Abstract
To investigate whether magnetization transfer imaging (MTI) is a useful detector of diffuse brain abnormalities in ‘premanifest’ carriers of the Huntington’s disease (HD) gene mutation. Furthermore we examined the relations between MTI, clinical measures and CAG repeat length. Sixteen premanifest carriers of the HD gene without motor manifestation and 14 non-carriers underwent a clinical evaluation and a MRI scan. MTI analysis of whole brain, grey matter and white matter was performed producing magnetization transfer ratio (MTR) histograms. A lower peak height of the grey matter MTR histogram in carriers was significantly associated with more UHDRS motor abnormalities. Furthermore, a lower peak height of the whole brain, grey and white matter was strongly associated with a longer CAG repeat length. MTI measures themselves did not differ significantly between carriers and non-carriers. In premanifest HD mutation carriers, a lower MTR peak height, reflecting worse histological brain composition, was related to subtle motor abnormalities and higher CAG repeat length. Although we could not detect altered MTI characteristics in carriers of the HD gene mutation without clinical manifestations, we did provide evidence that the MTR peak height might reflect genetic and subclinical disease burden and may be of value in monitoring further disease progression and provide insight in clinical heterogeneity.
Keywords: Huntington’s disease, Premanifest, Magnetization transfer imaging
Introduction
Huntington’s disease (HD) is a hereditary neurodegenerative disorder resulting in a progressive loss of motor and cognitive functioning and changes in mood and social behavior. The genetic defect leads to cell death especially in the basal ganglia. Recent studies demonstrated that the amount of white matter and cortical grey matter is found to be reduced as well, even early in the disease [5, 21, 23]. Improving the knowledge of brain changes in ‘premanifest’ carriers (further labeled as carriers) of the HD gene mutation (i.e. without overt clinical signs) is essential in the search for sensitive instruments suitable to monitoring HD onset and progression for future therapeutic trials with neuroprotective agents.
Volumetric MRI studies in carriers demonstrated smaller basal ganglia volumes even years before the onset of motor disturbances [4, 7, 17, 21]. Recent voxel based morphometry (VBM), diffusion tensor imaging (DTI) or positron emission tomography (PET) studies demonstrated abnormalities in white matter and cortical grey matter as well [8, 23, 24, 30]. Other quantitative MRI techniques, such as magnetization transfer imaging (MTI) may further improve our understanding of how diffuse brain changes in HD develop and how these are related to the genotype and the heterogeneous phenotype of the disease. In MTI the exchange of magnetization between bound protons and free water is represented by the magnetization transfer ratio (MTR), which can be demonstrated quantitatively in the MTR histogram. A low peak height of the MTR histogram indicates reduced capacity of the macromolecules in brain tissue to exchange magnetization with the surrounding water molecules, reflecting structural brain damage [14]. MTI has the advantage of being non-invasive and easy to administer, whilst having been proved to be sensitive for the first microstructural brain changes in different neurodegenerative disorders, like Alzheimer’s disease (AD) and Parkinson’s disease (PD), even before volumetric alterations [2, 16, 29, 33, 36, 37]. In mild cognitive impairment (MCI), which has become increasingly recognized as a transitional phase between normal old age and AD, abnormal MTR values of the brain parenchyma could be demonstrated without evidence of atrophy [33].
Although MTI was initially developed to investigate white matter changes in multiple sclerosis (MS), it was demonstrated to be sensitive for changes in tissue structure of the grey matter as well [13, 31, 37]. This, and the sensitivity for histopathological changes preceding atrophy, makes MTI an attractive tool in studying the earliest diffuse brain changes related to HD in both grey matter and white matter. To date, only one study investigated MTI parameters in predominantly symptomatic HD gene carriers [20]. No significant differences in mean MTR values between HD patients and controls were found. However, this study did not address the peak height of the MTR histograms, which has proven to be the most sensitive and distinctive MTI parameter for detecting brain damage in various neurodegenerative diseases [6, 22, 32, 37]. Lower peak heights of the histogram could be demonstrated in AD, MCI and MS, while mean MTR values were still within the normal range [31, 33, 36].
