Abstract
As a unique form of structural plasticity in the central nervous system, adult neurogenesis in the hippocampus alters network functions by continuously adding new neurons to the mature network, while at the same time is subjected to regulation by surrounding network activity. Here, we review the recently identified mechanisms through which network activity exerts its impacts on multiple steps of adult neurogenesis in rodents and culminates in the selective recruitment of new neurons. We also review recent progress on the study of cellular connectivity modified by new neurons in the dentate gyrus and its physiological functions in rodents. We believe that understanding these processes will allow eventual elucidation of the mechanisms controlling the development of balanced inputs and outputs for the adult-born neurons and reveal important insights into the cellular organization of learning and memory.
Introduction
It is well established that the dentate gyrus in the hippocampal formation is one of the two main structures in the brain with persistent neurogenesis in mammals [1]. In contrast to the other neural activity-dependent plasticity with structural modification within individual cells, adult neurogenesis represents a novel form of structural plasticity in the central nervous system by continuously adding new neurons to a mature network during adulthood. Among the plethora of intrinsic and extrinsic stimuli that modulate adult hippocampal neurogenesis [1], neural network activity has emerged as a prominent regulator in constantly sculpting the existing network through selective recruitment of new neurons, which in return serve to further refine network functions over time. Due to the well-recognized functions of hippocampus in learning, memory and emotion, and the strategically significant position of the dentate gyrus as the first station in the classic tri-synaptic structure of the hippocampus, the interplay between the neural network and neurogenesis in the adult hippocampus has been the subject of intensive study in the past decade. In this review, we aim to identify the emerging themes in this topic, including how network activity affects ongoing neurogenesis and how the integration of adult-born granule cells reshapes network functions. We want to emphasize that the literature we review here primarily comes from rodent studies and caution should be taken when extrapolating to other systems.
Impact of network activities on adult hippocampal neurogenesis
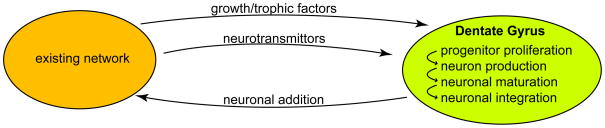
Ongoing neurogenesis in the dentate gyrus involves neural progenitor proliferation, neural production/differentiation/maturation and final integration into the surrounding network. How does the activity of the existing network affect neurogenesis in the adult hippocampus (Fig. 1)? The proposed excitation-neurogenesis coupling in the adult neural progenitors [2] can be generally achieved at multiple levels by either neurotransmittors or growth/trophic factors secreted from the neighboring network in a concerted manner. Even though heterogeneity of network activity may exist in the dentate gyrus, selective recruitment of adult-born neurons into the existing network can be achieved through distinct mechanisms.
Figure 1. Interplay between the neighboring network and ongoing neurogenesis in the dentate gyrus.
Neurogenesis in the dentate gyrus involves progressive sequences involving progenitor proliferation, neuron production, neuronal maturation and integration. The activities of the neighboring network can influence ongoing neurogenesis via growth/trophic factors or neurotransmittors. On the other hand, persistent neurogenesis constantly shapes the network by adding new granule neurons.
Neurotransmittors
Adult-born granule neurons follow a stereotyped and progressive development of neuronal morphology [3] – the apical dendrites of adult-born neurons reach the inner molecular layer 10 days post infection (dpi) with retrovirus (using a retrovirus that only infects dividing cells), the middle molecular layer at 14 dpi and the outer molecular layer at 21 dpi, whereas mossy fibers (the output axons of granule neurons) reach CA3 around 10dpi. During this timecourse the neighboring network plays an active role in regulating neuronal differentiation and maturation via neurotransmitters (in particular, GABA and NMDA) in partially overlapping sequences (see review in [4]): tonic ambient GABA activation (<1 wk old), excitatory dendritic GABAergic synaptic activation (1–2wks), NR1-dependent survival (2–3wks), perisomatic GABAergic synaptic inhibition (>3wks), NR2B-dependent plasticity (4–6wks).
