Abstract
The mouse incisor has two unusual features: it grows continuously and it is covered by enamel exclusively on the labial side. The continuous growth is driven in part by epithelial stem cells in the cervical loop region that can both self-renew and give rise to ameloblasts. We have previously reported that ectopic enamel is found on the lingual side of the incisor in mice with loss-of-function of sprouty (spry) genes. Spry2+/−; Spry4−/− mice, in which three sprouty alleles have been inactivated, have ectopic enamel as a result of upregulation of epithelial-mesenchymal FGF signaling in the lingual part of the cervical loop. Interestingly, lingual enamel is also present in the early postnatal period in Spry4−/− mice, in which only two sprouty alleles have been inactivated, but ectopic enamel is not found in adults of this genotype. To explore the mechanisms underlying the disappearance of lingual enamel in Spry4−/− adults, we studied the fate of the lingual enamel in Spry4−/− mice by comparing the morphology and growth of their lower incisors with wild type and Spry2+/−; Spry4−/− mice at several timepoints between the perinatal period and adulthood. Ameloblasts and enamel were detected on the lingual side in postnatal Spry2+/−; Spry4−/+ incisors. By contrast, new ectopic ameloblasts ceased to differentiate after postnatal day 3 in Spry4−/− incisors, which was followed by a progressive loss of lingual enamel. Both the posterior extent of lingual enamel and the time of its last deposition were variable early postnatally in Spry4−/− incisors, but in all Spry4−/− adult incisors the lingual enamel was ultimately lost through continuous growth and abrasion of the incisor.
The incisor in mice, as in other rodents, is used for gnawing. It is characterized by continuous growth, which is compensated for by continuous abrasion. This growth is fueled by stem cells in both the mesenchymal and epithelial compartments of the posterior (apical) end of incisor, whose progeny generate the various cell types in the tooth (Smith and Warshawsky, '75; Harada et al., '99). The labial (outer) surface of the incisor is more resistant to abrasion, because it is covered by hard enamel. In contrast, the lingual (inner) surface undergoes more abrasion because the dentin on this surface is not covered by enamel. Asymmetrical abrasion results in maintenance of a sharp gnawing edge at the incisor tip.
In the mouse incisor, enamel is produced by ameloblasts that are thought to differentiate from transit-amplifying cells (T-A cells). These T-A cells, in turn, arise from stem cells located in the posterior part of the incisor, which is called the cervical loop (Smith and Warshawsky, '75; Harada et al., '99; Wang et al., 2007). The lingual and labial portions of the cervical loop differ in their origin, morphology, cell-matrix interaction and gene expression (Smith and Warshawsky, '75; Kieffer et al., '99; Meyer et al., '99; Harada et al., 2002; Wang et al., 2007).
In wild-type mice, the inner dental epithelium on the lingual side does not contain ameloblasts (Smith and Warshawsky, '75). However, in mice with mutations that cause upregulation of the FGF signaling pathway either directly (Klein et al., 2008) or indirectly (Wang et al., 2007), ectopic enamel production occurs on the lingual side of the incisor. We have previously shown that sprouty genes, which encode intracellular antagonists of FGF and other receptor-tyrosine kinase signaling pathways (Hacohen et al., '98), prevent the generation of lingual ameloblasts by inhibiting an FGF-mediated epithelial-mesenchymal signaling loop on the lingual side (Klein et al., 2008). For reasons that are not yet understood, ameloblasts in these mutants are restricted to the labial and lingual surfaces and do not cover the lateral and medial surfaces of the incisor.
In the early postnatal period, the distribution of both ectopic lingual enamel and lingual ameloblasts is similar in mice lacking either two (Spry4−/−) or three (Spry2+/−; Spry4−/−) sprouty alleles. However, in adults lingual enamel is present only in Spry2+/−; Spry4−/− incisors, whereas it is absent on the lingual side of Spry4−/− incisors (Klein et al., 2008). Because it is not known precisely how and when the lingual enamel disappears in Spry4−/− mice, we compared the fate of the ectopic lingual ameloblasts and enamel in Spry4−/− and Spry2+/−; Spry4−/− mice from embryonic day (E) 18.5 through adulthood (3 months post partum).
Materials and Methods
Mouse lines and staging of embryos
Mouse lines carrying mutant alleles of Spry2 (Shim et al., 2005) and Spry4 (Klein et al., 2008) were maintained and genotyped as reported. Age-matched CD1 animals were used as wild-type controls in all cases; we have previously examined wild-type littermates of sprouty mutant embryos and have found that their dental development is very similar to CD1 embryos. To stage fetuses, noon of the day when a vaginal plug was detected was considered as E0.5. The pregnant mice were killed by cervical dislocation and wet body weight of the fetuses was determined immediately after their removal from the uterus. For postnatal animals, postnatal day zero (P0) was considered as the day of birth.
Histology
Heads of mice at E18.5, P0, P3, P5 and P14 were fixed in Bouin's fluid, decalcified in a mixture comprising equal parts of 50% formic acid and 0.68M sodium citrate in distilled water, embedded in paraffin, and cut into 7μm thick serial frontal or sagittal histological sections. Sections at prenatal stages were stained by a modified haematoxylin-eosin method and at postnatal stages by Heidenhein's aniline blue staining (Mallory, '42). At least three heads of each genotype (control, Spry4−/− and Spry2+/−; Spry4−/−) at each stage (E18.5, P0, P3, P5 and P14) were analyzed on histological sections.
Criteria for classification of ameloblasts on TEM sections (Warshawsky and Smith, '74) were adapted to light microscopy, as follows:
Presecretory ameloblasts
A low columnar epithelium with unpolarized cells (cell nuclei at different levels). Enamel is not present and stratum intermedium consists of 1–2 layers of flat cells.
Secretory ameloblasts
High, columnar and polarized cells (with nuclei at the cell base). Nuclei are elongated (long axes perpendicular to the predentin surface). Cytoplasm occupies more than 50% of the cell height. Enamel is present. Stratum intermedium is regular and is represented by a single layer of cuboidal cells.
Maturation ameloblasts
Lower polarized cells. Cell nuclei are localized basally and at the same level. Cytoplasm occupies less than 50% of the height of the cell. Enamel is present.