Therefore, in this explorative study, we investigated whether MTI based magnetization transfer ratios and histogram peak heights could reveal diffuse brain abnormalities in carriers in comparison with non-carriers by studying the total of brain parenchyma, the grey matter and the white matter. It is hypothesized that MTI may provide additional information on diffuse brain pathology and its relation with the genotype and the phenotype in the premanifest phase of HD.
Patients and methods
Seventeen carriers and 15 non-carriers were invited to participate in this study. All participants were recruited from the Leiden University Medical Center (LUMC) outpatient Neurological department. Participants had undergone gene testing according to international guidelines at an earlier time [1]. The median CAG repeat length in carriers was 42 (range 40–49) and in non-carriers 19 (range 16–24). The estimated probability of symptom onset within 5 years was determined [19]. Carriers were considered ‘premanifest’ in the absence of ‘definite’ motor signs on the unified Huntington’s disease rating scale (UHDRS), as assessed during their last visit to our outpatient department. Reassessment of motor functioning during study enrolment by a neurologist blind to genetic status, resulted in the exclusion of one carrier who was rated as definite HD. One non-carrier who showed evidence of overt cerebral damage on MRI was also excluded from analysis. Ultimately analyses were performed on 16 carriers and 14 non-carriers.
The study had been approved by the local Medical Ethical Committee. Written informed consent was obtained from all subjects.
Procedure
All participants were evaluated with the UHDRS and MRI of the brain [17, 18]. There were no more than 4 months between clinical assessment and MRI-scan (mean 30 days, SD 44 days).
From the motor part of the UHDRS the total motor score (TMS) was used (max 0–124), with higher scores representing more motor abnormalities. The cognitive and behavioral sections of the UHDRS were administered by a psychologist. The total behavioral score was obtained by adding the products of frequency and severity for each item.
Image acquisition
All imaging was performed on a whole body MR system operating at field strength of 3.0 T (Philips Medical Systems, Best, The Netherlands). MRI consisted of a 3D-T1-weighted and magnetization transfer imaging (MTI) scan. Acquisition parameters were as follows: 3D-T1-weighted: TR = 9.8 ms; TE = 4.6 ms; flip angle = 8°; section thickness = 1.2 mm; number of sections = 120; no section gap; whole brain coverage; FOV = 224 mm; matrix = 192, reconstruction matrix = 256; 3D-gradient echo MTI: TR = 100 ms; TE = 3.7 ms; flip angle = 8; section thickness = 7.2 mm; number of sections = 20; no section gap; whole brain coverage; FOV = 224 mm; matrix = 224, reconstruction matrix = 256. These scan parameters were chosen to minimize T1 and T2 weighting, resulting in a proton-density contrast in the absence of MT saturation pulses. Two consecutive sets of images were acquired; the first was performed in combination with the MT saturation pulse, and the second without. In the second scan a sinc-shaped saturation pulse 1,100 Hz below frequency of water was added.
Image postprocessing
Images were transferred to an offline LINUX workstation. All MTR processing steps were performed using the FMRIB’s software library (FSL) [25]. The MTR sequence was split into an m 0 dataset, which represents the signal intensity of voxels without saturation and an m 1 dataset, which represents the intensity of voxels with saturation. An MTR map was obtained by calculating the MTR value for each voxel using the formula; MTR = {(m 0 – m 1)/m 0} × 100% [11]. To obtain segmented grey and white matter the T1 weighted scans were segmented using the segmentation tool in FSL, FAST [38]. All segmentations were eroded one voxel in plane to minimize partial volume effects on the MTI parameters. A transformation matrix was used to mask the MTR map with the segmented whole brain, grey and white matter volume from the T1 weighted scan segmentation. From the resulting MTR maps of the whole brain, grey and white matter, histograms were created and finally normalized for the size of the region of interest. From these histograms the peak height was derived. Whole brain, grey and white matter volume was measured, using an automated method, the cross-sectional version of the Structural Image Evaluation of Normalized Atrophy (SIENAx) [27]. Furthermore we measured relative brain atrophy with SIENAx by accurately defining brain size with respect to skull size, normalized to a standard template, resulting in normalized brain volumes. This also reduces within group variations, making cross-group comparisons more sensitive [26]. Manual segmentation of basal ganglia volumes in these groups were described elsewhere [17].
