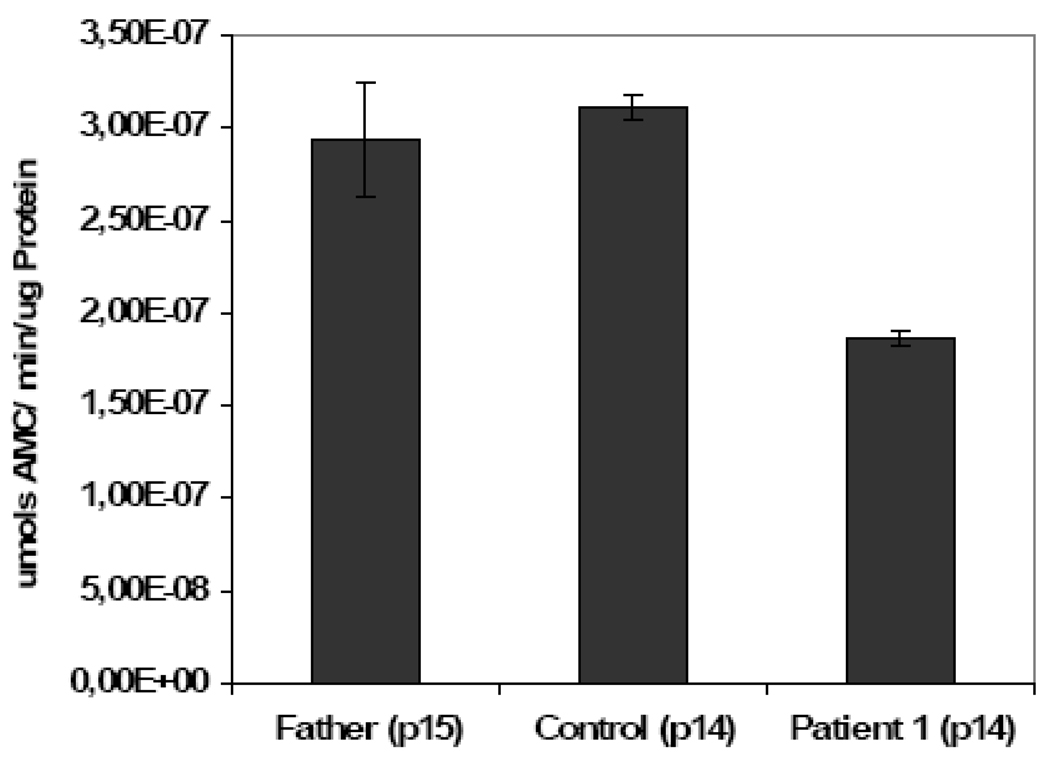
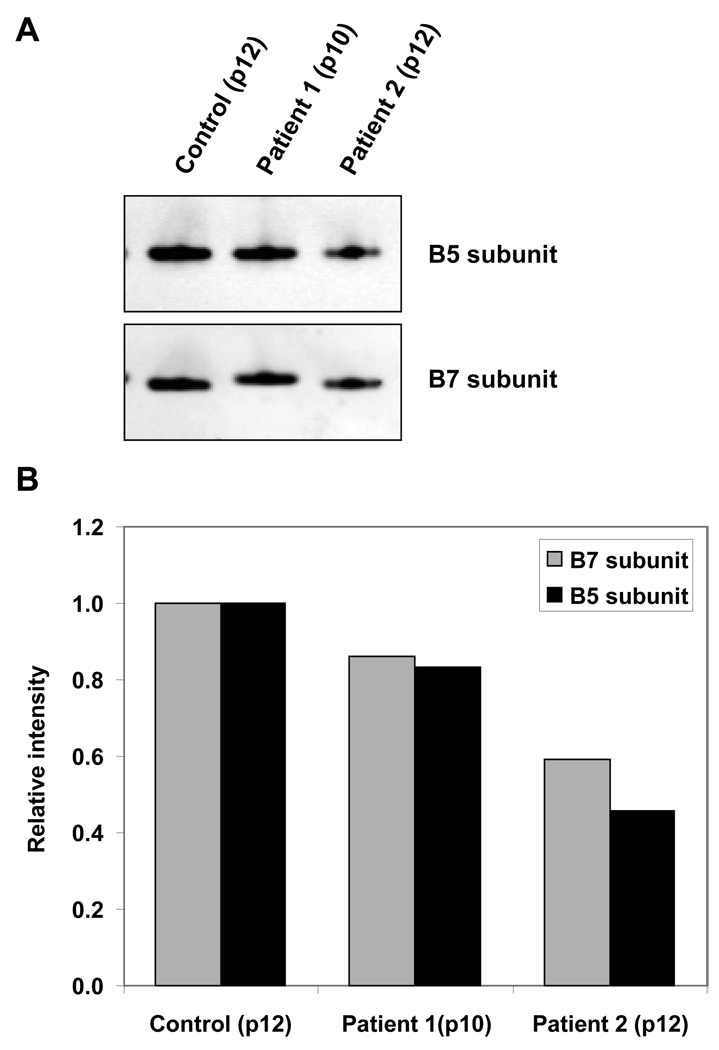
Protein degradation is central to the regulation of many important biological processes, including cell-cycle progression, apoptosis, and DNA repair (Wang et al., 2007). When macromolecules become damaged, the repair of oxidized DNA and degradation of oxidized protein helps maintain cellular integrity. The patient’s fibroblasts showed a 40% decrease in proteasomal caspase-like activity (Fig. 6); a decrease consistent with the accumulation of oxidatively-modified proteins. This decrease could be caused by impairment in the proteolytic activity of the proteasome system itself and/or to a reduced number of proteolytic complexes in the cells (Hwang et al., 2007). The levels of two subunits of the proteasome (β5 and β7) were analyzed with quantitative Western Blots and results showed up to 50% decreases in the levels of both subunits in progeria patients’ samples vs. age-matched healthy control (Fig. 7).
Figure 6.
Proteasome activity measurements in human dermal fibroblasts from controls and progeria patient. Caspase-like activity of the proteasome in the analyzed samples was assayed for 10 µg of protein crude extract using LLE-AMC as described in Materials and Methods.
Figure 7.