The sequential actions of neurotransmitters and ion channels are critical for the differentiation of neural progenitors into granule cells and the following maturation processes. GABA (mediated by GABAA receptor) selectively depolarizes neural precursors and immature neurons due to the high-level expression of NKCC1 (Cl− importer) and delayed expression of KCC2 (Cl− exporter). Precociously switching GABAergic depolarization to hyperpolarization by knocking down NKCC1 expression leads to reduced dendritic arborization and delayed GABAergic and glutamatergic synapse formation in adult-born granule cells [5]. At the next maturation stage, metabotropic G-protein coupled GABAB receptor mediated activation of inward-rectifying K+ channels (Kir) is hyperpolarizing (thus inhibitory). Ectopic expression of Kir induces mature firing properties in these young neurons, whereas in the presence of the Kir channel blocker Ba2+, mature neurons display increased excitability, mimicking young neurons [6]. Next, NR1 mediated excitatory glutamatergic synaptic activity is critical for the survival of new neurons and thus for their integration into the existing circuitry [7], whereas NR2B mediated plasticity in immature GCs has a characteristically lower threshold for excitation [8] and higher amplitude of long-term potentiation (LTP) [9], and thus likely plays an important role in encoding new inputs. These studies have established that the timely expression of proper GABA and NMDA receptors and related channels is essential for GC differentiation and maturation, even though little is known about the molecular mechanisms for controlling these orderly events.
Growth/trophic factors
Besides neurotransmittors, a cohort of growth or trophic factors have been shown to affect the behaviors of neural progenitors and neuronal maturation (see recent review [1]). However, how network activity regulates these factors is barely known. A recent study by Ma et al [10] explored epigenetic mechanisms in regulating these processes. Interestingly, in response to electroconvulsive treatment (ECT) or exercise, Gadd45b (a member of the Gadd45 family implicated in the DNA demethylation [11]) showed transient but robust expression in mature neurons and loss of Gadd45b significantly impaired proliferation and dendrite growth without affecting the basal level of proliferation. Mechanistically, Gadd45b induction resulted in upregulation of Fgf1 and Bdnf through demethylation at specific regulatory regions instead of causing global genomic demethylation. Therefore, this study identifies the mature GCs as one source for producing Fgf1 and Bdnf in an activity-dependent manner. While the in vivo importance of Fgf1 in the dentate still awaits confirmation, Bdnf regulates multiple aspects of dentate neurogenesis and maturation [1]. It will be interesting as well to determine whether similar mechanism also function cell-autonomously in progenitor proliferation and dendrite development in response to changes in activity. Importantly, this type of mechanism also allows for feedback mechanisms whereby more mature cells produce factors to feedback and regulate the production of new cells based potentially on local network properties and perhaps even in a topologically discrete manner.
Coordination of neurotransmitters and growth/trophic factors
How do the network activities coordinate the effects of growth/trophic factors and neurotransmittors? The pro-neural gene NeuroD1, which is essential for the differentiation of the adult-born granule cells in the adult dentate gyrus [12,13], may potentially play a role in this process. Calcium influx triggered by GABAergic depolarization in neuronal progenitors promotes neural differentiation by driving the expression of NeuroD1 [2,14]. Beyond transcriptional control of NeuroD1 expression, neural activity also modulates NeuroD1 function by CaMKII-mediated phosphorylation in a calcium-dependent manner as demonstrated in the dendritic morphogenesis of cerebellar granule cells [15] (although further investigation is needed to confirm whether the same mechanism also operates in the dentate gyrus). In addition, Wnt signaling mediated by β-catenin appears to be essential for NeuroD1 expression [13]. However, expression of degradation-resistant β-catenin in neural progenitors at perinatal ages tends to keep them in an undifferentiated state (our unpublished data) and basal Wnt activity also seems to be important for the survival of neural progenitors [13]. Therefore, too much or too little Wnt signaling tone apparently blocks differentiation, suggesting a moderate change of Wnt tone from the basal level is involved in physiologically relevant changes in NeuroD1 expression. While it is still quite enigmatic how network activity and Wnt tone are coupled to drive neuronal differentiation, nevertheless, NeuroD1 may be a convergent target regulated by both growth factors (such as Wnts) and neurotransmittors. Several other questions remain open in this regard, which include: (a) whether calcium influx triggered by neurotransmittors is sufficient to induce NeuroD1 expression in the absence of Wnt signaling; (b) whether Wnt signaling is sufficient to drive NeuroD1 expression when calcium influx is blocked; (c) mechanistically, how Wnt signaling and calcium-mediated pathways interact to regulate NeuroD1 expression and functions; and lastly (d) whether Wnt signaling itself can be regulated by network activity.