Statistical analysis
SPSS for Windows (release 16.0.) was used for data analysis. Group differences were analyzed with parametric or non-parametric tests when appropriate. To assess differences in age, education, UHDRS scores, brain volumes and MTI parameters we used independent t tests. Pearson correlation analysis (r) was used to investigate associations of MTI measures with UHDRS scores and CAG repeat length. The level of statistical significance was set at p ≤ 0.01. Values of 0.01 < p ≤ 0.05 were considered as a trend towards significance.
Results
Clinical characteristics
There were no significant differences between groups for sex, age, years of education and UHDRS motor, cognitive and behavioral functioning (Table 1).
Table 1.
Clinical characteristics
| Carriers (n = 16) | Non-carriers (n = 14) | |
|---|---|---|
| Male/femalea | 6/10 | 6/8 |
| Age (years) | 41.9 (10.0) | 47.2 (9.2) |
| Education (years) | 12.9 (2.7) | 12.6 (2.8) |
| CAG repeat lengthb | 42 (40–49) | 19 (16–24) |
| Probability of onset within 5 years (%)b,e | 17 (0–73) | |
| UHDRS | ||
| Total motor scorec,d | 3.5 (0–10) | 2.4 (0–6) |
| Verbal fluency | 38.5 (13.6) | 39.0 (10.4) |
| SDMT | 52.2 (11.8) | 60.6 (11.7) |
| Stroop color | 72.3 (13.0) | 80.9 (16.2) |
| Stroop word | 96.6 (10.6) | 100.1 (19.6) |
| Stroop interference | 42.3 (7.7) | 45.6 (6.8) |
| Total behavioral scored | 12.4 (11.4) | 15.8 (17.6) |
Values in the table are means (SD)
No significant differences were found between carriers and non-carriers, except for CAG repeat length (p = 0.00)
UHDRS unified Huntington’s disease rating scale, SDMT symbol digit modalities test
Independent t test analysis
aPearson’s χ2 test
bMedian (range)
cMean (range)
dHigher scores correspond with more abnormalities
eA greater probability of onset within 5 years corresponds with being closer to estimated onset of disease
MRI parameters
There were no significant differences between groups for whole brain, grey and white matter brain volumes and MTI parameters (Table 2). An association between smaller brain volume and higher age, was found only in non-carriers. Since the statistical assumption ‘homogeneity of regression slopes’ was violated, covarying for age was not feasible. Furthermore as groups did not differ significantly in age we decided not to correct for this variable. We also investigated brain volumes normalized for head size and found a tendency towards smaller whole brain volume (p = 0.05), especially white matter (p = 0.03) in carriers compared to non-carriers.
Table 2.
Volumes and MTI parameters of whole brain, grey matter and white matter
| Carriers (n = 16) | Non-carriers (n = 14) | P value | |
|---|---|---|---|
| Whole brain | |||
| Volume (cc) | 1150.1 (86.2) | 1205.2 (108.8) | 0.13 |
| NBV | 1492.8 (57.2) | 1536.6 (59.5) | 0.05* |
| MTRm | 39.8 (0.6) | 39.8 (0.9) | 0.94 |
| NPH | 88.8 (8.3) | 90.2 (5.2) | 0.59 |
| Grey matter | |||
| Volume (cc) | 654.0 (51.0) | 678.7 (70.2) | 0.28 |
| NBV | 849.6 (50.0) | 865.8 (62.3) | 0.43 |
| MTRm | 37.6 (0.6) | 37.7 (0.9) | 0.59 |
| NPH | 87.7 (9.4) | 90.1 (5.4) | 0.40 |
| White matter | |||
| Volume (cc) | 496.1 (47.6) | 526.5 (53.0) | 0.11 |
| NBV | 643.2 (34.3) | 670.9 (29.6) | 0.03* |
| MTRm | 43.4 (0.5) | 43.3 (1.1) | 0.72 |
| NPH | 147.6 (15.2) | 155.3 (16.7) | 0.20 |
Values in the table are means (SD)
The peak height was normalized for brain size of the region of interest (number of voxels on the peak divided by the total number of segmented voxels)
NBV normalized brain volume (normalized for skull size). MTRm mean MTR, NPH normalized peak height
Independent t tests were used for statistical analysis
*p < 0.05
The results on the basal ganglia volumes in the studied groups have been reported in an earlier study and showed smaller caudate, putamen and globus pallidus volumes in carriers [17].