Proteasome subunits content in human dermal fibroblasts from progeria patients and control. A. Proteasome subunits β5 and β7 content in 10 µg of protein per sample submitted to Western Blot techniques. B. Relative signal intensity of Western blots probed with anti-β5 and anti-β7 antibodies in human dermal fibroblasts.
ROS and Protein Oxidation in MEFs
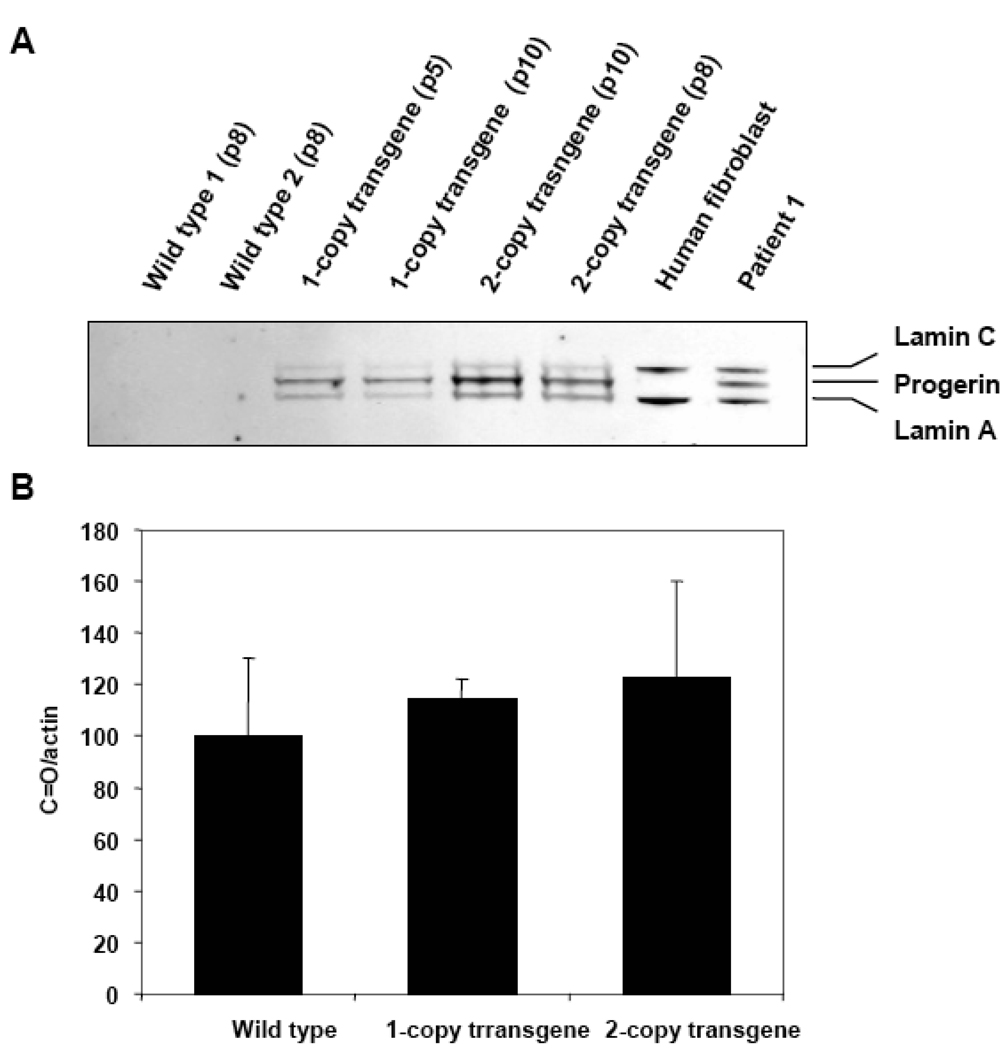
Mouse embryonic fibroblasts (MEFs) explanted from control, 1-copy, and 2-copy progeric transgenic mice [previously characterized (Varga et al., 2006)] were used as a disease model in our studies. The advantage of working with these particular cells is that they allow analysis of early-stage disease as well as clearly demonstrate the effects of progerin on the oxidant-antioxidant cell balance. Samples were obtained by growing the MEFs until passage 10; at which point the cells were collected and their protein extracted. Lamin A-reactive proteins were used to characterize the samples and their presence was determined with Western Blot. The signal, normalized to the amount of actin in the sample, showed a progerin increase in the transfected cells and, as expected, was higher in the two-copy transgene line (Fig. 8A). ROS levels and their effects on protein oxidation were analyzed using DCF-DA and Western Blot on three types of available MEFs. The spectra derived from the MEFs treated with DCF-DA did not show any significant difference in the amount of ROS from the cells despite the presence of progerin (data not shown). The protein carbonylation assay was used to determine the effect of the progerin on the level of oxidized proteins in the cells. Results did not show a significant increase in the oxidatively-modified proteins from cells containing either one or two copies of the defective gene (Fig. 8B).
Figure 8.
Lamin A and C presence in MEFs. A. Characterization of the MEFs samples by the lamin A, lamin C, and progerin expression levels and positive and negative controls. Lanes 1–2: MEF wild type passage 8, lanes 3–4: one-copy transgene MEFs passages 5 and 10, lanes 5–6: two-copy transgene MEFs passages10 and 8, lane 7: human fibroblast control sample (a negative control for progerin), and lane 8: Patient 1 (progerin expressing control). B. Relative carbonyl presence in proteins from mouse embryonic fibroblasts. Oxidized proteins were quantified by densitometry and normalized to the β–actin content in the samples. The oxidation level found for the wild type sample was set at 100% and the other samples were compared to that one.
