Abstract
Background
Translational research is needed to explore how people will respond to personal genetic susceptibility information related to common health conditions. Maximizing the rigor of this research will require that genetic test results be returned to study participants. Currently, there is no established method that guides the selection of genetic variants to be used in research with these objectives.
Methods and Results
To address this question, we designed a process to identify gene variants and health conditions to be included in a prototype genetic test for use in a larger research effort, the Multiplex Initiative. The intention of this exploration was to facilitate research that generates individual genetic test results that are returned to study participants. Inclusion criteria were developed as part of a transdisciplinary and iterative process that considered the weight of evidential support for genetic association with common health conditions, the appropriateness of use in human subjects research, and the recommendations of expert peer reviewers.
Conclusions
The selection process was designed to identify gene variants for the limited purpose of translational research and, therefore, should not be seen as producing a valid clinical test. However, this example of an applied selection process may provide guidance for researchers who are designing studies to evaluate the implications of genetic susceptibility testing through the return of personalized genetic information. As the rate of genomic discoveries increases, such research will be essential in steering the translation of this information towards the greatest public health benefit.
Key Words: Clinical utility, Common disease, Genetic epidemiology, Genetic testing
A primary objective of the Human Genome Project was to improve public health by creating an infrastructure that could speed the identification of genes associated with health and disease [1,2]. One possible medical benefit of finding such genes is that they could enable personalized risk prediction that, when provided to individuals, may motivate risk-reducing lifestyle changes. However, much research is needed to evaluate whether and how these risk communications might influence recipients. To this end, it is necessary to develop tools that could facilitate translational research related to genetic testing, that is, to explore the clinical utility, public uptake, and social and behavioral impact of such testing [3]. This research is needed prior to applying these genetic testing approaches if they are to inform and guide responsible integration of these applications into medical practice. Alternatively, findings of these studies could reveal that these tests have little clinical potential and thereby help deter their inappropriate use. The need for careful research on the implications of genetic susceptibility testing is underscored by a growing number of private companies that are bypassing the medical community to offer genetic testing services results directly to consumers [4,5,6].
To facilitate the rigorous research needed to evaluate the public health potential of genetic susceptibility testing, scientists must provide target groups with individual genetic test results. Experience tells us that using hypothetical genetic testing scenarios often produces results that are inconsistent with what is observed in practice [7]. Studies involving the return of actual genetic test results are common for high-penetrance genes that convey considerable risk for rare familial diseases [8]. However, there is limited research exploring the implications of conveying test results for low-penetrance genetic variants associated with common health conditions [see for example [9,10,11,12,13]]. Furthermore, these studies have generally focused on feedback related to a single genotype, which is an unlikely model for how these types of results would be conveyed in practice [14]. The following discussion will focus on the implications of genetic susceptibility testing for common health conditions, because these diseases have the largest impact on overall public health in the U.S. and, increasingly, worldwide.
A topic of considerable debate among health researchers is whether the small increases in genetic risk typically found for common health conditions (generally an odds ratio [OR] of <2) will inspire risk reductions that can improve on traditional behavioral interventions [15,16,17,18,19,20]. Although many questions that are central to this debate can best be answered through empirical studies, few have pursued this line of investigation. This is partially due to a perception that the evidential support for most gene-disease associations is insufficient to justify returning individual genetic test results from research and that such information might have insufficient benefits to outweigh potential psychological harm [21,22]. However, these specific concerns are coming into question as scientifically reproducible genetic risk factors are rapidly emerging [23,24,25] and numerous social and behavioral research studies have found no evidence of inappropriate psychological distress following positive genetic test results [26,27,28,29,30,31].
The return of individual genetic test results to research participants has many practical and ethical implications. Briefly, the debate over when it is appropriate to return results generally focuses on the likely impact on study participants, the quality of the evidence for a genetic risk, the health implications of the genetic risk, the capacity of participants to take action to avoid illness, and the context of the research [see for example [32,33,34,35,36,37,38,39,40]]. While some reports have provided informative criteria and guidance concerning returning individual genetic test results, these recommendations are often broad, are not focused on translational research, and have not been developed in the context of actual research applications [38,39,40]. Given this starting point, the question for clinical, social, and behavioral researchers seeking to conduct empirical research becomes how to determine and apply specific standards to produce a prototype genetic susceptibility test.
In this report, we describe the process, considerations, and challenges we addressed in developing a prototype genetic susceptibility test to be used as a translational research tool for the Multiplex Initiative [41]. As such a test would impact different areas of science and medicine, we used a transdisciplinary process to incorporate a variety of perspectives [42]. This approach included multiple rounds of review by an internal steering committee, an external working group, and peer reviewers. The objective of the process was to develop and implement a set of criteria for selecting health conditions and candidate genetic variants that could be included in a research prototype genetic susceptibility test. While the approach used in this study will not be directly applicable to all research objectives, it is intended to be an informative example of one attempt to move from theoretical considerations to actual research practice.
Methods
Process Overview
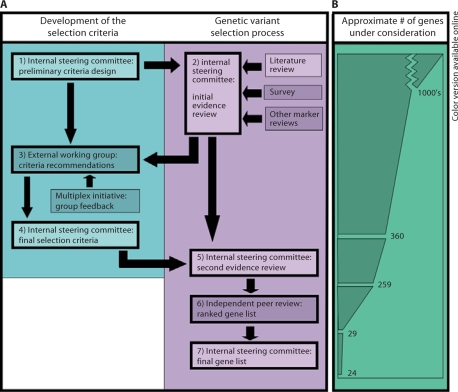
This project was part of the foundational work conducted to develop the Multiplex Initiative. The Multiplex Initiative has been described elsewhere [41], but in brief it is a transdisciplinary research effort that aims to begin exploring the public health implications of genetic susceptibility testing for common health conditions. Selecting gene variants and health conditions for the Multiplex Initiative involved a number of interactive steps, as outlined in figure 1A. Initially, working groups that included a broad representation of scientists from different disciplines engaged in deliberations to develop selection criteria and apply these criteria to the evidence base for genetic variants associated with common health conditions. Gene variants that met criteria identified by the working groups were subjected to an independent peer review by an expert panel whose recommendations were used to develop a final list of gene variants. In the following sections, we describe the specific steps that this process comprised.