Morphometry
Several components of the tooth germ were measured in a single incisor from control, Spry4−/−, and Spry2+/−; Spry4−/− animals. We measured the lingual part of the dental epithelium, the papilla and the labial part of the dental epithelium at E18.5 (Fig. 1), as well as the thickness of the enamel or ameloblast layer at P0, P3 and P5 (Figs. 2–5). Measurements were made along the longer axes of a frontal section of a lower incisor on frontal sections (Fig. 2C) using a Leica DMLB Microscope equipped with a drawing chamber. The measured distances on sections were taken in 35μm intervals along the antero-posterior length of the incisor. The antero-posterior length of the lower incisor was calculated based on the number of 7μm thick serial frontal histological sections comprising the entire enamel organ (Fig. 6).
Fig. 1.
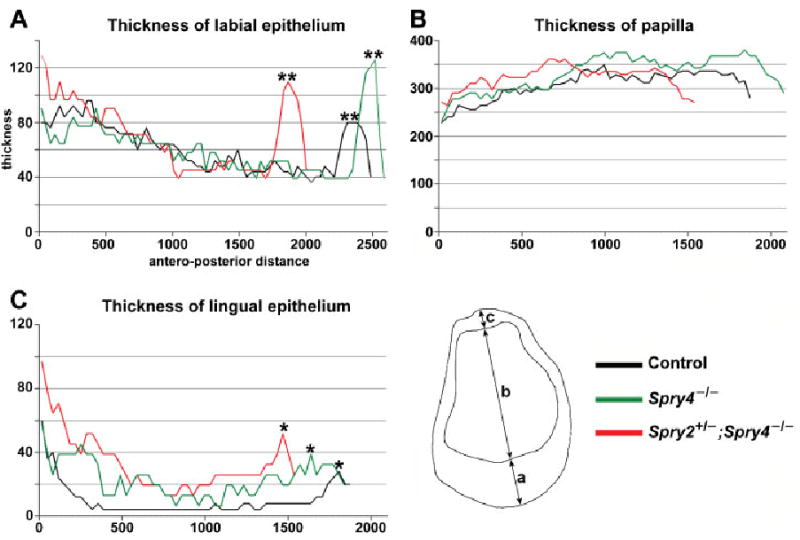
Graphs showing the antero-posterior dimensions of E18.5 lower incisor. The dimensions (a, b, c) were measured on frontal sections (compare to Fig. 2C). (A) Thickness of the inner dental epithelium on the labial side. (B) Thickness of the dental papilla. (C) Thickness of the inner dental epithelium on the lingual side. Note the elevation on the curves in the posterior part of the incisor corresponding to the thicker epithelium of the labial (**) and lingual (*) parts of the cervical loop. All measurements made in micrometers.
Fig. 2.
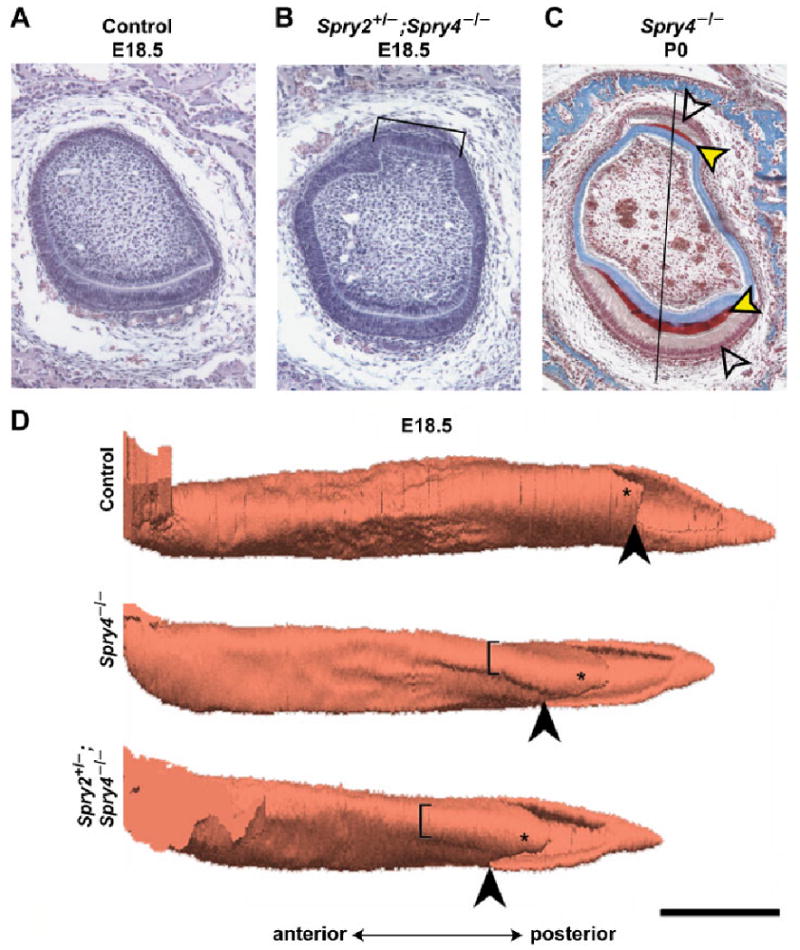
Histology and three-dimensional reconstructions of the lower incisor. Frontal histological section of (A) control and (B) Spry2+/−; Spry4−/− incisor at E18.5 (hematoxylin-eosin staining). The lingual “pouch” is indicated by the symbol ]. (C) Heidenhein's aniline blue staining of a Spry4−/− incisor at P0 shows the enamel in red and dentin and bone in blue. The red enamel (black/yellow arrowheads) is present on both labial (lower) and lingual (upper) side. Labial (lower) and lingual (upper) ameloblasts (blank arrowheads) are visible. The midline is located on the right side of the picture. The black line represents the middle axis along which measurements were made. (D) 3D reconstructions of the lower jaw incisor enamel organ at E18.5 in control, Spry4−/−, and Spry4−/−; Spry2+/− incisors. The lingual “pouch” is indicated by the symbol [. Such a pouch was not present in wild-type (control) incisors at the same stage. The lingual part of the cervical loop is indicated by an asterisk. The black arrowhead points to the split of the apical portion of the enamel organ into the lingual and labial parts of the cervical loop. Note the markedly longer lingual part of the cervical loop in sprouty mutant mice. Bar, 500 μm.
Fig. 5.