Relations between UHDRS and MTI parameters
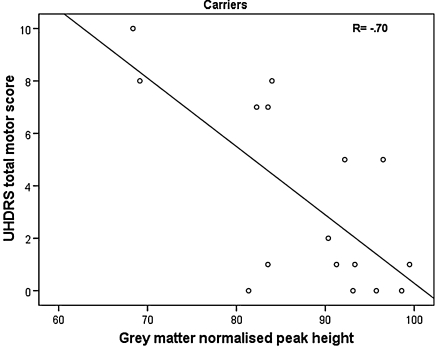
In gene carriers we found that a higher score on the UHDRS motor scale, reflecting more motor abnormalities, was significantly associated with a lower peak height of the grey matter MTR histogram (r = −0.70, p = 0.003) (Fig. 1) and marginally with a lower peak height of the whole brain MTR histogram (r = −0.57, p = 0.02). Associations remained after controlling for basal ganglia volume. From the UHDRS cognitive and behavioral assessment in carriers, a significant association was found between lower white matter mean MTR and better scores on the Stroop interference task (r = −0.63, p = 0.009) and marginally between lower whole brain peak height and worse scores on the Stroop color naming task (r = 0.56, p = 0.02). In non-carriers no significant associations with MTI parameters were found.
Fig. 1.
Significant association in carriers between higher score on the UHDRS motor scale and lower normalized peak height of the grey matter
Relations between CAG and MTI parameters
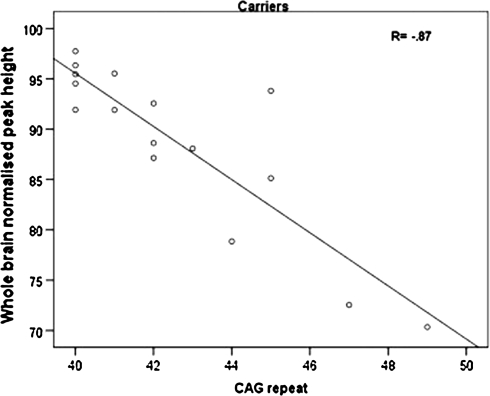
A higher CAG repeat length in carriers was strongly related to lower peak heights of the whole brain, grey and white matter (r = −0.87, p = 0.00; r = −0.86, p = 0.00; r = −0.68, p = 0.004) (see Fig. 2 for an example). A trend association was found between higher CAG repeat length and lower grey matter mean MTR (r = −0.55, p = 0.03). Furthermore, a greater probability of developing symptoms within 5 years was marginally related to smaller grey matter peak height (r = −0.58, p = 0.02).
Fig. 2.
Significant association in carriers between higher CAG repeat length and lower normalized peak height of the whole brain
Discussion
Regional brain changes, specifically in the striatum, have been demonstrated many years prior to the onset of clinical signs in HD [3, 17, 21]. The onset and progression of early diffuse brain changes in HD are imperfectly known. To the best of our knowledge, this is the first time that extensive MTI analysis has been applied to study grey and white matter in premanifest HD mutation carriers. In carriers lower MTR peak height was specifically related to decreased motor functioning and higher CAG repeat length. Although we could not demonstrate MTR differences between carriers and non-carriers, the strong associations with HD characteristics in carriers indicate that MTR values might reflect disease burden.
In line with one previous study, predominantly including symptomatic carriers, mean MTR values did not differ between carriers and non-carriers [20]. We also studied the more sensitive peak height of the MTR histogram and could not demonstrate any brain abnormalities in carriers with that parameter either. However, when volumes of the whole brain, grey matter and white matter were normalized for skull size, hereby measuring atrophy state, carriers had a smaller volume of the whole brain parenchyma and especially the white matter, indicating the presence of global brain changes in carriers [26]. This is in accordance with previous studies demonstrating early grey and white matter tissue loss in premanifest HD [21, 23].