Fig. 1.
Outline of the study process. A Flow diagram of the study. Boxes with heavy outlines represent major steps in the process. Lightened boxes indicate steps performed at NHGRI, while darkened boxes represent steps performed by external groups. B Representative chart showing the number of genetic variants under consideration at different points in the genetic variant selection process. Not to scale.
Step One
A subset of us (CHW, CMM, and LCB) served as an internal steering committee that outlined and oversaw the steps involved in developing criteria and candidates for a research prototype test. This committee has expertise in public health research, social and behavioral research, bioethics, and genetic epidemiologic research. The internal steering committee created a broad set of criteria to screen through the evidence for gene-disease associations. In brief, these criteria focused on health conditions that might plausibly have an impact on public health and for which there had been some evidence of association with common genetic variants.
Step Two
To identify candidate genetic variants from among the vast number of reported gene-disease associations, the internal steering committee used a multi-step process that drew on a range of informational resources. The goal of this process was not to identify every single genetic variant with the possibility of inclusion through a systematic review, but rather to explore the existing literature to identify a set of variants that would be appropriate for the research goals of the Multiplex Initiative. Additionally, while the process was aimed at identifying multiple variants that could be used in a multi-gene (multiplex) genetic test, we do not intend the risk information from these variants to be combined to create an overall disease risk, as there currently is little evidence clarifying how genetic risks interact. First, an informal online survey of genetic epidemiologists was conducted to solicit suggestions of gene variants that, in these experts’ opinions, had the ‘best’ evidence to support a robust association with common health conditions. This survey elicited responses from 25 researchers, with a resultant list of about 50 proposed genes. Additionally, the internal steering committee identified and integrated other gene variant lists that were publicly available [for example [43,44]] or that had been published in overviews of the genetic epidemiology of common health conditions. Finally, the internal steering committee surveyed the literature using references and PubMed searches between 7/05 and 4/06 (while information on the risk associations that were identified was updated, genetic risks reported subsequent to these dates did not go through the complete selection process). Examples of search terms included combinations of health condition names (e.g., osteoporosis), ‘genetic,’ ‘meta-analysis,’ ‘review,’ gene name (e.g., Calpain 10), gene symbol (e.g., CETP), and polymorphism (e.g., 677C>T, rs1801133). Because of the open-ended nature of the process and the broad informational needs of the study, this was not conducted as a formal literature review aimed at hypothesis testing. The search applied the broad initial selection criteria designated by the internal steering committee to find studies and meta-analyses, which were then used to identify other gene associations and evaluate the evidential support for the genes recommended in the survey and other review projects.
Step Three
An external working group was responsible for weighing the different issues relevant to determining which health conditions and gene-disease associations had a sufficient evidence base to be appropriate for returning individual genetic test results. The external working group was composed of 6 researchers in the fields of molecular biology, genetic counseling, nutrition, epidemiology, medical research, and genetic epidemiology. Group members were drawn from academic and government institutions. The external working group reviewed the steering committee's selection criteria and examples of genetic variants meeting the criteria. The group met regularly over a period of 4 months, developed the selection criteria, and integrated suggestions from a diverse group of 25 outside clinicians and researchers who were involved with the Multiplex Initiative. The external working group then assembled a report detailing the criteria and considerations for designing a prototype test.
Step Four
The internal steering committee used the recommendations of the external working group to decide on the final selection criteria (table 1). The criteria underwent further refinement at this point. For example, where the external working group suggested a range, the final criteria specified an exact cut-off value. The final criteria for selecting health conditions and genetic variants are as follows:
Table 1.
Examples of applied selection criteria
| Category | Criteria | Example of excluded disease/gene | Example of included disease/gene |
|---|---|---|---|
| Health condition | Large public health impact | Chronic beryllium disease | Lung cancer |
| Adult onset | Asthma | Osteoporosis | |
| Accepted clinical recommendations for prevention | Drug metabolism | Type 2 diabetes | |
| Applicable to everyone tested | Prostate and breast cancer | Colorectal cancer | |
| Genetic variant | Consistent replication of the disease association | CASR (osteoporosis) | MC1R (melanoma) |
| Studies include at least 500 cases | KIP2 (osteoporosis) | MTHFR (colorectal cancer) | |
| Minimum of 10% change in risk | NAT2 (lung cancer) | TCF7L2 (type 2 diabetes) | |
| Over 5% frequency in major US population | AAT (pulmonary disease) | GSTM1 (lung cancer) | |
| No pleiotropy with another disease without primary prevention measures | APOE4 (cardiovascular and Alzheimer's | CETP (cardiovascular disease) | |
Health Condition Selection Criteria. The common health conditions that were identified by the internal steering committee and external working group as being candidates for inclusion in a genetic susceptibility test were chosen based on several considerations (table 1). The first was that the health condition should have public health importance in the U.S. (i.e., based on prevalence, severity, and associated healthcare costs). Second, the health condition had to be adult-onset, since return of individual genetic susceptibility test results to children would be too controversial and, all agreed, premature. Another consideration was that the health condition be preventable, that is, there be accepted clinical recommendations for reducing risk for the health condition. A final consideration was that the health condition be relevant to a broad population, and not specific to a gender or ethnic group.