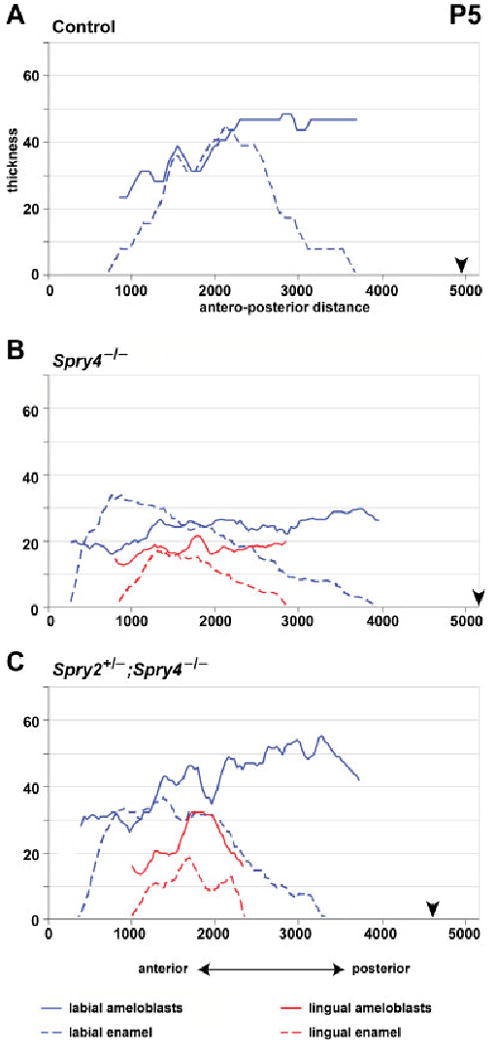
Graphs showing the thickness of the ameloblasts and enamel layers in P5 incisors. Note the decrease in height of both lingual and labial ameloblasts in Spry4−/− incisors (B). The antero-posterior extent and thickness of the lingual enamel is similar in both Spry4−/− and Spry2+/−; Spry4−/− incisors. Black arrowhead indicates posterior end of the enamel organ (posterior limit of the labial part of the cervical loop). All measurements made in micrometers.
Fig. 6.
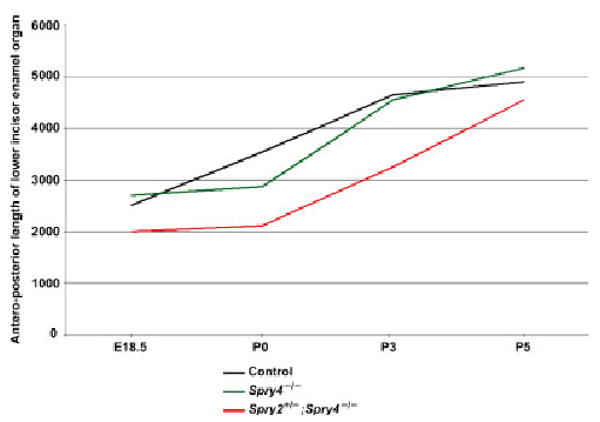
Graph showing the antero-posterior length of the lower incisor enamel organ of control, Spry4−/−, and Spry4−/−; Spry2+/− mice at E18.5, P0, P3 and P5. Length was measured in micrometers.
For each component of an evaluated incisor, the average value was calculated from all anteroposterior measurements. This average value, although not sufficient for a statistical evaluation, provided a semiquantitative assessment of a specific component. The ameloblast layer was measured (Figs. 3–5) where there were elongated secretory ameloblasts, which was chosen as a region where the height of the nucleus was less than 50% of the height of the whole cell. The enamel layer was measured (Figs. 3–5) in a region where specifically stained enamel was well visible on a histological section. The extent of both polarized and unpolarized (presecretory) ameloblasts is shown in Fig. 7A.
Fig. 3.
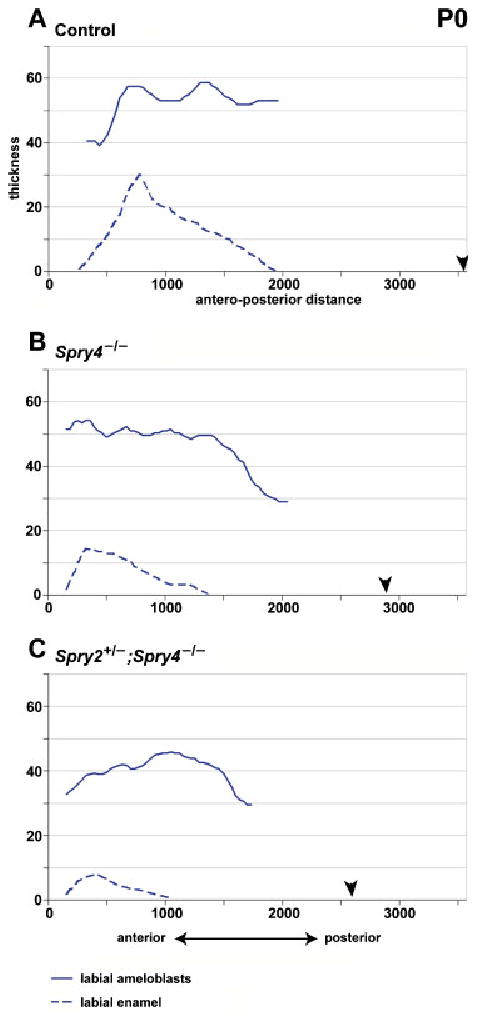
Graphs showing the thickness of the labial ameloblasts and labial enamel layer in P0 incisors. Black arrowhead indicates posterior end of the enamel organ (posterior limit of the labial part of the cervical loop). All measurements made in micrometers.
Fig. 7.