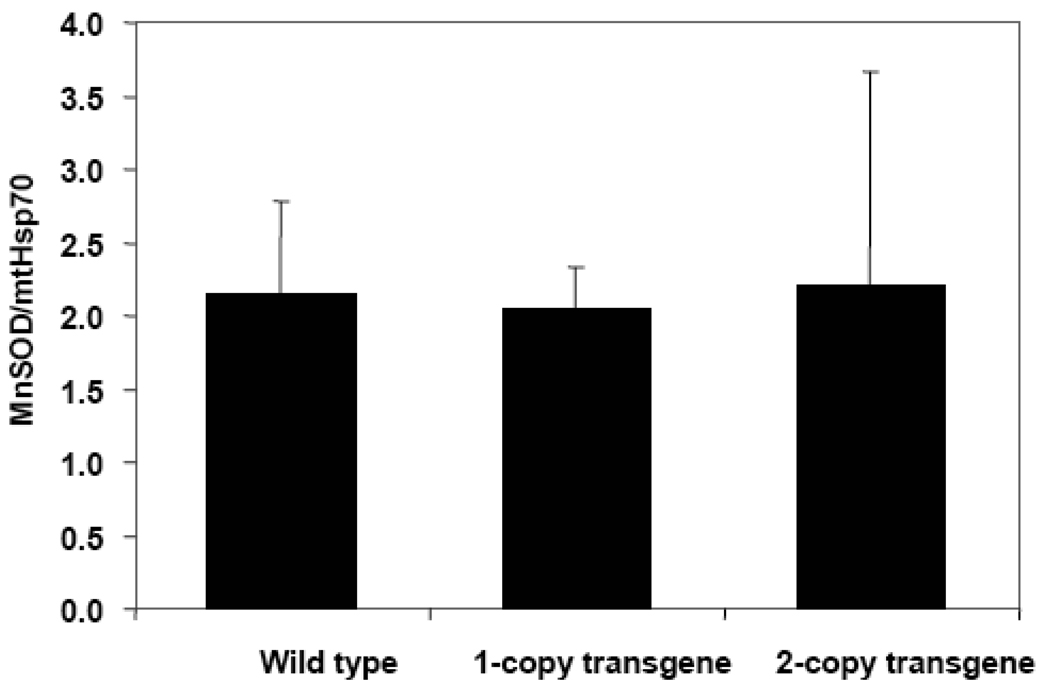
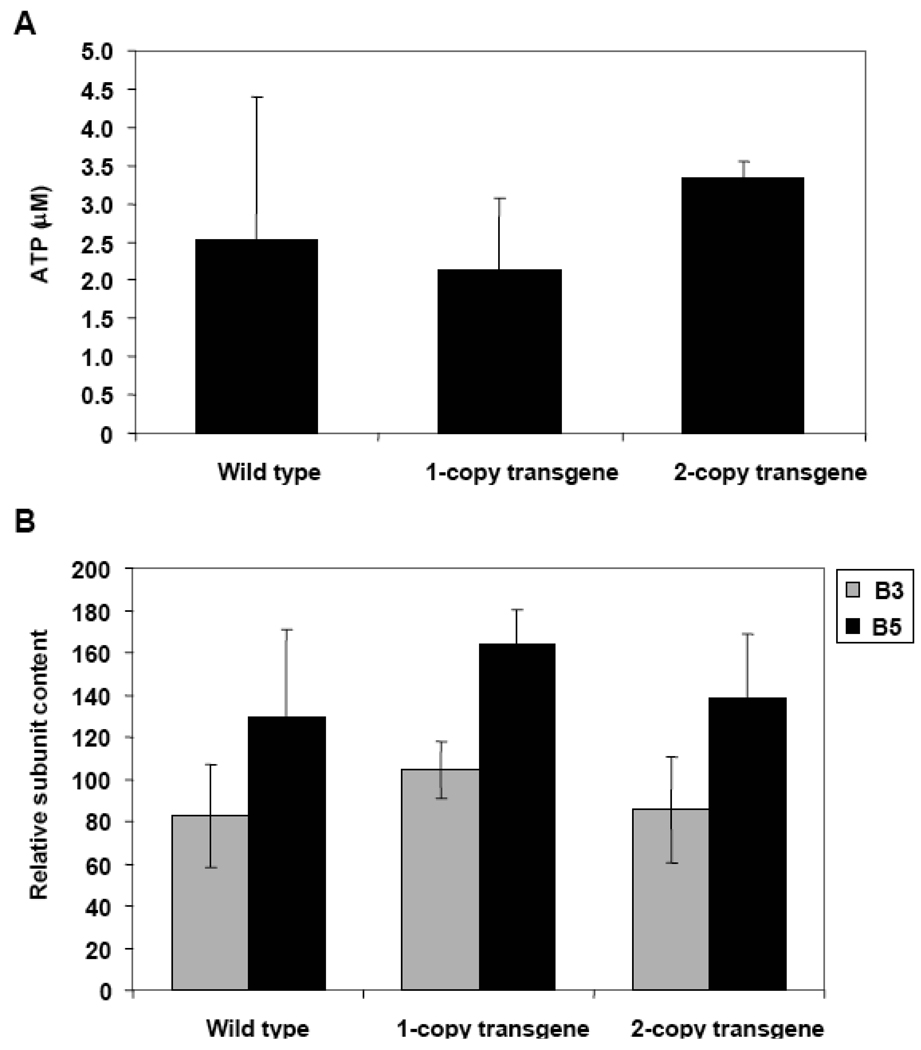
Cell extracts were also analyzed for MnSOD to better understand the effects of progerin in the early stages of HGPS. Western Blot techniques were used and the results were normalized to mtHsp70 content. No significant increases in the MnSOD content accompanied the presence of lamin A and progerin in either one-copy or two-copy transgene MEFs (Fig. 9). These differences between human dermal fibroblasts and MEFs might occur because the former come from already-aged tissue while the latter are taken from embryonic cells. Thus, we decided to age the MEFs and perform additional analyses. Cells were split and grown several times until all reached 30 population doublings. We then confirmed that the aging process had not altered expression of the LMNA with a Western Blot analysis using anti-human lamin A+C antibody (data not shown). The ATP content of the different MEFs at passages close to 30 was analyzed as described in Materials and Methods. No significant decrease in the amount of ATP was found in either the one copy or two copy transgenic MEFs (Fig. 10A).
Figure 9.
Relative content of MnSOD in wild type, one-copy transgene, and two-copy transgene mouse embryonic fibroblasts at passage 10. Samples were analyzed by Western Blot. The enzyme content in the samples was normalized to the Hsp70 content found in them.
Figure 10.
Measurments of ATP and proteasome subunits in MEFs. A. Content of ATP present in wild type, one-copy transgene, and two-copy transgene mouse embryonic fibroblasts at passage 30. The results presented are the average of three measurements performed on the same number of viable cells. B. Relative proteasome subunits content in wild type, one-copy transgene, and two-copy transgene MEFs. The signal from each subunit was normalized to the actin content in the sample.