Genetic Variant Selection Criteria. We specified minimum criteria for genetic variants to be considered appropriate for inclusion in any prototype test (step 3 and 4, table 1). First, the genetic association must have robust evidence in the literature, including replication of the risk association. Second, we required that the evidence base include studies of sufficient size to support the association, optimally derived from a meta-analysis, but with the criterion of including a minimum of 500 cases. Whenever possible, the epidemiologic quality of the research and existence of plausible biological explanations for the association were also evaluated. Third, the minimum strength of association was selected based on the range of associations likely to be observed for genetic risks for common health conditions, and therefore associated risks were required to have at least a 10% increase or decrease in susceptibility to be considered (excepted from this criterion were statistical measures of biological states, such as cholesterol levels). As the intention was to select gene variants for a test to be used in population-based research, the risk gene variant needed to be carried by a reasonably large proportion of the population (>5% in a major U.S. population). We used the risk unit reported in the original association papers (usually a single genotype, but sometimes reports combined genotypes) in determining whether the genetic variant met the magnitude of the association and the population prevalence requirements. Finally, we excluded genetic variants that were associated with multiple health conditions (i.e., pleiotropic) if any of the conditions lacked evidence-based prevention strategies.
Step Five
In step 5, the internal steering committee applied the final selection criteria to identify candidate conditions and genes for test inclusion. Each gene was ranked based on their appropriateness for returning individual test results to study participants. This process resulted in a gene variant list that was stratified into four tiers, with the top two tiers containing the strongest candidates for inclusion.
Step Six
The internal steering committee solicited peer reviews of the candidate gene variants from six leaders in the field of genetic epidemiology. Using the established marker selection criteria, the peer reviewers were instructed to evaluate the weight of the evidence for the association of each gene variant on the list and submit their own rankings on a range from ‘unacceptable’ to ‘strong.’ Any gene variant that on average received an ‘acceptable,’ ‘good,’ or ‘strong’ ranking was kept on the list, while those receiving lower ranking on average were removed.
Step Seven
The recommendations of the independent peer reviewers were then used by the internal steering committee to develop a final list of gene variants meeting all selection criteria.
Results
Identification of Genetic Variants
To start the processes, an initial information-gathering step was conducted that examined the breadth of data available on genetic associations with disease. It was deliberately designed to cast a wide net that allowed the inclusion of health conditions and gene variants covering a range of public health significance. While the processes for identifying genetic variants was not intended to be exhaustive of all possible candidates, it did use a range of different types of informational sources aimed at exploring the many associations in the literature (see Methods). In total, 360 gene variants with potential for inclusion based on reported associations with increased risk for a health condition (figure 1B) were identified.
Health Condition Criteria
Application of the final selection criteria for health conditions developed by the internal steering committee (step 5) reduced the pool of variants to those associated with the largest public health threats. Following the application of these selection criteria, 259 genetic variants associated with several health conditions (e.g., cardiovascular disease, obesity, type 2 diabetes, osteoporosis, and several cancers) remained under consideration. To illustrate the deliberative nature of this selection process, we present a series of examples where the criteria were used in assessing various health conditions (see below and table 1).
Health Condition Criterion 1. Health conditions were expected to have a substantial public health impact, as measured by prevalence, severity, and healthcare costs. For example, health conditions such as chronic beryllium disease may be addressed by genetic susceptibility testing for those who have an environmental exposure to beryllium [45]. However, chronic beryllium disease is uncommon, affecting ∼1% of a distinct group, generally industrial metal workers exposed to beryllium dust [45]. Thus, while genetic testing might be appropriate for this targeted group, chronic beryllium disease was excluded from consideration for a research genetic susceptibility test that is focused on the general population. By comparison, lung cancer is a relatively rare disease in the absence of smoking, but given the high prevalence of tobacco use, there is an approximately 7% lifetime risk of developing the disease in the U.S. population [46]. Additionally, the 5-year survival rate is only about 15%. Therefore, lung cancer was found to meet this criterion.
Health Condition Criterion 2. Requiring that the diseases be adult-onset resulted in the elimination of several health conditions. Asthma, although levying a major public health burden, was excluded because it generally presents in childhood [47]. In contrast, osteoporosis was included because it generally develops in people over the age of 50 [48]. Although it could be argued that preventive strategies for osteoporosis are most effective when initiated in childhood, almost all adult-onset health conditions would have been omitted if held strictly to this standard.
Health Condition Criterion 3. Availability of accepted clinical recommendations for prevention excluded gene variants associated with drug metabolism, often considered under the rubric of pharmacogenomics. Although the evidence base in this domain is perhaps the best- explored topic for genetic variation research, there are few well-developed drug dose recommendations based on genetic test results [49]. By comparison, genetic variants associated with type 2 diabetes were included as it is accepted that weight loss, exercise, and diet restrictions significantly reduce the risk of developing the disease [50].
Health Condition Criterion 4. Requiring the health conditions to be relevant to a broad range of the population (defined as everyone having a significant likelihood of developing a disease) excluded a number of diseases. For example, prostate and breast cancer were not included because they are gender-specific. However, gene variants associated with colorectal cancer, which has similar incidence rates between men and women, were included for consideration [46].
Genetic Variant Criteria
The exercise of applying selection criteria for gene variants met several challenges. First and foremost was the need to evaluate a vast and diverse body of evidence in the scientific literature. Initial selection of genetic variants was carried out by the internal steering committee over a period of 6 months (step 5). The results of these efforts were subsequently reviewed by the independent peer reviewers (step 6). The recommendations of this external review were then applied in the final selection (step 7) with the result that only 24 of the 259 gene variants considered met the selection criteria described in this report (for further information see supplementary table 1, www.karger.com/doi/10.1159/000236061). To illustrate this process, examples of the deliberations over which genetic variants to include based upon the selection criteria are provided below.