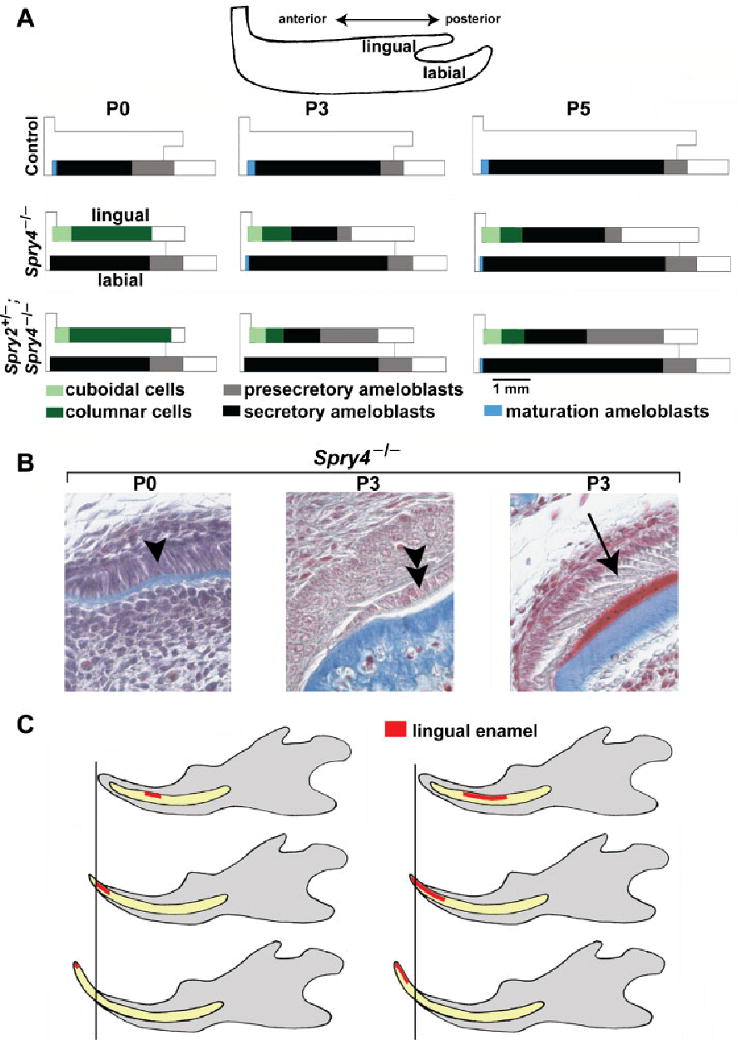
Schematic representation and frontal sections of postnatal lower incisor. (A) The relative length of the area of different types of epithelial cells on the lingual and labial surfaces is shown along the antero-posterior axes. The relative extent of the region of presecretory, secretory or maturation ameloblasts and of cuboidal or columnar cells were calculated as a proportion from the total antero-posterior length of the corresponding incisor enamel organ. The representative length of an incisor enamel organ at P0, P3 or P5 was calculated as the average of the length of the enamel organs of all the control, Spry4−/− and Spry2+/−; Spry4−/− incisor. On the lingual surface of the mutant incisor tip, cuboidal and low columnar cells are present. Compared with the labial surface, ameloblast polarization and secretion are retarded on the lingual side of mutant incisors. Note loss of presecretory ameloblasts during P3 and P5 in Spry4−/− mice. (B) Three types of epithelial cells on the lingual side of a Spry4−/− lower incisor are documented on frontal histological sections (Heidenhein's aniline blue staining): columnar cells (single arrowhead) at P0, cuboidal-low columnar cells (double arrowhead) in the anterior part of the lower incisor enamel organ at P3 and secretory ameloblasts (arrow) in the middle part of the lower incisor enamel organ at P3. The Spry4−/− incisor at P3 shows the enamel in red and dentin and bone in blue. (C) A model for how ectopic lingual enamel shifts anteriorly on the surface of the erupting incisor in Spry4−/− mice. The timing of disappearance of the lingual enamel depends on the posterior extent of the ectopic enamel zone. Variability was detected in the phenotype, such that when the length of the enamel zone was short, the enamel was abraded (disappeared) earlier (left) than in cases when the enamel zone was longer in the posterior direction (right).
Volumes of the labial and lingual parts of a cervical loop of one incisor of each genotype (control, Spry4−/− and Spry2+/−; Spry4−/−) at E18.5 were evaluated using ImageJ 1.37v software (Wayne Rasband, National Institute of Mental Health, Bethesda, MD). The anterior limit of the measured area was chosen as the point at which the apical part of the enamel organ split into the lingual and labial parts of the cervical loop (Fig. 2D). Projection drawings of the cervical loop epithelium on frontal sections were made in 35 μm intervals along the antero-posterior course of the tooth, scanned and the cross-sectional area of a cervical loop was measured. The volume of the lingual or labial part of the cervical loop in each segment between two evaluated sections was calculated by multiplying the cross-sectional area of the cervical loop (in μm2) by the length of the segment (35μm). The volume of the cervical loop was assessed by adding all volumes of the various segments.
Three-dimensional reconstructions
The contours of E18.5 incisor epithelium were drawn from serial frontal sections (7μm intervals). The superposition of drawings was made by a best-fit method, and three-dimensional (3D) images were generated as previously described (Lesot et al., '96).
Postnatal analysis
The heads of 1-, 1.5-, 2- and 3-month-old mice were frozen and put into 1% potassium hydroxide in distilled water for removal of soft tissues. Subsequently, the lower incisors were extracted and stained with azocarmine solution. Twenty four Spry4−/−, 12 Spry2+/−; Spry4−/−, and ten wild-type control heads of various ages were analyzed.
Results
To determine the time course of lingual enamel disappearance in Spry4−/− mice, and the cellular mechanisms by which this process occurs, we compared the distribution of ectopic lingual ameloblasts and enamel in Spry4−/− and Spry2+/−; Spry4−/− mice from embryonic day (E) 18.5 through adulthood (3 months post partum). We began by comparing morphological and quantitative parameters in the lower incisor tooth germ between control and sprouty mutant mice using histological sections (Fig. 2A,B,C) and 3D reconstructions (Fig. 2D), in order to search for qualitative or quantitative abnormalitiesin the mutants that were present before there was evidence of ectopic lingual ameloblasts and enamel.
At E18.5, we focused on three components of the lower incisor germ: the labial part of the dental epithelium, the lingual part of the dental epithelium and the papilla mesenchyme. We measured the thickness of these components along the middle axis of the frontally sectioned incisor germ (Fig. 1) as well as the volumes of both lingual and labial parts of the cervical loop. The 3D reconstructions revealed that both the labial and, more strikingly, the lingual parts of the cervical loop were markedly larger and longer in both Spry4−/− and Spry2+/−; Spry4−/− incisors than in controls (Fig. 2D). Compared with controls, the labial dental epithelium was thicker in the posterior regions that contained the cervical loop in Spry4−/− and Spry2+/−; Spry4−/− incisors (Fig. 1). The lingual dental epithelium was thicker in Spry4−/− and Spry2+/−; Spry4−/− incisors along the entire antero-posterior extension compared with controls. Volumetric measurements confirmed the larger cervical loops in sprouty mutant mice than in controls. The volume of the lingual part of the cervical loop of the control incisor was 0.03 mm3, compared with 1.2 and 1.5 mm3 in Spry4−/− and Spry2+/−; Spry4−/− incisors, respectively. The volume of the labial part of the cervical loop was 4.5 mm3 in control, compared with 7.6 and 9.0 mm3, respectively, in Spry4−/− and Spry2+/−; Spry4−/− incisors.