Bdnf and its receptor TrkB also play a critical role in this coordination. Depletion of Bdnf from mature GCs affects neurogenesis, perhaps by impairing the composition of GABAA receptors in neural progenitors [16], whereas removal of TrkB from neural progenitors and their progeny eliminates neurogenesis-dependent LTP mediated by NR2B [17]. Since neuronal activity regulates Bdnf cell biology at multiple levels [18], the future characterization of the spatiotemporal distribution of Bdnf is a prerequisite for elucidating its actions on the expression and functions of the receptors for neurotransmittors such as GABA and NMDA.
Network activity in the dentate gyrus: uniform vs. regionalized
Do network activities occur in a uniform or a region-specific manner in the dentate gyrus? At the level of genetic makeup, recent genome-wide in situ hybridization data reveals molecular regionalization within the DG [19]. In this study three regions were defined by the expression of Cyp7b1 (cytochrome P450, family 7, subfamily b, polypeptide 1) and Trhr (thyrotropin releasing hormone receptor): Cyp7b1 in the septal region, Trhr in the temporal region and their overlap in the middle. At the level of the cellular constituents, the differential distributions of hilar interneurons [20,21] and mossy cells [22] along the septotemporal axis can also increase the heterogeneity of network activity in the DG. At the level of neuroanatomical connections in rodents, entorhinal cortex and the medial septal nucleus are the two major input structures for the DG, whereas the hippocampal CA3 field is the main output for the DG [23]. Detailed studies of entorhinal-to-dentate projections show that the septal levels of the DG mainly receive projections from the cells located laterally and caudally in the entorhinal cortex, whereas temporal levels receive projections from cells located medially and rostrally [24]. Similar topographic organizations are also observed in the projections from medial septum to DG and from DG to hippocampal CA3 field (see review [25]). At a functional level studies in rodents with lesions in the dorsal or ventral hippocampus [26,27] imply that spatial learning and memory may be more relevant to the DG septal pole and emotion/anxiety to the temporal pole [25]. As indicated by immediate early gene expression (Arc or c-Fos), neural activity in the DG during spatial exploration shows differential distribution along the dorsoventral axis [28] or in the upper/lower blades [29]. Therefore, multiple levels of evidence support the idea that network activities in the adult dentate gyrus are regionalized by neuroanatomical connectivity.
The regional heterogeneity of neural activity in the DG may be further compounded by the observation that spatial information is sparsely encoded in the DG [30]. In other words, unlike gene expression patterns [19], the distribution pattern of DG neurons activated by spatial clues is not continuous [29,31]. Since the majority of the studies so far have used a portion of or the entire DG for quantification analysis, whether the regional or patterned network activities result in differential neurogenesis awaits future substantiation.