Genetic Variant Criterion 1. Requiring that genetic associations should be replicated in most follow-up studies to indicate sufficiently stable evidence to justify the return of results excluded the CASR A986S polymorphism. Despite promising initial findings of an association of this polymorphism with bone density [51,52], subsequent reports did not confirm this association [53,54,55]. In contrast, there is a highly consistent association with the RHC (red hair color) variants that disrupt the function of the MC1R gene and an increased risk of melanoma [56,57,58].
Genetic Variant Criterion 2. Requiring that the evidence to support associations be based on studies with at least 500 cases excluded the CDKN1C (KIP2) homozygous deletion genotype that has been associated with osteoporosis [59]. Although one study showed a significant association of this genotype with osteoporosis, the sample size was only 154 participants [59]. Without further published work to support this finding, CDKN1C did not meet our criterion for robust evidence and so was excluded from consideration. By comparison, the significant association of the MTHFR 677C>T polymorphism with increased risk for colorectal cancer has been examined in studies totaling over 8,000 cases and was included in the final list [60].
Genetic Variant Criterion 3. Requiring gene variants to be associated with at least a 10% increase or decrease in risk for the health condition eliminated the NAT2 slow acetylation polymorphism from consideration as a risk marker for lung cancer. Although some studies have found an association of this polymorphism with lung cancer, the NAT2 slow acetylation polymorphism does not appear to have a strong overall impact on disease risk, with an OR of 1.04 (0.96–1.14) [61]. However, an example of a robust association included in the final list is the TCF7L2 rs12255372 variant which has a per allele OR of 1.48 (1.37–1.60) for type 2 diabetes [62].
Genetic Variant Criterion 4. Requiring that the genetic risk variants have a population prevalence of at least 5% eliminated the Z variant of alpha-1 antitrypsin gene (AAT). Although this variant is a well-established risk factor for chronic obstructive pulmonary disease, the Z allele is found in only 2–4% of the U.S. population [63]. In contrast, the homozygous GSTM1 null-null genotype, which is associated with lung cancer risk, has a prevalence of approximately 50% in the U.S. [64] and was included in the final list.
Genetic Variant Criterion 5. Requiring that the gene variant should not have pleiotropic associations with health conditions with no established prevention measures eliminated the APOE e4 variant. APOE e4 has been associated with increased risk of cardiovascular disease and with Alzheimer's disease. As there are no validated prevention strategies for Alzheimer's disease, APOE e4 was excluded from consideration. In contrast, the cardiovascular disease risk variant, CETP Taq1B, has been studied in the context of several health conditions [65], but was included in the final list because it does not yet appear to have a sufficiently strong association with other non-preventable health condition to warrant exclusion.
An example of an optimal variant to be included in a prototype genetic susceptibility test would be KCNJ11. Research indicates that KCNJ11 is associated with type 2 diabetes, a significant public health problem that is the 6th leading cause of mortality in the U.S. [66] and has a lifetime risk of approximately 35% [67]. The KCNJ11 gene encodes a component of the ATP-sensitive K+ channel, which is involved in glucose-induced insulin secretion [68]. The function of this channel has been shown to be disrupted by the E23K polymorphism, providing it with biological plausibility as a possible factor in disease development [69]. Case-control studies have broadly replicated the E23K polymorphisms association with type 2 diabetes, with more than 7,700 subjects examined in over 9 studies [68]. Additionally, the increase in risk is significant, with an OR of 1.12 and 1.44 for heterozygotes and homozygotes, respectively. This association with type 2 diabetes has recently been confirmed in several genome-wide association studies [70]. Given this evidence, KCNJ11 is representative of a gene variant selected by our deliberative process that constitutes an ideal candidate for inclusion in a genetic susceptibility test where research subjects are to receive information about their personal genetic risks.
Discussion
If the knowledge gained from the Human Genome Project is going to be used to improve public health, translational research exploring clinical applications will be required. Unfortunately, in contrast to the rapid pace of discovery of gene-disease associations for common health conditions, research to understand the clinical potential of these findings has lagged. This gap in knowledge could slow appropriate uses of genetic susceptibility testing and fail to deter the premature application of tests that have little clinical utility.
The transdisciplinary and iterative process we used to arrive at a prototype genetic susceptibility test for translational research illustrates one approach for advancing a field of investigation. We do not suggest this approach as the definitive process for determining which gene variants are appropriate for conveying test results. Rather, this process has both strengths and limitations expected in an initial exploration of a complex research question. Among the strengths of this study is the fact that it used multiple information sources, including expert recommendations, the scientific literature, and other related research. Also, input on the criteria for the test was elicited from experts in a range of disciplines and institutions at multiple steps in the process. This approach was intended to increase the likelihood that considerations relevant to the project would be effectively addressed. Weaknesses include the fact that we used an evolving and exploratory approach to our investigation of the literature to account for new suggestions and input throughout the process, rather than a formal literature review. Additionally, we relied heavily on expert opinion and did not exclude meta-analysis with significant heterogeneity. Others considering similar efforts may also want to consider alternative approaches. These may range from the decision to select a single information source (e.g., scientific publications) or analysis group (e.g., an expert panel) to an expansive approach that attempts to integrate as much data and perspectives as possible. One could also choose between a more informal process and a comprehensive analysis combined with multiple rounds of review. Regardless of the approach chosen to meet the needs of a particular study, our experience provided us with a perspective and an operational framework that may be informative for future projects.