Additional experiments were done to assess the carbonyl content of the proteins as a measurement of the cellular oxidation processes; MnSOD and proteasome subunit contents were also evaluated. All assessments were done using Western Blot techniques. Results do not show a significant increase in oxidation levels of the cell extracts. In contrast to human fibroblasts, we did not find a significant increase in MnSOD levels nor a decrease in proteasome subunit content in MEFs (Fig. 10B).
Discussion
Previous research consistently demonstrates a progressive, almost exponential increase in oxidatively-damaged proteins, lipids, and nucleic acids during aging, whereas younger people, those under age 60, have relatively stable oxidized protein levels (Oliver et al., 1987; Stadtman, 2001; Miyoshi et al., 2006). In contrast to other young people, an accelerated accumulation of oxidatively-modified proteins, much like those seen in normal aging, has been found in fibroblast samples from progeric patients (Oliver et al., 1987).
Our research focuses on HGPS, considered a “segmental progeroid syndrome” because it incompletely reproduces the aging process, i.e. no increase in some age-associated features including tumor susceptibility, cataract formation, and cognitive degeneration (Stewart et al., 2007). The results of our studies provide new clues to the biochemical changes underlying the pathology in this disorder. DCF-DA experiments, for example, reveal an increased concentration of ROS in fibroblasts from HGPS patients vs. age-matched controls. Increased levels of oxidized proteins in the HGPS samples were also noted, demonstrating that the escalating oxidative assault could not be controlled by the cell’s antioxidant defense mechanisms. And, importantly, cellular ATP levels were decreased by ~50%.