Integration of adult-born GCs into circuits
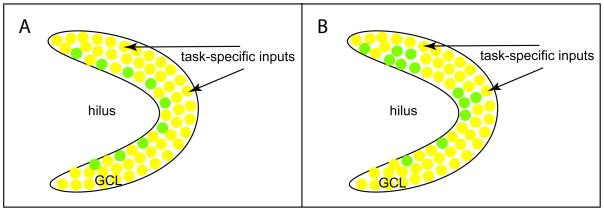
Expression of immediate-early genes (IEGs) such as c-Fos, Arc, Zfp268 are highly correlated with neuronal firing, therefore task relevant neural circuits can be mapped by the expression of IEGs induced by task performance [32,33]. Previous studies have showed that adult-born GCs are activated in response to behavioral stimuli, as indicated by the expression of IEGs [34,35]. How does the system control the maximum recruitment of the newly produced adult-born GCs into circuits specific for a corresponding task? Running and environmental enrichment have been well-documented to globally promote proliferation, survival and neuron production [36,37], however it is not clear whether both task-specific and non-task-specific activities may just generally increase the reserve of new GCs for future integration (Fig. 2A), or whether tasks involving specific circuits might selectively enhance proliferation or neurogenesis focally in neurogenic regions adjacent to the activated circuits (Fig. 2B).
Figure 2. The effects of task-specific inputs on neurogenesis.
In response to task-specific inputs, progenitor proliferation, cell survival and neuronal differentiation can occur either globally (A) or specifically in the neurogenic regions adjacent to the inputs (B). Yellow cells represent the existing granule cells and green cells represent the new granule cells produced as a result of progenitor proliferation, cell survival and neuronal differentiation. GCL, granule cell layer.
The spatial constraint from the task-specific circuits alone may not be sufficient to lead to selective recruitment of adult-born GCs, but its combination with the temporal constraint from the progressive maturation of adult-born GCs may help solve this issue. Indeed, the different timing of the task-specific circuit activity has differential effects. The studies on spatial exploration or environment enrichment suggest enhanced survival of adult-born GCs occurred when the GCs were one- to two-week old [31,38]. If adult-born GCs of 4- to 8-week old were trained for the water maze, preferential recruitment could be achieved upon recall by the same task [31]. These two critical windows for optimal survival and recruitment seem to coincide with the physiological and morphological maturation of adult-born GCs [3,7,9]. Therefore, step-wise temporal actions on the maturing adult-born GCs by the spatially defined task-specific circuit activity may represent a distinct mechanism to achieve specific recruitment of adult-born GCs into the corresponding circuits.
Impact of hippocampal neurogenesis on the existing network: from cellular perspectives to physiological functions
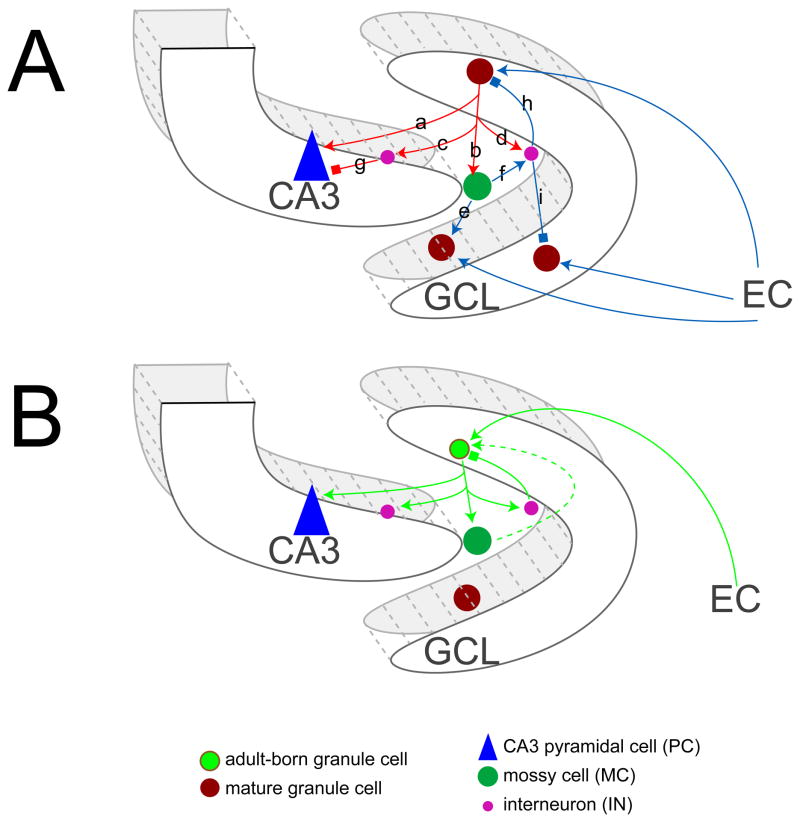