Our selection criteria have similarities to recommendations reported by others in that we engaged with a set of questions about appropriate genetic evidence and disease impact. The existing differences in perspective hinge primarily on the fact that other reports focused on genetic epidemiological studies (where returning individual genetic test results was a secondary concern), while we focused on developing a translational research test where the very purpose of the study was to provide study participants with their individual genetic test results. In the context of clinical, social, and behavioral research objectives, different criteria were seen as important. For example, we were willing to consider returning individual test results for genetic variants that have a comparatively small impact on risk for a health condition (as opposed to criteria for genetic epidemiology studies where a previously suggested cutoff was an OR of 2.0 [38]). This decision recognizes that relative risks associated with genetic variants for common health conditions will remain low even as evidence continues to accrete and that translational research should reflect this reality [71]. Additionally, we added criteria eliminating health conditions without preventive measures, along with genetic variants that had pleiotropic risks for these types of health conditions. These criteria made sense for research studying the use of genetic risk factors in the prevention of common health conditions because there should be an appropriate behavior change recommendation.
The criteria for this study were selected with the goals of the Multiplex Initiative in mind and the understanding that each study may find it necessary to develop customized approaches. Under certain circumstances, it may make sense to disregard some of our criteria or to make them more stringent. For instance, if the target population had been men, it might have made sense to include more gender-specific markers, such as those that increase risk for prostate cancer, and exclude markers indicating osteoporosis risk. Alternatively, if the objective is to provide direct medical recommendations of treatments or interventions that are made on the basis of a specific genotype, then it may be necessary to require more stringent criteria for evidence that more closely resemble what would be expected from a clinical test. It also may be helpful to create other criteria, be they population-specific (e.g., requiring that a variant has been studied in groups with similar ancestry to the study participants), disease-specific (e.g., the variant must be involved in the metabolic syndrome), platform-specific (e.g., the variant can be tested using Illumina bead arrays), age-specific (e.g., the average age of disease onset should be between 20 and 40), culture-specific (e.g., the variants should not have implications that could influence the stigmatization of a group), or any of a range of other considerations relevant to a study. Furthermore, it may be appropriate to select multiple or single gene variants for one or several health conditions. For example, of the genetic variants identified in this paper, the genetic test used in the Multiplex Initiative contains 15 genetic variants for several overarching health conditions [41].
One of the major challenges of this project was to identify gene variants in an environment where new disease-gene associations are reported almost weekly. In fact, such reviews are likely to become more difficult as further gene-gene and gene-environment interactions are uncovered. As a consequence of these changes, lists of genetic variants appropriate for test inclusion may have a fairly short shelf life. For example, subsequent to our initial selection process there has been a torrent of published genome-wide association (GWA) studies [25]. As of early 2008, GWA studies found associations with 8 of the genes identified in our initial review that reached genome-wide significance [23,70,72,73,74,75,76,77,78,79,80,81,82,83]. Interpreting the implications for the other genetic variants examined that did not show an association is complicated by the fact that most GWA studies have a low power to detect genetic variants with a modest impact on disease risk [84]. It remains to be seen how many of the other genetic variants will be verified as future GWA studies examine more disease states and further replicate associations. In addition to these findings, many previously unknown genetic associations have been reported in GWA studies, such that a review similar to the one we performed would produce more genetic variants if conducted today (we estimate that our criteria would include another 30 genes if applied to GWA studies published through early 2008). While the pace of discovery using this method may slow once the most easily identified variants are found, we expect that the majority of common disease phenotypes will have genes associated with them in the near future. These rapid changes are notable for those planning research because as a prospective study is in process, new markers may need to be added or removed from the test and scientific updates communicated to study participants in the form of newsletters or other correspondence.
It will also be important to consider the implications of research that clarifies gene-gene interactions and results in combined genetic risk estimates for different diseases. For example, one study that looked at type 2 diabetes risk with three genetic markers found a gradient of risk that increased with the number of genetic risk variants carried [85]. This risk ranged up to a nearly 6-fold increase in those carrying 6 risk alleles, which is much larger than the 20–30% increases expected for most individual variants. Such research may increase the relevance of testing for genetic susceptibility variants in the context of public health, because it can identify those individuals with high combined genetic risks [14].
This study may have implications in a broader scientific and policy context. The process described here uses selection criteria that were intended only for this limited exercise of developing a prototype genetic test to be used for translational research. The genetic variants selected should not be taken as appropriate for clinical testing. However, it is worth noting that many of the genetic markers on our list are already available through commercial genetic tests. In fact, of the 24 genetic markers identified in the initial review, more than half are now available for purchase by individual consumers. Given this context, research using these markers may have implications for the wider debate over the appropriateness of direct-to-consumer genetic testing. Furthermore, while a genetic susceptibility test derived from our set of genetic markers will not fully represent the characteristics of the tests that could eventually be used in a clinical context, it may be able to model the type of information that could arise from personal genome sequencing. Such research holds the potential to uncover the crucial factors that allow genetic susceptibility testing to be directed towards the greatest public health benefit.
Acknowledgements
We would like to thank the members of the external working group, Tanya Agurs-Collins, Holly Peay, Karen Mohlke, and Giovanni Cizza. Additionally, we appreciate the recommendations of our peer reviewers, Muin Khoury, Janet Stanford, Timothy Rebbeck, Alisa Goldstein, and Elaine Ostrander. We also thank Elizabeth Gillanders and other participants in the Multiplex Initiative planning meeting for their useful comments. This research was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
No statement in this article should be construed as an official position of the National Human Genome Research Institute, National Institutes of Health or Department of Health and Human Services.