Our data show that in these areas of reduced grey and white matter tissue MTR values were not abnormal, indicating that the integrity of the tissue was not significantly altered on a group level. It might be that the specific HD pathologic process does not result in altered tissue integrity, confirmed by the study of Mascalchi et al. [20] who could not demonstrate MTI abnormalities in basal ganglia of HD patients, despite smaller basal ganglia volumes compared to controls. In other neurological diseases a reduction in MTR has been especially associated to myelin loss, axonal loss, gliosis and inflammation [9, 13, 33]. Since there is no unequivocal substrate underlying MTR, the technique may be less sensitive for detecting other microscopic changes, such as those in HD. Nevertheless, the remarkably strong association between lower MTR peak height and a higher CAG repeat length, indicates that the integrity of the tissue reflects underlying genetic disease burden. Furthermore, clinical relevance of the MTR was also demonstrated; lower whole brain and grey matter peak height in carriers, reflecting less cerebral homogeneity, was found to be strongly related to more motor abnormalities. Motor functioning has been linked to striatal changes in previous premanifest studies [3, 7, 17]. Since associations remained after controlling for basal ganglia volumes, the findings might indicate the involvement of more diffuse grey matter changes in altered motor functioning.
It can be argued that with advancing motor abnormalities and pathological burden the MTR peak heights continue to decrease and will significantly differ from controls. This is strengthened by the finding that MTR peak heights tend to be somewhat lower in carriers in this study. Furthermore, lower grey matter peak heights were associated with a greater probability of developing symptoms within 5 years.
Various studies in other diseases, like Alzheimer’s disease and multiple sclerosis, related MTI measurements to cognitive functioning, however results are inconsistent [10, 31, 33, 35]. Surprisingly, we showed in carriers that a lower mean value of white matter MTR, indicating worse histological brain composition, was associated with better performance on the Stroop interference task. Since minimum variation is found in mean MTR values in both carriers and non-carriers this result has to be interpreted with caution. Furthermore MTR peak heights proved a better reflection of abnormal brain structure in other MTI studies of neurodegenerative diseases [6, 22, 32, 37]. Van der Flier et al. [34] demonstrated a strong association between diffuse brain damage as measured with global MTR peak heights and neuropsychological test results in mild cognitive impairment and AD. We did show that lower whole brain peak height was related to worse scores on the Stroop color naming task. Snowden et al. [28] emphasized that relatively automatic speed based tasks, such as this Stroop condition, are most sensitive for HD related cognitive changes.
Strengths of the current study include the automated segmentation methods for MRI data. Furthermore, we associated MTI values with the full spectrum of HD characteristics. Potential limitations include the small sample size, which might have contributed to the absence of a statistical difference in MTI characteristics between carriers and non-carriers. Also the rather thick section thickness of the MTI sequence might have contributed to less sensitivity. Furthermore, carriers seemed quite far from onset based on probability of onset estimations and on the relatively low motor scores compared to other studies in premanifest HD [12, 15]. A follow up of the current cohort and a larger sample, including manifest HD carriers as well, should shed more light on diffuse MTR abnormalities and motor and cognitive correlates in HD.
In conclusion, although we could not detect altered MTI characteristics in carriers of the HD gene mutation without clinical manifestations, we did provide evidence that the MTR peak height might reflect genetic and subclinical disease burden. Whether MTI parameters are sensitive for HD related brain changes and associations with the phenotype in more advanced stages of disease remains to be investigated.
Acknowledgments
The authors thank Dr. Y.A.M. Grimbergen for her help in motor assessments and L. van de Wiel for her help in assessing the protocols. We are grateful to Dr. B. Emmer for his help in making the MTR script.