Analysis with electron-microscopy (EM) in combination with retroviral GFP labeling suggests that adult-born neurons in the dentate gyrus compete with existing GCs not only for their excitatory and inhibitory inputs [39] but also for the targets of their mossy fibers, including CA3 neurons, interneurons and hilar mossy cells [40,41], making cellular connections very similar to mature GCs (Fig. 3A). Emerging from these studies, three modes of connectivity made by the adult-born neurons have been identified for both their inputs and outputs: making new connections, overlapping with or replacing existing connections [39,41]. Adult born neurons, when maturing and integrating into the existing circuitry, are equivalent to developmentally born neurons in terms of their physiological properties and capabilities to develop afferent/efferent connections [39–43]. However, from the cellular perspective, the generation of new neurons can significantly change the nature and flow of the input information because they are going through this process and being added to already formed circuits in some manner.
Figure 3. Basic connections of the granule cells of the dentate gyrus.
(A) Mature granule cells (GCs) send outputs to: CA3 pyramidal cells (PCs, a), hilar mossy cells (MCs, b), and interneurons (INs, c and d). MCs can activate GCs located (e) or INs (f). INs can further mediate feedforward inhibition on CA3 PCs (g) or feedback inhibition on GCs (h or i). Mature GCs receive inputs from entorhinal cortex (EC) and local neurons including MCs (e) and INs (h or i).
(B) Adult-born GCs receive inputs from EC, INs and possibly MCs. In the meantime, they send axons to targets including CA3 PCs, MCs and INs.
The “vee” arrows stand for stimulation and “box” arrows for inhibition. Dashed line is the unconfirmed connection. GCL, granule cell layer.
Cellular perspective
Depending on the nature of the input sources and output targets, outcomes of neuronal addition to the GCL may be very complicated. In the following section, we will discuss this cellular complexity based upon current knowledge about mature GCs in the neural circuitry (Fig. 3A) of the dentate gyrus and the findings that adult-born GCs target neurons (Fig. 3B) in a manner similar to mature GCs.
Targeting CA3
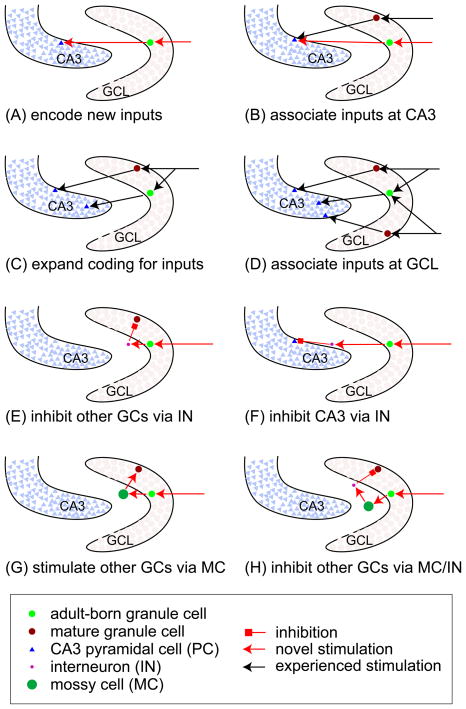
During the early life of individuals, it is very likely that postnatally born GCs encoding new inputs may directly innervate naïve CA3 pyramidal neurons [40,41] (Fig. 4A). A study examining immediate-early gene expression in animals with spatial exploration [31] supports the idea that adult-born GCs can encode new inputs preferentially over mature GCs, which can be explained by the findings that immature GCs bear unique electrophysiological properties including lower threshold for excitation [8] and higher amplitude of LTP [9], and function as the substrate to elicit LTP with a 100-Hz stimulation protocol in the absence of the GABAA-R blocker [44,45]. In older animals, adult-born GCs activated by novel inputs may compete for the same CA3 neurons with the mature GCs encoding the experienced inputs [40,41] (Fig. 4B). Thus, different inputs (distant in time) may be associated by overlapping activity patterns in the CA3 field (Fig. 4B).