References
- 1.Collins FS, Green ED, Guttmacher AE, Guyer MS, US National Human Genome Research Institute A vision for the future of genomics research. Nature. 2003;422:835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 2.Collins FS, McKusick VA. Implications of the Human Genome Project for medical science. JAMA. 2001;285:540–544. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 3.McBride CM. Blazing a trail: a public health research agenda in genomics and chronic disease. Prev Chronic Dis. 2005;2:A04. [PMC free article] [PubMed] [Google Scholar]
- 4.Haga SB, Khoury MJ, Burke W. Genomic profiling to promote a healthy lifestyle: not ready for prime time. Nat Genet. 2003;34:347–350. doi: 10.1038/ng0803-347. [DOI] [PubMed] [Google Scholar]
- 5.Janssens AC, Gwinn M, Bradley LA, Oostra BA, van Duijn CM, Khoury MJ. A critical appraisal of the scientific basis of commercial genomic profiles used to assess health risks and personalize health interventions. Am J Hum Genet. 2008;82:593–599. doi: 10.1016/j.ajhg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geransar R, Einsiedel E. Evaluating online direct-to-consumer marketing of genetic tests: informed choices or buyers beware? Genet Test. 2008;12:13–23. doi: 10.1089/gte.2007.0024. [DOI] [PubMed] [Google Scholar]
- 7.Persky S, Kaphingst KA, Condit CM, McBride CM. Assessing hypothetical scenario methodology in genetic susceptibility testing analog studies: a quantitative review. Genet Med. 2007;9:727–738. doi: 10.1097/gim.0b013e318159a344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beery TA, Williams JK. Risk reduction and health promotion behaviors following genetic testing for adult-onset disorders. Genet Test. 2007;11:111–123. doi: 10.1089/gte.2006.0527. [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, Wilfond B, Louben G, Caporaso N. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking-related cognitions, emotions, and behavior change. Health Psychol. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Matsuo K, Wakai K, Saito T, Kumimoto H, Okuma K, Tajima K, Hamajima N. An intervention study of smoking cessation with feedback on genetic cancer susceptibility in Japan. Prev Med. 2006;42:102–108. doi: 10.1016/j.ypmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.McBride CM, Bepler G, Lipkus IM, Lyna P, Samsa G, Albright J, Datta S, Rimer BK. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11:521–528. [PubMed] [Google Scholar]
- 12.Sanderson SC, Humphries SE, Hubbart C, Hughes E, Jarvis MJ, Wardle J. Psychological and behavioural impact of genetic testing smokers for lung cancer risk: a phase II exploratory trial. J Health Psychol. 2008;13:481–494. doi: 10.1177/1359105308088519. [DOI] [PubMed] [Google Scholar]
- 13.Harvey-Berino J, Gold EC, West DS, Shuldiner AR, Walston J, Starling RD, Nolan A, Silver K, Poehlman ET. Does genetic testing for obesity influence confidence in the ability to lose weight? A pilot investigation. J Am Diet Assoc. 2001;101:1351–1353. doi: 10.1016/S0002-8223(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Khoury MJ, Botto L, Friedman JM, Flanders WD. Improving the prediction of complex diseases by testing for multiple disease-susceptibility genes. Am J Hum Genet. 2003;72:636–649. doi: 10.1086/367923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell J. Predicting disease using genomics. Nature. 2004;429:453–456. doi: 10.1038/nature02624. [DOI] [PubMed] [Google Scholar]
- 16.Holtzman NA. What role for public health in genetics and vice versa? Community Genet. 2006;9:8–20. doi: 10.1159/000090688. [DOI] [PubMed] [Google Scholar]
- 17.Khoury MJ, Gwinn M. What role for public health in genetics and vice versa? Community Genet. 2006;9:282. doi: 10.1159/000094481. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan AV, Weiss KM, Fullerton SM. Dissecting complex disease: the quest for the Philosopher's Stone? Int J Epidemiol. 2006;35:562–571. doi: 10.1093/ije/dyl001. [DOI] [PubMed] [Google Scholar]
- 19.Khoury MJ, Gwinn M. Genomics, epidemiology, and common complex diseases: let's not throw out the baby with the bathwater! Int J Epidemiol. 2006;35:1363–1364. doi: 10.1093/ije/dyl214. [DOI] [PubMed] [Google Scholar]
- 20.Burke W, Psaty BM. Personalized medicine in the era of genomics. JAMA. 2007;298:1682–1684. doi: 10.1001/jama.298.14.1682. [DOI] [PubMed] [Google Scholar]
- 21.McBride CM, Brody LC. Genetic risk feedback for common disease: time to test the waters. Cancer Epidemiol Biomarkers Prev. 2007;16:1724–1726. doi: 10.1158/1055-9965.EPI-07-0102. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PA. Counterpoint: genetic risk feedback for common disease – time to test the waters. Cancer Epidemiol Biomarkers Prev. 2007;16:1727–1729. doi: 10.1158/1055-9965.EPI-07-0213. [DOI] [PubMed] [Google Scholar]
- 23.Florez JC. The new type 2 diabetes gene TCF7L2. Curr Opin Clin Nutr Metab Care. 2007;10:391–396. doi: 10.1097/MCO.0b013e3281e2c9be. [DOI] [PubMed] [Google Scholar]
- 24.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 25.Seng KC, Seng CK. The success of the genome-wide association approach: a brief story of a long struggle. Eur J Hum Genet. 2008;16:554–564. doi: 10.1038/ejhg.2008.12. [DOI] [PubMed] [Google Scholar]