High levels of ROS and protein oxidation require efficient repair and elimination systems to control the damage inflicted on cell components. The proteasomal system is critical to this process, responsible for degrading abnormal and oxidatively-modified proteins (Hwang et al., 2007). It has been shown that proteasomal degradation increases during mild oxidation but decreases at higher levels. In fact, previous research has shown a decrease in proteasome activity and subunit content in aged human epidermal keratinocytes (Petropoulos et al., 2000) and senescent fibroblasts (Chondrogianni et al., 2003). Although the mechanisms for this have been well studied in the aging process (Ahmed et al., 2007; Breusing and Grune, 2008) and age-associated diseases (Di et al., 2007; Shah and Di, 2007), they have not been investigated in progeric cells. Our investigations show a significant decrease in the protein-degrading capability of progeric proteasomes; an impairment that occurs, in part, as a consequence of a decreased number of subunits required to assemble the proteolytic complex. However, direct oxidative damage to the proteasome should not be ruled out (Breusing and Grune, 2008). Several segmental progeroid systems have been related to the ubiquitin-proteasome system, for example through regulation of of LMNA in HGPS by ubiqutinylation (Grillari et al., 2006). Inhibition of the proteasome has been shown to induce mitochondrial dysfunction, increase generation of reactive oxygen species, and increase the levels of oxidatively modified DNA, RNA, and protein. Our observation of increased ROS generation and decreased proteasome activity in progeria fibroblasts provides a mechanistic basis for their increased content of oxidatively modified proteins.
ATP is a key molecule for energy-dependent as well as ATP-mediated signal transduction processes inside the cell; important among them is degradation of targeted proteins by the oxidative-stress sensitive 26S proteasome. Proteasome complexes are abundant in the cell, present in both the nucleus and cytoplasm (Coux et al., 1996). Studies confirm that the ratio of 26S to 20S proteasomes is greater in the nucleus than in the cytosol (Breusing and Grune, 2008). The decrease in ATP content of progeria fibroblasts may negatively impact the ability of nuclear proteasomes to degrade ubiquitinylated proteins. This finding also raises questions about other metabolic activities taking place in HGPS cells. However, this deficit in energy is not sufficient to block apoptotic cell death since it has been shown that blocking this pathway requires a 75% decrease of the ATP content in cells (Lee and Shacter, 1999).
A normal cell has the ability to enzymatically detoxify superoxide radicals. Superoxide dismutase (SOD), along with catalase, glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase help maintain optimal intracellular concentrations of antioxidant defense molecules, glutathione, and NADPH. Both PCR and Western Blot analyses revealed increased mRNA and protein levels for MnSOD in the progeria samples that proved strongly dependent on the cells’ passage number. We used PCR and Western Blot to assess the level of mRNA and the amounts of several antioxidant proteins in the cells of patients with HGPS. A previous study reported contrary results, that is a decreased amount of MnSOD in progeria fibroblast cells (Yan et al., 1999). . We note that Yan et al analyzed a whole cell lysate while we analyzed purified mitochondria and included a loading control.
The different results yielded by MEFs and the ones observed in the patients’ fibroblasts may reflect the embryonic nature of the MEFs, which have not been through the same aging process as human fibroblasts. This belief is reinforced by the clinical course of HGPS-patients appear normal at birth, but soon develop the multiple phenotypes of premature aging (DeBusk, 1972; Yang et al., 2008). Furthermore, MEFs were explanted from the mouse model of HGPS and even though several symptoms of the rodent disease resemble human progeria; for example, progressive loss of vascular smooth muscle cells, elastic fiber breakage, thickening of the adventitia and medial layer, proteoglycans accumulation, and collagen deposition, the mouse does not show all the symptoms experienced by patients with progerias (Varga et al., 2006). It seems likely that human dermal fibroblasts and mouse embryonic fibroblasts do not behave the same way in vitro either. Another possibility is that MEFs require more than the 30 consecutive divisions allowed in our experiments in order to resemble human dermal progeric fibroblasts.
In summary, the results of this study show that HGPS fibroblast cells have a reduced ATP content and an increased presence of MnSOD; there are high levels of ROS and protein oxidation as well as a decrease in active proteasome subunits and total proteasome activity. All of these factors combine to encourage protein modification and accumulation, causing cellular dysfunction.