Figure 4. Cellular connections altered by addition of adult-born GCs in the DG.
(A and B) Adult-born granule cells (GC) can preferentially encode new inputs by making connections to naïve CA3 pyramidal cells (PC) (A), or by making connections to the CA3 PCs activated by the experienced input (B), thus experienced and new inputs can be associated at the CA3 level.
(C and D) The experienced inputs can also activate adult-born GCs that make new connections to CA3 PCs (C). When two different inputs are present close in time, the adult-born GC can associate both and make overlapping projection to the CA3 field (D).
(E and F) Upon stimulation, the adult-born GCs can inhibit other GCs through hilar interneurons (INs)
(E) or suppress CA3 PCs through INs in the strata pyramidale and lucidum of the CA3 area (F).
(G and H) The adult-born GCs can stimulate the hilar mossy cells (MCs), which can either activate GCs at other levels (G) or inhibit other GCs via INs (H).
GC, granule cell; GCL, granule cell layer; PC, pyramidal cell; IN, interneuron; MC, mossy cell.
In contrast to input association at the CA3 level, input association can also occur at the level of GCs as proposed by the Gage’s group [46,47]. In this case, adult-born neurons may rather broadly respond to the entorhinal inputs activated at similar times, therefore these different inputs (close in time) will be temporally associated by similar sets of adult-born neurons with overlapping outputs to the CA3 (Fig. 4C).
Mature GCs may share experienced inputs with the new adult-born GCs [41], which potentially make new connections with a different pool of CA3 neurons, leading to extra CA3 neurons to encode the experienced inputs at the CA3 level (Fig. 4D).
Targeting interneurons
Adult-born GCs also can target hilar interneurons [41], stimulation of which potentially enhances feedback inhibition on the inputs or outputs of both adult-born and neighboring mature GCs (Fig. 4E). Adult-born GCs can also synapse with interneurons located in the strata pyramidale and lucidum of the CA3 area [41] (Fig. 4F), possibly leading to enhanced feedforward inhibition of the CA3 neurons [48]. Previous studies [49,50] have demonstrated that activation of large spatial areas in the entorhinal cortex leads to activation of only a small population of GCs, which is believed to be critical for the DG function in pattern separation. This organization may be due in part to this phenomenon of the new adult-born GCs inhibiting the firing of mature GCs or CA3 pyramidal cells. Indeed, different classes of interneurons are present in the DG [20,21] and they modulate GCs and CA3 neurons by forming synapses onto distinct perisomatic or dendritic regions [51,52]. From the point of view of network stability, homeostatic mechanisms engaged by harnessing interneurons have been proposed to prevent potential adverse effects caused by adding large numbers of excitatory GCs [53].
Targeting hilar mossy cells
Adult-born neurons can also form synapses with the hilar mossy cells (MCs) [41], which then recurrently innervate other GCs over long distance longitudinally and contralaterally [54]. As a consequence of this, inputs that activate adult-born GCs would spuriously stimulate other irrelevant GCs via MCs (Fig. 4G). Alternatively, MCs can also innervate hilar interneurons (INs) that inhibit GCs [54]. Thus, adult-born GCs can potentially suppress other GCs through sequential actions of MCs and INs (Fig. 4H).