Conflict of interest statement
We state that there are no potential conflicts of interest, including any financial, personal or other relationships with people or organizations that could inappropriately influence the current study. The study has been approved by the LUMC Medical Ethical Committee.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.American College of Medical Genetics/American Society of Human Genetics Huntington Disease Genetic Testing Working Group ACMG/ASHG statement Laboratory guidelines for Huntington disease genetic testing. Am J Hum Genet. 1998;62:1243–1247. doi: 10.1086/301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anik Y, Iseri P, Demirci A, Komsuoglu S, Inan N. Magnetization transfer ratio in early period of Parkinson disease. Acad Radiol. 2007;14:189–192. doi: 10.1016/j.acra.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch Neurol. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 4.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 5.Bates G, Harper PS, Jones L (2002) Huntington’s disease. Oxford University Press, NY
- 6.Bosma GP, Rood MJ, Zwinderman AH, Huizinga TW, van Buchem MA. Evidence of central nervous system damage in patients with neuropsychiatric systemic lupus erythematosus, demonstrated by magnetization transfer imaging. Arthr Rheum. 2000;43:48–54. doi: 10.1002/1529-0131(200001)43:1<48::AID-ANR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Campodonico JR, Aylward E, Codori AM, Young C, Krafft L, Magdalinski M, Ranen N, Slavney PR, Brandt J. When does Huntington’s disease begin? J Int Neuropsychol Soc. 1998;4:467–473. doi: 10.1017/S1355617798455061. [DOI] [PubMed] [Google Scholar]
- 8.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47:215–222. [PubMed] [Google Scholar]
- 9.Davies GR, Ramio-Torrenta L, Hadjiprocopis A, Chard DT, Griffin CM, Rashid W, Barker GJ, Kapoor R, Thompson AJ, Miller DH. Evidence for grey matter MTR abnormality in minimally disabled patients with early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:998–1002. doi: 10.1136/jnnp.2003.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deloire MS, Salort E, Bonnet M, Arimone Y, Boudineau M, Amieva H, Barroso B, Ouallet JC, Pachai C, Galliaud E, Petry KG, Dousset V, Fabrigoule C, Brochet B. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:519–526. doi: 10.1136/jnnp.2004.045872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmer BJ, Steup-Beekman GM, Steens SC, Huizinga TW, van Buchem MA, van der Grond J. Correlation of magnetization transfer ratio histogram parameters with neuropsychiatric systemic lupus erythematosus criteria and proton magnetic resonance spectroscopy: association of magnetization transfer ratio peak height with neuronal and cognitive dysfunction. Arthritis Rheum. 2008;58:1451–1457. doi: 10.1002/art.23452. [DOI] [PubMed] [Google Scholar]
- 12.Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, Paulsen JS, Dhawan V, Eidelberg D. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130:2858–2867. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippi M, Agosta F. Magnetization transfer MRI in multiple sclerosis. J Neuroimaging. 2007;17(suppl 1):22S–26S. doi: 10.1111/j.1552-6569.2007.00132.x. [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca MA, Comi G. The use of quantitative magnetic-resonance-based techniques to monitor the evolution of multiple sclerosis. Lancet Neurol. 2003;2:337–346. doi: 10.1016/S1474-4422(03)00408-3. [DOI] [PubMed] [Google Scholar]
- 15.Ghilardi MF, Silvestri G, Feigin A, Mattis P, Zgaljardic D, Moisello C, Crupi D, Marinelli L, Dirocco A, Eidelberg D. Implicit and explicit aspects of sequence learning in pre-symptomatic Huntington’s disease. Parkinsonism Relat Disord. 2008;14:457–464. doi: 10.1016/j.parkreldis.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanyu H, Asano T, Sakurai H, Takasaki M, Shindo H, Abe K. Magnetisation transfer measurements of the subcortical grey and white matter in Parkinson’s disease with and without dementia and in progressive supranuclear palsy. Neuroradiology. 2001;43:542–546. doi: 10.1007/s002340100558. [DOI] [PubMed] [Google Scholar]