Besides the changes in the cervical loop, we identified the presence of an unsual structure in sprouty mutants. On sections and 3D reconstructions, a protrusion located anteriorly to the cervical loop was apparent on the lingual side of the tooth germ in both Spry4−/− and Spry2+/−; Spry4−/− incisors (Fig. 2B,C,D), but not in controls (Fig. 2A,D). Because of its shape, we refer to this protrusion as the “lingual pouch” (Fig. 2). The “pouch” consisted of a protrusion of papillary mesenchyme and was covered with dental epithelium (Fig. 2B,C).
Because our previous studies showed that ameloblasts are present in the perinatal Spry4−/− incisors but disappear in the adults, we studied the inner dental epithelium on both the lingual and labial sides early in the postnatal period (P0, P3, P5 and P14). We measured the height of the secretory ameloblasts and the thickness of the enamel layer (Figs. 3–5), and we assessed the distribution of presecretory, secretory and maturation ameloblasts (Fig. 7).
At P0, in control incisors, tall secretory ameloblasts and enamel were present on the labial side (Figs 3A, 7A). Anteriorly and posteriorly to the secretory ameloblasts, maturation and presecretory ameloblasts were present, respectively (see definitions in “Material and Methods”). On the lingual side, there was a low, single layer of inner dental epithelium without differentiated stratum intermedium.
In sprouty mutant incisors, the secretory ameloblasts and enamel were also only visible on the labial side (Figs. 3B,C, 7A). On the lingual side, there was a layer of columnar cells with nuclei located at different distances from the basal cell surface. These cells were adjacent to irregular stratum intermedium comprising 1–2 layers of flat cells. No enamel was visible. The average height of the inner dental epithelium on the lingual side reached about 50% of the height of labial ameloblasts in both Spry4−/− and Spry2+/−; Spry4−/− incisors (Table 1). The height of the inner dental epithelium became progressively reduced in the anterior direction, where first lower columnar and then cuboidal epithelial cells were observed adjacent to the lingual surface of dentin at the anterior incisor tip in both Spry4−/− and Spry2+/−; Spry4−/− incisors (Fig. 7A). The antero-posterior length of the zone of the cuboidal cells was approximately 400 μm, and the extent remained similar at P3 and P5.
TABLE 1. Enamel thickness and height of ameloblasts (in micrometers) in lower incisors.
| P0 | P3 | P5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | S4−/− | S2+/− S4−/− | WT | S4−/− | S2+/− S4−/− | WT | S4−/− | S2+/− S4−/− | ||
| Labial ameloblasts (secretory) | Mean | 48.1 | 43.0 | 40.6 | 44.2 | 38.8 | 43.7 | 23.4 | 23.3 | 42.0 |
| SD | 9.5 | 10.4 | 4.5 | 9.6 | 9.9 | 7.2 | 12.1 | 4.5 | 9.5 | |
| Max | 58.0 | 55.0 | 45.0 | 55.0 | 55.0 | 52.0 | 48.0 | 30.0 | 55.0 | |
| Labial enamel | Mean | 14.4 | 8.6 | 4.7 | 19.9 | 30.0 | 11.7 | 35.9 | 18.6 | 22.7 |
| SD | 7.8 | 4.9 | 2.2 | 9.3 | 9.9 | 4.5 | 13.3 | 9.9 | 10.9 | |
| Max | 30.0 | 15.0 | 8.0 | 32.0 | 34.0 | 17.0 | 45.0 | 34.0 | 36.0 | |
| Lingual ameloblasts (secretory) | Mean | – | – | – | – | 19.8 | 28.8 | – | 17.3 | 27.4 |
| SD | – | – | – | – | 7.2 | 7.1 | – | 2.5 | 6.5 | |
| Max | – | – | – | – | 30.0 | 35.0 | – | 22.0 | 32.0 | |
| Lingual enamel | Mean | – | – | – | – | 4.3 | 3.5 | – | 9.8 | 10.8 |
| SD | – | – | – | – | 1.5 | 0.9 | – | 4.8 | 4.5 | |
| Max | – | – | – | – | 7.0 | 4.0 | – | 18.0 | 20.0 | |
WT, control; S4−/−, Spry4−/−; S2+/− S4−/−, Spry2+/−; Spry4−/−.
In control incisors at P3, the height of the labial ameloblasts and the thickness of labial enamel remained similar to that seen at P0 (Fig. 4, Table 1). Anteriorly and posteriorly to the secretory ameloblasts, maturation and presecretory ameloblasts were present, respectively. The zone of secretory ameloblasts was elongated in the posterior direction compared to P0 (Fig. 7A).
Fig. 4.
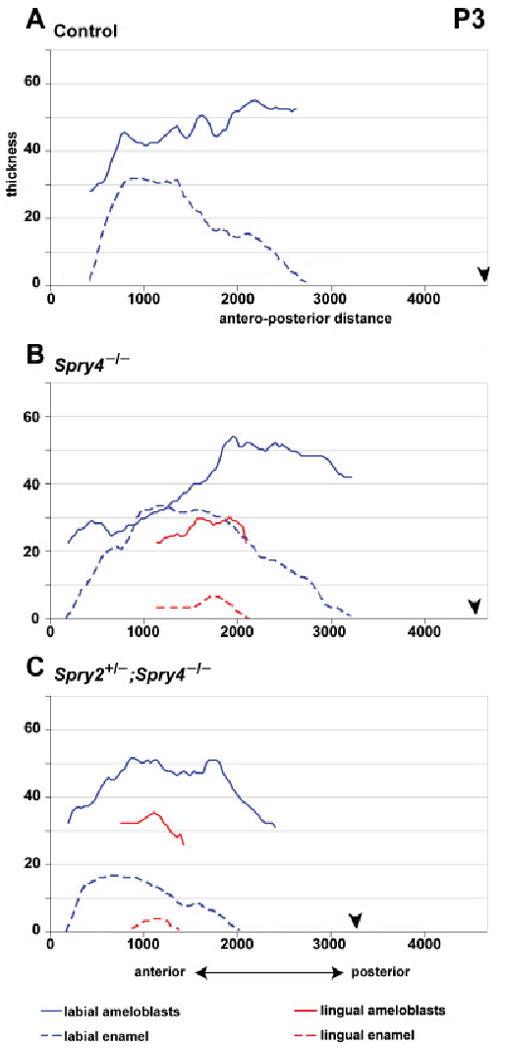
Graphs showing the thickness of the ameloblasts and enamel layers in P3 incisors. The layer of labial ameloblasts is thinner in the anterior part of the lower incisor in Spry4−/− compared to Spry2+/−; Spry4−/− and control incisors. Lingual ameloblasts are of similar height in both Spry4−/− and Spry2+/−; Spry4−/− incisors, but this height is lower than the height of labial ameloblasts. Lingual enamel is very thin, and is restricted to the middle part of the enamel organ in mutant incisors. Black arrowhead indicates posterior end of the enamel organ (posterior limit of the labial part of the cervical loop). All measurements made in micrometers.