- 26.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10:19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- 27.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 28.Meiser B, Dunn S. Psychological effect of genetic testing for Huntington's disease: an update of the literature. West J Med. 2001;174:336–340. doi: 10.1136/ewjm.174.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology. 2005;14:1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 30.Meiser B, Collins V, Warren R, Gaff C, St John DJ, Young MA, Harrop K, Brown J, Halliday J. Psychological impact of genetic testing for hereditary non-polyposis colorectal cancer. Clin Genet. 2004;66:502–511. doi: 10.1111/j.1399-0004.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Green RC. Genetic risk assessment for adult children of people with Alzheimer's disease: the Risk Evaluation and Education for Alzheimer's Disease (REVEAL) study. J Geriatr Psychiatry Neurol. 2005;18:250–255. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez CV, Kodish E, Weijer C. Informing study participants of research results: an ethical imperative. IRB. 2003;25:12–19. [PubMed] [Google Scholar]
- 33.Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- 34.Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294:737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- 35.Meltzer LA. Undesirable implications of disclosing individual genetic results to research participants. Am J Bioeth. 2006;6:28–30. doi: 10.1080/15265160600935811. author reply W10–12. [DOI] [PubMed] [Google Scholar]
- 36.Ossorio PN. Letting the gene out of the bottle: a comment on returning individual research results to participants. Am J Bioeth. 2006;6:24–25. doi: 10.1080/15265160600935555. author reply W10–12. [DOI] [PubMed] [Google Scholar]
- 37.Clayton EW, Ross LF. Implications of disclosing individual results of clinical research. JAMA. 2006;295:37. doi: 10.1001/jama.295.1.37-a. author reply 37–38. [DOI] [PubMed] [Google Scholar]
- 38.Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, Koski G, Motulsky A, Wilfond B, Manolio TA, Fabsitz RR, Luepker RV. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140:1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renegar G, Webster CJ, Stuerzebecher S, Harty L, Ide SE, Balkite B, Rogalski-Salter TA, Cohen N, Spear BB, Barnes DM, Brazell C. Returning genetic research results to individuals: points-to-consider. Bioethics. 2006;20:24–36. doi: 10.1111/j.1467-8519.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 40.Ravitsky V, Wilfond BS. Disclosing individual genetic results to research participants. Am J Bioeth. 2006;6:8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- 41.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Putting science over supposition in the arena of personalized genomics. Nat Genet. 2008;40:939–942. doi: 10.1038/ng0808-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi BC, Pak AW. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin Invest Med. 2006;29:351–364. [PubMed] [Google Scholar]
- 43.Epidemiology and Surveillance Work Group (2004) Disease Database, North Carolina Task Force on Genomics and Public Health. Available at http://statgen.ncsu.edu/ggibson/nc_gph/index.html
- 44.National Office of Genomics and Disease Prevention: Genomics and Populations Health: United States, 2003, in National Office of Genomics and Disease Prevention CDC, Atlanta, CDC, 2004. Available at http://www.cdc.gov/genomics/activities/OGDP/2003/chap01.htm
- 45.Silver K, Sharp RR. Ethical considerations in testing workers for the -Glu69 marker of genetic susceptibility to chronic beryllium disease. J Occup Environ Med. 2006;48:434–443. doi: 10.1097/01.jom.0000200878.16077.3b. [DOI] [PubMed] [Google Scholar]
- 46.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK (eds): SEER Cancer Statistics Review, 1975–2006, National Cancer Institute, Bethesda, MD. Available at http://seer.cancer.gov/csr/1975_2006/
- 47.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146:888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 48.Melton LJ, 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–1010. doi: 10.1002/jbmr.5650070902. [DOI] [PubMed] [Google Scholar]
- 49.Walgren RA, Meucci MA, McLeod HL. Pharmacogenomic discovery approaches: will the real genes please stand up? J Clin Oncol. 2005;23:7342–7349. doi: 10.1200/JCO.2005.03.0825. [DOI] [PubMed] [Google Scholar]
- 50.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorentzon M, Lorentzon R, Lerner UH, Nordstrom P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur J Endocrinol. 2001;144:257–261. doi: 10.1530/eje.0.1440257. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamoto K, Orimo H, Hosoi T, Miyao M, Ota N, Nakajima T, Yoshida H, Watanabe S, Suzuki T, Emi M. Association of bone mineral density with polymorphism of the human calcium-sensing receptor locus. Calcif Tissue Int. 2000;66:181–183. doi: 10.1007/pl00005835. [DOI] [PubMed] [Google Scholar]
- 53.Bollerslev J, Wilson SG, Dick IM, Devine A, Dhaliwal SS, Prince RL. Calcium-sensing receptor gene polymorphism A986S does not predict serum calcium level, bone mineral density, calcaneal ultrasound indices, or fracture rate in a large cohort of elderly women. Calcif Tissue Int. 2004;74:12–17. doi: 10.1007/s00223-002-0066-1. [DOI] [PubMed] [Google Scholar]
- 54.Young R, Wu F, Van de Water N, Ames R, Gamble G, Reid IR. Calcium sensing receptor gene A986S polymorphism and responsiveness to calcium supplementation in postmenopausal women. J Clin Endocrinol Metab. 2003;88:697–700. doi: 10.1210/jc.2002-020355. [DOI] [PubMed] [Google Scholar]
- 55.Takacs I, Speer G, Bajnok E, Tabak A, Nagy Z, Horvath C, Kovacs K, Lakatos P. Lack of association between calcium-sensing receptor gene A986S polymorphism and bone mineral density in Hungarian postmenopausal women. Bone. 2002;30:849–852. doi: 10.1016/s8756-3282(02)00741-x. [DOI] [PubMed] [Google Scholar]
- 56.Palmer JS, Duffy DL, Box NF, Aitken JF, O'Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 58.Landi MT, Kanetsky PA, Tsang S, Gold B, Munroe D, Rebbeck T, Swoyer J, Ter-Minassian M, Hedayati M, Grossman L, Goldstein AM, Calista D, Pfeiffer RM. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J Natl Cancer Inst. 2005;97:998–1007. doi: 10.1093/jnci/dji176. [DOI] [PubMed] [Google Scholar]