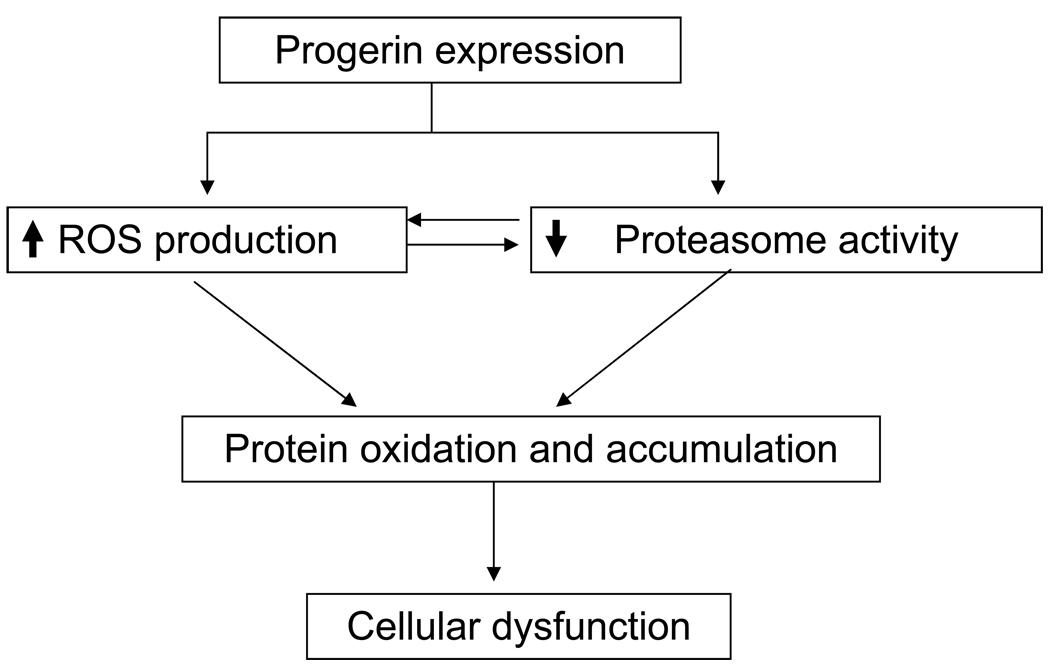
The proposed mechanism for oxidized protein accumulation in HGPS dermal fibroblasts is summarized in Fig. 11. So far, we do not know where and how the ATP decrease fits in the proposed scheme of causality of cellular dysfunction in progeria cells and it has not been included. The mechanism by which progerin expression triggers an ATP decrease and MnSOD overexpression remains unclear and is the focus of continuing research.
Figure 11.
Scheme of the proposed mechanism of modified protein accumulation in human dermal fibroblasts from progeria patients.
Acknowledgments
We thank the members of the Laboratory of Biochemistry, National Heart, Lung, and Blood Institute for valuable suggestions during the course of this study. The MEFs used in this study were kindly shared by Dr. Kan Cao and Mr. Michael Erdos, respectively, from the Molecular Genetics Section of the National Human Genome Research Institute. Marcy Stone provided guidance in the editing of the manuscript. Our special thanks to Dr. Rodney Levine for valuable discussions and comments. This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp. Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capell BC, Erdos MR, Madigan JP, Fiordalisi JJ, Varga R, Conneely KN, Gordon LB, Der CJ, Cox AD, Collins FS. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12879–12884. doi: 10.1073/pnas.0506001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Rusinol A, Sinensky M, Wang Y, Zou Y. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J. Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerace L, Burke B. Functional organization of the nuclear envelope. Annu. Rev. Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. 335–374. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- 7.Schoneich C. Protein modification in aging: an update. Exp. Gerontol. 2006;41:807–812. doi: 10.1016/j.exger.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006;12:3521–3533. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- 10.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 11.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- 12.Hipkiss AR. Accumulation of altered proteins and ageing: causes and effects. Exp. Gerontol. 2006;41:464–473. doi: 10.1016/j.exger.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, Capell BC, Cheng J, Faddah D, Perkins S, Avallone H, San H, Qu X, Ganesh S, Gordon LB, Virmani R, Wight TN, Nabel EG, Collins FS. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 17.Iwaki T, Iwaki A, Fukumaki Y, Tateishi J. Alpha B-crystallin in C6 glioma cells supports their survival in elevated extracellular K+: the implication of a protective role of alpha B-crystallin accumulation in reactive glia. Brain Res. 1995;673:47–52. doi: 10.1016/0006-8993(94)01393-v. [DOI] [PubMed] [Google Scholar]
- 18.Soskic V, Groebe K, Schrattenholz A. Nonenzymatic posttranslational protein modifications in ageing. Exp. Gerontol. 2008;43:247–257. doi: 10.1016/j.exger.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Cimen MY. Free radical metabolism in human erythrocytes. Clin. Chim. Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. 22–38. [DOI] [PubMed] [Google Scholar]
- 21.Maulik N, Das DK. Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim. Biophys. Acta. 2008;1780:1368–1382. doi: 10.1016/j.bbagen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Guerrero C, Kaiser P, Huang L. Proteomics of proteasome complexes and ubiquitinated proteins. Expert. Rev. Proteomics. 2007;4:649–665. doi: 10.1586/14789450.4.5.649. [DOI] [PubMed] [Google Scholar]