In both Spry4−/− and Spry2+/−; Spry4−/− incisors, ameloblasts and enamel were present on both lingual and labial sides. The height of the labial ameloblasts remained similar to P0. The thickness of the labial enamel increased compared to P0 (Fig. 4, Table 1). In the Spry4−/− incisor, the labial enamel was similar in thickness to controls, whereas in the Spry2+/−; Spry4−/− incisors it was thinner (Fig. 4, Table 1).
The antero-posterior extent of the zone of lingual presecretory ameloblasts was shorter in Spry4−/− than in Spry2+/−; Spry4−/− incisors (Fig. 7). In contrast to P0, at P3 we found secretory ameloblasts on the lingual side of both Spry4−/− and Spry4−/−; Spry2+/− incisors. The secretory ameloblasts were adjacent to a well-differentiated stratum intermedium, and enamel was present. In Spry4−/− incisors, the zone of the lingual secretory ameloblasts was longer in the posterior direction and the presecretory ameloblast region decreased in length in the posterior direction (Fig. 7A) compared with Spry4−/−; Spry2+/− incisors. Anteriorly to the lingual secretory ameloblasts, lower columnar cells were present. These cells were similar to the inner dental epithelium at P0. At the anterior tip, cuboidal epithelial cells covered the lingual surface of dentin, similar to P0 (Fig. 7B).
At P5, on the labial side of all control and sprouty mutant animals, the thickness of the enamel and the posterior extension of polarized ameloblasts increased in comparison to previous stages (Fig. 5, Table 1). At the anterior tip, maturation ameloblasts were present, similar to previous stages (Fig. 7A).
In all Spry4−/− incisors, the enamel as well as the ameloblasts were present on both lingual and labial sides. The height of both labial and lingual ameloblasts was lower in comparison to Spry2+/−; Spry4−/− incisors at P5 and to lingual ameloblasts of the same genotype at earlier stages (Fig. 5, Table 1). In Spry2+/−; Spry4−/− incisors, the enamel and ameloblasts were also present on the labial and lingual side. The height of both lingual and labial ameloblasts remained similar to the earlier stages (Fig. 5, Table 1).
On the lingual side in both Spry4−/− and Spry2+/−; Spry4−/− incisors, the antero-posterior length of the anterior zone of cuboidal cells and lower columnar cells remained similar to the previous stage (Fig. 7A). The zone of lingual presecretory and secretory ameloblasts was shorter and its posterior limit was located more anteriorly in Spry4−/− compared to Spry2+/−; Spry4−/− incisors (Fig. 7A).
At P0, P3 and P5, a well-differentiated stratum intermedium was present under secretory ameloblasts on both the labial and lingual side of the incisor in all specimens. An irregular stratum intermedium was present under presecretory ameloblasts.
On sagittal histological sections at P14, lingual enamel was observed in both Spry2+/−; Spry4−/− and Spry4−/− animals.
At 1–2 months, we assessed for the presence of lingual enamel in extracted lower jaw incisors. In Spry2+/−; Spry4−/− incisors, lingual enamel was found on all extracted incisors. In contrast, in Spry4−/− incisors, lingual enamel was only apparent in 60, 50 and 40% of the incisors in 1-, 1.5- and 2-month-old mice, respectively (Table 2). We also observed that in some animals, lingual enamel was present on one incisor but not on the contralateral one. Thus, after incisor eruption, which normally occurs around P10, the ectopic lingual enamel progressively disappeared in Spry4−/− incisors, whereas it was maintained in Spry2+/−; Spry4−/− incisors.
TABLE 2. Presence of lingual enamel in Spry4−/− incisors.
| Postnatal age | Total number of incisors | Number of incisors with lingual enamel | Proportion |
|---|---|---|---|
| 1 month | 12 | 7 | 0.58 |
| 1.5 months | 12 | 6 | 0.50 |
| 2 months | 12 | 5 | 0.40 |
| 3 months | 12 | 0 | 0 |
We examined extracted incisors from several adult animals 3 months post partum and older (Fig. 8) and found lingual enamel in all Spry2+/−; Spry4−/− incisors. However, this enamel was thinner than the regular enamel on the labial side of the tooth and tended to detach from the dentin during tooth extraction. In contrast, none of the incisors from Spry4−/− animals had lingual enamel, and these incisors were hypoplastic (thinner and shorter—Table 3, Fig. 8) when compared with control and Spry2+/−; Spry4−/− teeth.
Fig. 8.
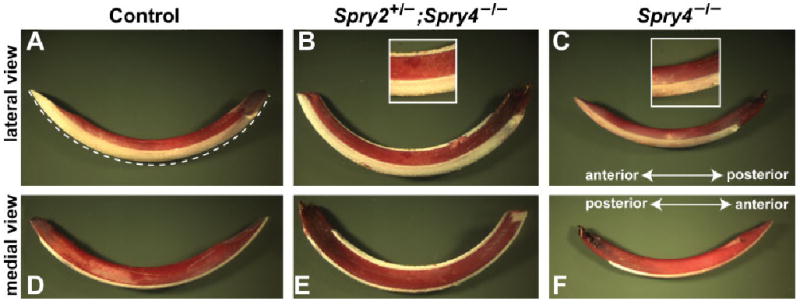
Lower incisors of adult control, Spry4−/− and Spry4−/−; Spry2+/− mice. Dentin (red) is stained with azocarmine G, whereas the enamel is white (unstained). Note the labial and lingual layer of enamel in Spry4−/−; Spry2+/− incisors and the hypoplastic incisor of Spry4−/− animals. Dashed line (A) indicates the measured length reported in Table 3.