- 59.Urano T, Hosoi T, Shiraki M, Toyoshima H, Ouchi Y, Inoue S. Possible involvement of the p57(Kip2) gene in bone metabolism. Biochem Biophys Res Commun. 2000;269:422–426. doi: 10.1006/bbrc.2000.2306. [DOI] [PubMed] [Google Scholar]
- 60.Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci. 2005;96:535–542. doi: 10.1111/j.1349-7006.2005.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borlak J, Reamon-Buettner SM. N-acetyltransferase 2 (NAT2) gene polymorphisms in colon and lung cancer patients. BMC Med Genet. 2006;7:58. doi: 10.1186/1471-2350-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, van Dam RM, Hu FB. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55:2645–2648. doi: 10.2337/db06-0643. [DOI] [PubMed] [Google Scholar]
- 63.Dahl M, Hersh CP, Ly NP, Berkey CS, Silverman EK, Nordestgaard BG. The protease inhibitor PI*S allele and COPD: a meta-analysis. Eur Respir J. 2005;26:67–76. doi: 10.1183/09031936.05.00135704. [DOI] [PubMed] [Google Scholar]
- 64.Engel LS, Taioli E, Pfeiffer R, Garcia-Closas M, Marcus PM, Lan Q, Boffetta P, Vineis P, Autrup H, Bell DA, Branch RA, Brockmoller J, Daly AK, Heckbert SR, Kalina I, Kang D, Katoh T, Lafuente A, Lin HJ, Romkes M, Taylor JA, Rothman N. Pooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: a HuGE review. Am J Epidemiol. 2002;156:95–109. doi: 10.1093/aje/kwf018. [DOI] [PubMed] [Google Scholar]
- 65.Boekholdt SM, Sacks FM, Jukema JW, Shepherd J, Freeman DJ, McMahon AD, Cambien F, Nicaud V, de Grooth GJ, Talmud PJ, Humphries SE, Miller GJ, Eiriksdottir G, Gudnason V, Kauma H, Kakko S, Savolainen MJ, Arca M, Montali A, Liu S, Lanz HJ, Zwinderman AH, Kuivenhoven JA, Kastelein JJ. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 2005;111:278–287. doi: 10.1161/01.CIR.0000153341.46271.40. [DOI] [PubMed] [Google Scholar]
- 66.National Center for Health Statistics: Health, United States, 2006, in: Statistics N.C.H.S., U.S. Government Printing Office, Hyattsville, 2006. Available at http://www.cdc.gov/nchs/data/hus/hus06.pdf
- 67.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 68.van Dam RM, Hoebee B, Seidell JC, Schaap MM, de Bruin TW, Feskens EJ. Common variants in the ATP-sensitive K+ channel genes KCNJ11 (Kir6.2) and ABCC8 (SUR1) in relation to glucose intolerance: population-based studies and meta-analyses. Diabet Med. 2005;22:590–598. doi: 10.1111/j.1464-5491.2005.01465.x. [DOI] [PubMed] [Google Scholar]
- 69.Schwanstecher C, Meyer U, Schwanstecher M. KIR6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K+ channels. Diabetes. 2002;51:875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 70.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8:657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 71.Ioannidis JP, Trikalinos TA, Khoury MJ. Implications of small effect sizes of individual genetic variants on the design and interpretation of genetic association studies of complex diseases. Am J Epidemiol. 2006;164:609–614. doi: 10.1093/aje/kwj259. [DOI] [PubMed] [Google Scholar]
- 72.Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Palsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- 73.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marcano AC, Hajat C, Burton P, Deloukas P, Brown M, Connell JM, Dominiczak A, Lathrop GM, Webster J, Farrall M, Spector T, Samani NJ, Caulfield MJ, Munroe PB. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82:139–149. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kathiresan S, Manning AK, Demissie S, D'Agostino RB, Surti A, Guiducci C, Gianniny L, Burtt NP, Melander O, Orho-Melander M, Arnett DK, Peloso GM, Ordovas JM, Cupples LA. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;19(suppl 1):S17. doi: 10.1186/1471-2350-8-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, Novartis Institutes of BioMedical Research, Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Råstam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjögren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 76.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 78.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 80.Salonen JT, Uimari P, Aalto JM, Pirskanen M, Kaikkonen J, Todorova B, Hyppönen J, Korhonen VP, Asikainen J, Devine C, Tuomainen TP, Luedemann J, Nauck M, Kerner W, Stephens RH, New JP, Ollier WE, Gibson JM, Payton A, Horan MA, Pendleton N, Mahoney W, Meyre D, Delplanque J, Froguel P, Luzzatto O, Yakir B, Darvasi A. Type 2 diabetes whole-genome association study in four populations: the DiaGen Consortium. Am J Hum Genet. 2007;81:338–345. doi: 10.1086/520599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 82.Meigs JB, Manning AK, Fox CS, Florez JC, Liu C, Cupples LA, Dupuis J. Genome-wide association with diabetes-related traits in the Framingham Heart Study. BMC Med Genet. 2007;19(suppl 1):S16. doi: 10.1186/1471-2350-8-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maeda S, Osawa N, Hayashi T, Tsukada S, Kobayashi M, Kikkawa R. Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney Int Suppl. 2007;106:S43. doi: 10.1038/sj.ki.5002385. [DOI] [PubMed] [Google Scholar]
- 84.Iles MM. What can genome-wide association studies tell us about the genetics of common disease? PLoS Genet. 2008;4:e33. doi: 10.1371/journal.pgen.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, Rayner NW, Shields B, Owen KR, Hattersley AT, Frayling TM. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3:e374. doi: 10.1371/journal.pmed.0030374. [DOI] [PMC free article] [PubMed] [Google Scholar]