- 17.Jurgens CK, van de Wiel L, van Es AC, Grimbergen YM, Witjes-Ané MN, van der Grond J, Middelkoop HA, Roos RA. Basal ganglia volume and clinical correlates in ‘preclinical’ Huntington’s disease. J Neurol. 2008;255:1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- 18.Huntington Study Group Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 19.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;66:81. doi: 10.1111/j.0009-9163.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 20.Mascalchi M, Lolli F, Della NR, Tessa C, Petralli R, Gavazzi C, Politi LS, Macucci M, Filippi M, Piacentini S. Huntington disease: volumetric, diffusion-weighted, and magnetization transfer MR imaging of brain. Radiology. 2004;232:867–873. doi: 10.1148/radiol.2322030820. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, Nopoulos PC. Brain structure in preclinical Huntington’s disease. Biol Psychiatry. 2006;59:57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Rosano C, Sigurdsson S, Siggeirsdottir K, Phillips CL, Garcia M, Jonsson PV, Eiriksdottir G, Newman AB, Harris TB, van Buchem MA, Gudnason V, Launer LJ (2008) Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2008.08.004 [DOI] [PMC free article] [PubMed]
- 23.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, Caplan K, Marek K, Seidman LJ, Makris N, Jenkins BG, Goldstein JM. Evidence for more widespread cerebral pathology in early HD—an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 24.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang YY, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Rao A, De Stefano N, Jenkinson M, Schott JM, Matthews PM, Fox NC. Longitudinal and cross-sectional analysis of atrophy in Alzheimer’s disease: cross-validation of BSI, SIENA and SIENAX. Neuroimage. 2007;36:1200–1206. doi: 10.1016/j.neuroimage.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Zhang YY, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 28.Snowden J, Craufurd D, Griffiths H, Thompson J, Neary D. Longitudinal evaluation of cognitive disorder in Huntington’s disease. J Int Neuropsychol Soc. 2001;7:33–44. doi: 10.1017/S1355617701711046. [DOI] [PubMed] [Google Scholar]
- 29.Tambasco N, Pelliccioli GP, Chiarini P, Montanari GE, Leone F, Mancini ML, Paciaroni M, Gallai V. Magnetization transfer changes of grey and white matter in Parkinson’s disease. Neuroradiology. 2003;45:224–230. doi: 10.1007/s00234-002-0925-5. [DOI] [PubMed] [Google Scholar]
- 30.Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 31.van Buchem MA, McGowan JC, Grossman RI. Magnetization transfer histogram methodology: its clinical and neuropsychological correlates. Neurology. 1999;53:S23–S28. [PubMed] [Google Scholar]
- 32.van Buchem MA, Udupa JK, McGowan JC, Miki Y, Heyning FH, Boncoeur-Martel MP, Kolson DL, Polansky M, Grossman RI. Global volumetric estimation of disease burden in multiple sclerosis based on magnetization transfer imaging. AJNR Am J Neuroradiol. 1997;18:1287–1290. [PMC free article] [PubMed] [Google Scholar]
- 33.van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, Bollen EL, Westendorp RG, van Buchem MA, Middelkoop HA. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann Neurol. 2002;52:62–67. doi: 10.1002/ana.10244. [DOI] [PubMed] [Google Scholar]
- 34.van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, Spilt A, Bollen EL, Westendorp RG, Middelkoop HA, van Buchem MA. Cognitive decline in AD and mild cognitive impairment is associated with global brain damage. Neurology. 2002;59:874–879. doi: 10.1001/archneur.59.5.874. [DOI] [PubMed] [Google Scholar]
- 35.van der Hiele K, Vein AA, van der Welle A, van der Grond J, Westendorp RG, Bollen EL, van Buchem MA, van Dijk JG, Middelkoop HA. EEG and MRI correlates of mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28:1322–1329. doi: 10.1016/j.neurobiolaging.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 36.van Es AC, van der Flier WM, Admiraal-Behloul F, Olofsen H, Bollen EL, Middelkoop HA, Weverling-Rijnsburger AW, van der Grond J, Westendorp RG, van Buchem MA. Lobar distribution of changes in gray matter and white matter in memory clinic patients: detected using magnetization transfer imaging. AJNR Am J Neuroradiol. 2007;28:1938–1942. doi: 10.3174/ajnr.A0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Es AC, van der Flier WM, Admiraal-Behloul F, Olofsen H, Bollen EL, Middelkoop HA, Weverling-Rijnsburger AW, Westendorp RG, van Buchem MA. Magnetization transfer imaging of gray and white matter in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2006;27:1757–1762. doi: 10.1016/j.neurobiolaging.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]