TABLE 3. Length (in millimeters) of extracted lower incisors of adult mice.
| Genotype | Number of incisors | Length (mean/SD) | Diameter (mean/SD) |
|---|---|---|---|
| Control | 8 | 12.00/0.4 | 0.95/0.08 |
| Spry2+/−; Spry4−/− | 8 | 13.00/0.5 | 1.10/0.10 |
| Spry4−/− | 8 | 10.00/0.4 | 0.80/0.07 |
Discussion
We have previously shown that ectopic enamel is regularly found on the lingual side of incisors in both Spry4−/− and Spry2+/−; Spry4−/− mice in the early postnatal period (Klein et al., 2008). In sprouty mutants, the ameloblasts are restricted to the labial and lingual surfaces and do not cover the lateral and medial surfaces of the incisor. Lingual enamel has also been demonstrated in the lower incisors of mice that are null for follistatin, a TGF-β signaling antagonist (Wang et al., 2007). In the case of both sprouty and follistatin mutants, it appears that lingual enamel production is induced by upregulation of FGF signaling. Interestingly, lingual enamel production is only maintained in Spry2+/−; Spry4−/− mice, whereas it is lost in Spry4−/− incisors. This has been explained by a downregulation of FGF signaling in the lingual part of the cervical loop of Spry4−/− incisors after birth (Klein et al., 2008).
The onset of ectopic lingual enamel production in sprouty mutants is delayed compared with the labial side. It starts in Spry2+/−; Spry4−/− lower incisors at P3 and lasts through adulthood (Klein et al., 2008). In Spry4−/− mice, lingual enamel production in lower incisors also started at P3 and was observed in the middle part of the incisor enamel organ, similarly to Spry2+/−; Spry4−/− incisors (Figs. 4B, 7A). But in contrast to the Spry2+/−; Spry4−/− mice, the lingual ameloblasts decreased in height in Spry4−/− animals between P3 and P5. This likely corresponds with the cessation of production of ectopic lingual enamel and its subsequent absence in adult Spry4−/− incisors. Interestingly, the labial ameloblasts also decreased in height in Spry4−/− incisors at P5 (Fig. 5), which correlated with the hypoplastic labial enamel observed in these teeth in adult animals. We do not currently know why the labial ameloblasts are affected in Spry4−/− incisors.
Our data document that both the enamel and the layer of ameloblasts are always thinner on the lingual than on the labial side of sprouty mutant incisors (Figs. 2–5). Based on electron microscopy criteria, ameloblasts in adult rodents have been divided into three main types—presecretory, secretory and maturation (Warshawsky and Smith, '74). The patterns of these three cell populations were investigated in histological sections. In all control, Spry4−/− and Spry2+/−; Spry4−/− incisors the labial side was predominantly covered by secretory ameloblasts, except for a short region of presecretory ameloblasts near the cervical loop and a very small region of maturation ameloblasts at the incisor tip (Fig. 7A). All secretory ameloblasts were adjacent to a single continuous layer of stratum intermedium cells, which typically accompany ameloblasts on the incisor labial surface (Warshawsky and Smith, '74). Stratum intermedium is a thin layer of cells, and though its function is still not fully understood, it is thought to be important for enamel formation (Zeichner-David et al., '95), possibly through transport of phosphate into the enamel organ (Woltgens et al., '95). Another role of the stratum intermedium may be production of BMP-2 (Nadiri et al., 2004), as functional tests have demonstrated that BMP-2 can induce ameloblast differentiation (Coin et al., '99). Our histological data indicate that loss of sprouty gene function is accompanied by ectopic differentiation of the ameloblasts and stratum intermedium and by secretion of abnormal, fragile enamel on the lingual side of the mouse incisor. However, we do not yet know whether the thinness and fragility of the lingual enamel in sprouty mutant mice is caused by primary abnormalities in the ectopic ameloblasts, if the stratum intermedium is abnormal and causes secondary defects in the ameloblasts, or if some combination of these occurs.
We observed interesting differences in the ameloblasts along the antero-posterior length of the lingual surface of the sprouty mutant incisors. The lingual side of control incisors consisted of a layer of flat epithelial cells, and no stratum intermedium was present there. In contrast, in the sprouty mutant incisors, ectopic lingual ameloblasts were polarized on the lingual side, and they secreted enamel and were adjacent to a well-differentiated stratum intermedium. However, in the anterior part of the lingual surface of the incisor, cells with a cuboidal or columnar morphology were present (Fig. 7A) in Spry4−/− and Spry2+/−; Spry4−/− incisors. These cells were not adjacent to a differentiated stratum intermedium and did not appear to be postsecretory ameloblasts, because no enamel was present there. This observation suggests that ameloblasts do not differentiate anteriorly on the lingual side of embryonic sprouty mutant incisors, but rather that lingual ameloblasts only arise from migrating progeny of stem cells at later stages. This is in line with our previous finding of a delay in expression of Shh, a preameloblast marker, and its posterior restriction on the lingual side of sprouty mutant incisors when compared with the labial side (Klein et al., 2008). This may also explain the delayed onset of lingual enamel secretion, characterized by a more posteriorly located anterior border of secretory ameloblasts in sprouty mutants (Figs. 3–5,7).
The inner dental epithelium on the lingual side of the mouse incisor is normally devoid of ameloblasts. After a minimal number of cell cycles, preameloblasts can be induced to differentiate into ameloblasts when in contact with predentin–dentin (Karcher-Djuricic et al., '85) or with BMPs (Coin et al., '99). The equivalent cells from the lingual side neither transcribe nor accumulate amelogenin mRNA even when put in contact with a favorable microenvironment (i.e. labial predentin), suggesting that when extracted from wild-type adult animals they are not competent to differentiate into functional ameloblasts (Amar et al., '89). This suggests that, though it is possible to induce embryonic lingual epithelium to form ameloblasts, as indicated by studies in sprouty and follistatin mutants, the competence to differentiate along the ameleoblast lineage is later lost in lingual epithelium in rodent incisors.