Future studies to decipher the cellular connectivity by adult-born GCs
The alterations in the network made by adult-born GCs we’ve suggested here are mainly based on what is known about the neuroanatomy of mature GCs [23] and the connections made by adult-born GCs [39–41]. In order to understand the functional significance of adult hippocampal neurogenesis, it is essential to decipher how these adult-born GCs modify the existing network at the level of cellular connections. The advent of optogenetic tools makes it possible to use light inducible chanelrhodopsin-2 (ChR2) [55] to label adult-born GCs [41] and temporally control their firing with cell-type specificity. When combined with retrograde or anterograde neural tracers [56] to follow afferents and efferents, it will open up new avenues to probe the cellular connections made by the adult-born GCs. As the proper balance of excitatory and inhibitory synaptic inputs are increasingly appreciated in other systems [57,58], it will be intriguing to uncover the mechanisms for maintaining this balance for adult-born GCs within the mature network. This balance may be disturbed under pathological conditions, for instance, GCs born after seizures receive more inhibitory and fewer excitatory inputs [59]. Since adult-born GCs also target neurons with drastically different properties [41], it is also important to investigate how balanced outputs can be made by the adult-born GCs in the future studies.
Physiological functions
How do the adult-born GCs affect the functions of the existing network? A way to get at this in part is to ask: when are adult-born GCs functionally important - when they are young and more excitable or when they are old and fully integrated? Examining the functional consequences when the numbers of adult-born GCs are altered or when the integration of adult-born GCs is aberrant is the most straightforward way to ask this question thus far.
Down-regulation of neurogenesis by genetic ablation or focal irradiation demonstrated that LTP elicited by a 100-Hz stimulation protocol without using GABAA receptor blocker was neurogenesis-dependent [44]. However, the outcomes of behavioral studies on rodents with neurogenesis perturbed in this way are quite controversial [1]. It seems likely that answering this question authoritatively will entail both circuit-specific manipulation of neurogenesis and carefully designed behavioral assays for the corresponding circuit functions. In a lot of cases, the circuits responsible for the behaviors are poorly defined at the cellular level at this point. Despite these caveats, recent studies do indeed shed some light on this issue by using somewhat improved methodologies to manipulate neurogenesis and using novel assays of hippocampus-dependent tasks.
In one recent study, reversible manipulation of neurogenesis was performed with double transgenic lines (nestin-rtTA and TetO-Bax) by Tet induction. Loss of neurogenesis impaired spatial relational Morris water maze (MWM) performance when the starting point was changed at each trial, but not the standard MWM with constant starting point [60]. More strikingly, when neurogenesis was suppressed by low-dose x-irradiation or stereotaxic lentiviral injection of dominant negative Wnt, mice displayed impairments in spatial discrimination using more challenging behavioral assays: delayed nonmatching to place radial arm maze and mouse touch screen [61]. These sophisticated new studies suggest that neurogenesis is required for optimally performing hippocampus-dependent activities in more demanding tasks, even though there is a caveat that the manipulations in these studies act broadly to block neurogenesis and are not specific for the circuits mediating the tasks.
At the cellular level, if the adult-born GCs developing abnormal outputs or receiving unbalanced inputs may have profound consequences. One recent study [17] showed that mutant animals with conditional ablation of TrkB in the progenitors of the adult DG developed anxiety-like behaviors in open field and elevated plus maze tests [17]. Meanwhile, selective removal of TrkB in mature GCs in another study [62] showed increased number of mossy fiber bouton filopodia which make contact with inhibitory interneurons, suggestive of an enhanced feedforward inhibition of CA3 neurons. It brings up the issue of whether the alteration in the cellular outputs of adult-born GCs is responsible for the behavioral phenotypes. On the other hand, adult-born GCs can develop adjusted inputs under seizure conditions [59]. Therefore, it will be interesting to thoroughly investigate the behavioral consequences caused by altered integration of adult-born GCs in the future.
