TO THE EDITOR
Organ transplant recipients have a 100-fold increased risk of developing cutaneous squamous cell carcinoma (SCC) compared to the general population (Euvrard et al., 1993). Compared to immunocompetent patients, organ transplant recipients often develop more invasive and aggressive SCCs, with increased multiplicity, recurrence, and risk for metastasis. In addition to SCCs, organ transplant recipients develop more keratoacanthomas (KAs), Bowen’s disease, and actinic keratoses. A potential reason for the development of these tumors is that posttransplant immunosuppressive medications result in enhanced susceptibility to infection. Histologically, many of the lesions in these patients demonstrate the hallmarks of viral infection such as the presence of koilocytes and verruciform epidermal hyperplasia (Euvrard et al., 1993; Hsi et al., 1997; Harwood et al., 2006).
Study of viruses in skin tumors of transplant patients has previously focused on human papilloma virus (HPV). PCR assays for epidermodysplasia verruciformis and mucosal serotypes of HPV have revealed that high risk mucosal HPVs are relatively infrequent in these cutaneous tumors. The frequency of epidermodysplasia verruciformis HPV infection ranges from 39–92% in SCCs from transplant patients (Berkhout et al., 1995; Boxman et al., 1997; Hopfl et al., 1997). However, the presence of epidermo-dysplasia verruciformis HPV can also be found in plucked hairs and forehead swabs from healthy individuals and skin tumors from immunocompetent patients (Boxman et al., 1997; Hsi et al., 1997; McGregor et al., 1997; Hazard et al., 2007). Thus, the exact etiologic role that these viruses play in skin cancer in organ transplant recipients remains unclear. Other viruses may also contribute to tumorigenesis in organ-transplant patients. Recently, the presence of polyomavirus has been detected in 80% of Merkel cell carcinomas (MCCs; Feng et al., 2008). In these tumors, two viral genomes, designated MCV339 and MCV350, were identified that are distantly related to the known human polyomaviruses. Polyomavirus infection in immuno-competent individuals is harmless. However, in immunocompromised individuals, BK virus infection most often leads to nephropathy whereas JC polyomavirus infection commonly causes multifocal leukoencephalopathy (Drachenberg et al., 2007; Weiner and Narayan, 1973). MCC is a rare but potentially aggressive primary neuroen-docrine carcinoma of the skin that presents most commonly on sun-exposed areas. Increased incidence is associated with immunosuppression, and MCCs are more prevalent in patients with HIV infection, leukemias, and organ transplantation (Miller and Rabkin, 1999; Engels et al., 2002; Kanitakis et al., 2006; Pectasides et al., 2007).
To investigate the potential role of polyomavirus in nonmelanoma skin cancer and actinic keratoses, we used the PCR-based assay described by Feng et al. (2008) to detect the presence of polyomavirus in 156 nonmelanoma skin cancers from organ transplant recipients. The study was approved by the Institutional Review Board of the University of California, San Francisco. The study was conducted according to the Declaration of Helsinki Principles and written consent was obtained from the participants. Genomic DNA was extracted from formalin-fixed paraffin-embedded tumors and the DNA concentration was estimated by Taq-man analysis (Ginzinger et al., 2000). A 400 base pair fragment of β-globin was amplified as a positive control to determine the presence of PCR-amplifiable DNA. The PCR assay was validated on a cohort of MCCs and the results showed that 54% (7/13) of tumors were positive for the presence of polyomavirus. Genomic DNA from a MCC was used as a positive control in all subsequent PCR assays. We examined 85 SCCs, 37 KAs, 28 Bowen’s disease, and 6 actinic keratoses for the presence of polyomavirus DNA. Genomic DNA (50 ng) from each tumor was used to amplify for the LT1, LT3, and VP1 genomic DNA of the polyomavirus and the PCR products were separated on 2% agarose gels. Polyomavirus fragments were detected in 1 KA (2.7%). However, the bands were significantly fainter than in MCCs (Figure 1). Sequencing of the polyomavirus PCR products from the KA revealed that the LT3 and VP1 PCR products fwere identical to the MCV339 virus found in MCC tumors; whereas the sequence of the LT1 PCR product differed by 2 base pairs. With respect to the MCV350 virus, also detected in MCC tumors, the KA-derived LT3 product was identical but the LT1 and VP1 PCR products differed by 5 and 1 base pairs, respectively. The presence of polyomavirus in the KA was not due to contamination because the viral sequence was different to the polyomavirus sequences detected in our cohort of MCC. The pathogenetic relevance of polyomavirus in this single KA is unclear.
Figure 1. Detection of polyomavirus in a single keratoacanthoma.