- 25.Hwang JS, Hwang JS, Chang I, Kim S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:490–499. doi: 10.1093/gerona/62.5.490. [DOI] [PubMed] [Google Scholar]
- 26.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, Capell BC, Cheng J, Faddah D, Perkins S, Avallone H, San H, Qu X, Ganesh S, Gordon LB, Virmani R, Wight TN, Nabel EG, Collins FS. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J. Biol. Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- 28.Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J. Biol. Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- 30.Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp. Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JS, Hwang JS, Chang I, Kim S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J Gerontol. A Biol. Sci. Med. Sci. 2007;62:490–499. doi: 10.1093/gerona/62.5.490. [DOI] [PubMed] [Google Scholar]
- 32.Petropoulos I, Conconi M, Wang X, Hoenel B, Bregegere F, Milner Y, Friguet B. Increase of oxidatively modified protein is associated with a decrease of proteasome activity and content in aging epidermal cells. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B220–B227. doi: 10.1093/gerona/55.5.b220. [DOI] [PubMed] [Google Scholar]
- 33.Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J. Biol. Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed EK, Picot CR, Bulteau AL, Friguet B. Protein oxidative modifications and replicative senescence of WI-38 human embryonic fibroblasts. Ann. N. Y. Acad. Sci. 2007;1119:88–96. doi: 10.1196/annals.1404.020. 88–96. [DOI] [PubMed] [Google Scholar]
- 35.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 2008;389:203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 36.Di NM, Shah IM, Stewart DA. Molecular pathways and genetic aspects of Parkinson's disease: from bench to bedside. Expert. Rev. Neurother. 2007;7:1693–1729. doi: 10.1586/14737175.7.12.1693. [DOI] [PubMed] [Google Scholar]
- 37.Shah IM, Di NM. The ubiquitin-proteasome system and proteasome inhibitors in central nervous system diseases. Cardiovasc. Hematol. Disord. Drug Targets. 2007;7:250–273. doi: 10.2174/187152907782793572. [DOI] [PubMed] [Google Scholar]
- 38.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 2008;389:203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 39.Grillari J, Katinger H, Voglauer R. Aging and the ubiquitinome: traditional and non-traditional functions of ubiquitin in aging cells and tissues. Exp. Gerontol. 2006;41:1067–1079. doi: 10.1016/j.exger.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. 801–847. [DOI] [PubMed] [Google Scholar]
- 41.Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J. Biol. Chem. 1999;274:19792–19798. doi: 10.1074/jbc.274.28.19792. [DOI] [PubMed] [Google Scholar]
- 42.Yan T, Li S, Jiang X, Oberley LW. Altered levels of primary antioxidant enzymes in progeria skin fibroblasts. Biochem. Biophys. Res. Commun. 1999;257:163–167. doi: 10.1006/bbrc.1999.0423. [DOI] [PubMed] [Google Scholar]
- 43.DeBusk FL. The Hutchinson-Gilford progeria syndrome. Report of 4 cases and review of the literature. J. Pediatr. 1972;80:697–724. doi: 10.1016/s0022-3476(72)80229-4. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Qiao X, Farber E, Chang SY, Fong LG, Young SG. Eliminating the synthesis of mature lamin A reduces disease phenotypes in mice carrying a Hutchinson-Gilford progeria syndrome allele. J. Biol. Chem. 2008;283:7094–7099. doi: 10.1074/jbc.M708138200. [DOI] [PubMed] [Google Scholar]