Conclusions
In recent years significant progress has been made in understanding the molecular and cellular mechanisms for the exquisitely concerted interactions between the hippocampal network and ongoing neurogenesis in the dentate gyrus. However, largely for reasons of technical limitation, a majority of these previous studies have treated dentate neurogenesis as a unitary entity proceeding in a homogenous structure. Due to the heterogeneous nature of the neuroanatomical connections of the dentate gyrus and the cellular complexity of neural addition to the dentate circuitry, future research must emphasize the modality specific manipulation of neurogenesis and subsequent examination of its functional consequences at the cellular circuit level in the hippocampus. We believe that a new frontier for understanding the functions of adult hippocampal neurogenesis is to elucidate the mechanisms that control the development of the inputs/outputs of the adult-born GCs, and the subsequent behavioral consequences. Clarification of these issues will tremendously enhance our understanding of the roles of ongoing DG neurogenesis in learning, memory and motivation, and also potentially provide the therapies for neurological disorders with evidence for disordered adult neurogenesis.
Acknowledgments
Drs. Li and Pleasure were funded by NIMH, NIDA and CIRM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. This is a thorough and updated review on adult neurogenesis in general. [DOI] [PubMed] [Google Scholar]
- 2.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 9.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. This study identifies an epigenetic mechanism linking transient network activity to the change of proliferation and the maturation of newborn neurons in the dentate gyrus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12 :1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- 16.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, et al. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 21.Jinno S, Kosaka T. Cellular architecture of the mouse hippocampus: a quantitative aspect of chemically defined GABAergic neurons with stereology. Neurosci Res. 2006;56:229–245. doi: 10.1016/j.neures.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Fujise N, Liu Y, Hori N, Kosaka T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience. 1998;82:181–200. doi: 10.1016/s0306-4522(97)00261-3. [DOI] [PubMed] [Google Scholar]
- 23.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. This is a systematic study of the neuroanatomical connections between the entorhinal cortex and the dentate gryus, indicating functional segmentation along the septotemporal axis in the dentate gyrus. [PubMed] [Google Scholar]
- 25.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 26.Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99 :10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13 :3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15 :579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 30.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 31.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. This is a detailed analysis about the relationship between the temporal actions of spatial exploration on the adult-born granule cells of different ages and their preferential recruitment. [DOI] [PubMed] [Google Scholar]
- 32.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 37.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 38.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27 :3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 40.Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. 40 and 41 together show a detailed study with retroviral labeling, electron microscopy and electrophysiology, demonstrating that newborn neurons in the dentate gyrus can form synapses with multiple target cells including CA3 neurons, hilar mossy cells and interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laplagne DA, Kamienkowski JE, Esposito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25:2973–2981. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- 44.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 46.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. A very interesting paper describes the role of adult neurogenesis in the context of the cellular network altered by adult-born neurons. It is the first paper to propose the temporal association of events through ongoing neurogenesis. [DOI] [PubMed] [Google Scholar]
- 47.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 50.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 51.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 52.Ribak CE, Shapiro LA. Ultrastructure and synaptic connectivity of cell types in the adult rat dentate gyrus. Prog Brain Res. 2007;163:155–166. doi: 10.1016/S0079-6123(07)63009-X. [DOI] [PubMed] [Google Scholar]
- 53.Meltzer LA, Yabaluri R, Deisseroth K. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 2005;28:653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Henze DA, Buzsaki G. Hilar mossy cells: functional identification and activity in vivo. Prog Brain Res. 2007;163:199–216. doi: 10.1016/S0079-6123(07)63012-X. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 56.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr Biol. 2005;15 :R203–205. doi: 10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. This study shows that adult-born granule cells can receive adjusted inputs under seizure conditon. [DOI] [PubMed] [Google Scholar]
- 60.Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. In this paper, the authors greatly improve the behavioral assays for studying the functions of hippocampal neurogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danzer SC, Kotloski RJ, Walter C, Hughes M, McNamara JO. Altered morphology of hippocampal dentate granule cell presynaptic and postsynaptic terminals following conditional deletion of TrkB. Hippocampus. 2008;18:668–678. doi: 10.1002/hipo.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]