The mouse upper incisor grows at a rate of about 1mm per week, such that the incisor, including enamel, is completely renewed in 1.5 months (Coady et al., '67). In our study, the approximate length of the adult lower incisor was 12mm in control mice, and 13 or 10mm in Spry2+/−; Spry4−/− or Spry4−/− animals, respectively. If the production of ectopic enamel is stopped early in the postnatal period, as demonstrated here, then the lingual enamel should move forward as a result of the continuous growth of the incisor (Fig. 7C). Given the rate of incisor growth, the ectopic lingual enamel should be at the incisal tip 10–12 weeks after its deposition, depending on the incisor size. This corresponds to our observation that no lingual enamel was present in Spry4−/− mice at 3 months. We do not yet know what molecular or structural differences account for the observation that production of the lingual, but not labial, enamel ceases, but it is likely that differential signaling contributes to this effect.
If the termination of lingual ameloblast activity and incisor abrasion were similar in all Spry4−/− mice, then the disappearance of the lingual enamel should occur at the same time point in all Spry4−/− mice. However, we found a wide variability in the timing of the lingual enamel disappearance, to the extent that we found in more than one case that in a single animal on the left side lingual enamel was present and on the right side it was not. At 1-month post partum, 60% of Spry4−/− incisors still had lingual enamel. The disappearance of lingual enamel progressively increased at 1.5-, 2- and 3-month-old mice (Table 2). The variability in the lingual enamel disappearance may reflect variability in the cytodifferentiation of the new lingual ameloblasts and/or their activity in the lower incisors of Spry4−/− mice.
In summary, the anterior boundary of ectopic ameloblasts and enamel are stable in sprout mutants during the early postnatal period. In contrast, the posterior extent of lingual enamel and the timing of its last deposition are variable in Spry4−/− mutant mice. This is caused by a cessation of ameloblast function, as indicated by the shortening of the posterior extent of the area of lingual presecretory ameloblasts in Spry4−/− mice at P5. Subsequently, there is a loss of ectopic lingual enamel production posteriorly, and the existing lingual enamel progressively disappears during anterior incisor growth and abrasion. In contrast, the generation of lingual presecretory ameloblasts, their differentiation into secretory ameloblasts and secretion of enamel continue in Spry2+/−; Spry4−/− mice throughout adulthood.
Acknowledgments
This study was supported by the Grant Agency of the Czech Republic (grant number 304/07/0223), Academy of Sciences of the Czech Republic (grant number AVOZ 503905512), Ministry of Education, Youth and Sports of the Czech Republic (projects MSM 0021620843 and COST B23.002), and by the U.C.S.F Sandler Family Foundation. We thank Iva Koppova and Zdena Markova for excellent technical assistance. O.K. was supported by a grant from the NIH (K08-DE017654). The authors thank Gail Martin for providing guidance and mentorship during these studies.
Grant sponsor: Grant Agency of the Czech Republic; Grant number: 304/07/0223; Grant sponsor: Academy of Sciences of the Czech Republic; Grant number: AVOZ 503905512; Grant sponsor: Ministry of Education, Youth and Sports of the Czech Republic; Grant numbers: MSM 0021620843 and COST B23.002; Grant sponsor: U.C.S.F Sandler Family Foundation.; Grant sponsor: NIH (O.K.); Grant number: K08-DE017654.
Literature Cited
- Amar S, Luo W, Snead ML, Ruch JV. Amelogenin gene expression in mouse incisor heterotopic recombinations. Differentiation. 1989;41:56–61. doi: 10.1111/j.1432-0436.1989.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Coady JM, Toto PD, Santangelo MV. Histology of the mouse incisor. J Dent Res. 1967;46:384–388. doi: 10.1177/00220345670460021201. [DOI] [PubMed] [Google Scholar]
- Coin R, Haikel Y, Ruch JV. Effects of apatite, transforming growth factor beta-1, bone morphogenetic protein-2 and interleukin-7 on ameloblast differentiation in vitro. Eur J Oral Sci. 1999;107:487–495. doi: 10.1046/j.0909-8836.1999.eos107611.x. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129:1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- Karcher-Djuricic V, Staubli A, Meyer JM, Ruch JV. Acellular dental matrices promote functional differentiation of ameloblasts. Differentiation. 1985;29:169–175. doi: 10.1111/j.1432-0436.1985.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Kieffer S, Peterkova R, Vonesch JL, Ruch JV, Peterka M, Lesot H. Morphogenesis of the lower incisor in the mouse from the bud to early bell stage. Int J Dev Biol. 1999;43:531–539. [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesot H, Vonesch JL, Peterka M, Tureckova J, Peterkova R, Ruch JV. Mouse molar morphogenesis revisited by three-dimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int J Dev Biol. 1996;40:1017–1031. [PubMed] [Google Scholar]
- Mallory FB. Pathological technique. Philadelphia: W. B. Saunders Co.; 1942. p. 154. [Google Scholar]
- Meyer JM, Bodier-Houlle P, Cuisinier FJ, Lesot H, Ruch JV. Initial aspects of mineralization at the dentino-enamel junction in embryonic mouse incisor in vivo and in vitro: a tem comparative study. In Vitro Cell Dev Biol Anim. 1999;35:159–168. doi: 10.1007/s11626-999-0019-3. [DOI] [PubMed] [Google Scholar]
- Nadiri A, Kuchler-Bopp S, Haikel Y, Lesot H. Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first lower molar. J Histochem Cytochem. 2004;52:103–112. doi: 10.1177/002215540405200110. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Smith CE, Warshawsky H. Histological and three dimensional organization of the odontogenic organ in the lower incisor of 100 gram rats. Am J Anat. 1975;142:403–429. doi: 10.1002/aja.1001420402. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky H, Smith CE. Morphological classification of rat incisor ameloblasts. Anat Rec. 1974;179:423–446. doi: 10.1002/ar.1091790403. [DOI] [PubMed] [Google Scholar]
- Woltgens JH, Lyaruu DM, Bronckers AL, Bervoets TJ, Van Duin M. Biomineralization during early stages of the developing tooth in vitro with special reference to secretory stage of amelogenesis. Int J Dev Biol. 1995;39:203–212. [PubMed] [Google Scholar]
- Zeichner-David M, Diekwisch T, Fincham A, Lau E, MacDougall M, Moradian-Oldak J, Simmer J, Snead M, Slavkin HC. Control of ameloblast differentiation. Int J Dev Biol. 1995;39:69–92. [PubMed] [Google Scholar]