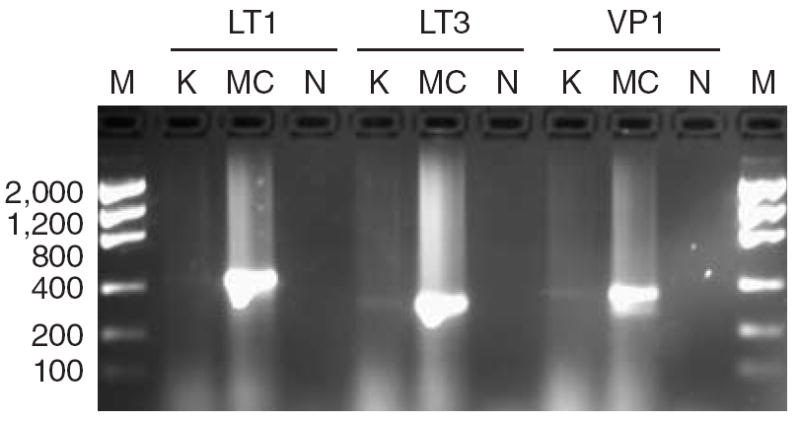
Genomic DNA (50 ng) from a keratoacanthoma (K) or a Merkel cell carcinoma (MC) were amplified with primers specific for the LT1, LT3, or VP1 genes of polyomavirus associated with Merkel cell carcinomas (MCCs). N, non template negative control; M, low mass DNA ladder (fragment length in base pairs is indicated).
To conclude, the MCC polyomaviruses are an infrequent finding in nonmelanoma skin cancers of organ transplant patients.
Abbreviations
- HPV
human papilloma virus
- KA
keratoacanthoma
- MCC
Merkel cell carcinoma
- SCC
squamous cell carcinoma
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
- Berkhout RJ, Tieben LM, Smits HL, Bavinck JN, Vermeer BJ, ter Schegget J, et al. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J Clin Microbiol. 1995;33:690–5. doi: 10.1128/jcm.33.3.690-695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman IL, Berkhout RJ, Mulder LH, Wolkers MC, Bouwes Bavinck JN, Vermeer BJ, et al. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J Invest Dermatol. 1997;108:712–5. doi: 10.1111/1523-1747.ep12292090. [DOI] [PubMed] [Google Scholar]
- de Jong-Tieben LM, Berkhout RJ, Smits HL, Bouwes Bavinck JN, Vermeer BJ, van der Woude FJ, et al. High frequency of detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J Invest Dermatol. 1995;105:367–71. doi: 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- Drachenberg CB, Hirsch HH, Papadimitriou JC, Gosert R, Wali RK, Munivenkatappa R, et al. Polyomavirus Bk versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84:323–30. doi: 10.1097/01.tp.0000269706.59977.a5. [DOI] [PubMed] [Google Scholar]
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–8. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- Euvrard S, Chardonnet Y, Pouteil-Noble CP, Kanitakis J, Thivolet J, Touraine JL. Skin malignancies and human papillomaviruses in renal transplant recipients. Transplant Proc. 1993;25(1 Part 2):1392–3. [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzinger DG, Godfrey TE, Nigro J, Moore DH, II, Suzuki S, Pallavicini MG, et al. Measurement of DNA copy number at microsatellite loci using quantitative PCR analysis. Cancer Res. 2000;60:5405–9. [PubMed] [Google Scholar]
- Harwood CA, Proby CM, McGregor JM, Sheaff MT, Leigh IM, Cerio R, et al. Clinicopathologic features of skin cancer in organ transplant recipients: a retrospective case-control series. J Am Acad Dermatol. 2006;54:290–300. doi: 10.1016/j.jaad.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Hazard K, Andersson K, Dillner J, Forslund O. Human papillomavirus subtypes are not uncommon. Virology. 2007;362:6–9. doi: 10.1016/j.virol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfl R, Bens G, Wieland U, Petter A, Zelger B, Fritsch P, et al. Human papillomavirus DNA in non-melanoma skin cancers of a renal transplant recipient: detection of a new sequence related to epidermodysplasia verruciformis associated types. J Invest Dermatol. 1997;108:53–6. doi: 10.1111/1523-1747.ep12285630. [DOI] [PubMed] [Google Scholar]
- Hsi ED, Svoboda-Newman SM, Stern RA, Nickoloff BJ, Frank TS. Detection of human papillomavirus DNA in keratoacanthomas by polymerase chain reaction. Am J Dermatopathol. 1997;19:10–5. doi: 10.1097/00000372-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Kanitakis J, Euvrard S, Chouvet B, Butnaru AC, Claudy A. Merkel cell carcinoma in organ-transplant recipients: report of two cases with unusual histological features and literature review. J Cutan Pathol. 2006;33:686–94. doi: 10.1111/j.1600-0560.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- McGregor JM, Berkhout RJ, Rozycka M, ter Schegget J, Bouwes Bavinck JN, Brooks L, et al. p53 mutations implicate sunlight in post-transplant skin cancer irrespective of human papillomavirus status. Oncogene. 1997;15:1737–40. doi: 10.1038/sj.onc.1201339. [DOI] [PubMed] [Google Scholar]
- Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–8. [PubMed] [Google Scholar]
- Pectasides D, Papaxoinis G, Pectasides E, Galani H, Razi E, Katodrytis N, et al. Merkel cell carcinoma of the skin: a retrospective study of 24 cases by the Hellenic Cooperative Oncology Group. Oncology. 2007;72:211–8. doi: 10.1159/000112944. [DOI] [PubMed] [Google Scholar]
- Weiner LP, Narayan O. A papovavirus isolated from patients with progressive multifocal leukoencephalopathy. Ann Clin Res. 1973;5:279–82. [PubMed] [Google Scholar]


