Abstract
Background
While geographic disparities in stroke mortality are well documented, there are no data describing geographic variation in recurrent stroke. Accordingly, we evaluated geographic variations in 1-year recurrent ischemic stroke rates in the USA with adjustment for patient characteristics.
Methods
One-year recurrent stroke rates for ischemic stroke (International Classification of Diseases, 9th Revision codes 433, 434 and 436) following hospital discharge were calculated by county for all fee-for-service Medicare beneficiaries from 2000 to 2002. The rates were standardized and smoothed using a bayesian conditional autoregressive model that was risk-standardized for patients’ age, gender, race/ethnicity, prior hospitalizations, Deyo comorbidity score, acute myocardial infarction, congestive heart failure, diabetes, hypertension, dementia, cancer, chronic obstructive pulmonary disease and obesity.
Results
The overall 1-year recurrent stroke rate was 9.4% among 895,916 ischemic stroke patients (mean age: 78 years; 56.6% women; 86.6% White, 9.7% Black and 1.2% Latino/Hispanic). The rates varied by geographic region and were highest in the South and in parts of the West and Midwest. Regional variation was present for all racial/ethnic subgroups and persisted after adjustment for individual patient characteristics.
Conclusions
Almost 1 in 10 hospitalized ischemic stroke patients was readmitted for an ischemic stroke within 1 year. There was heterogeneity in recurrence patterns by geographic region. Further work is needed to understand the reasons for this regional variability.
Key Words: Stroke, recurrent; Small-area analysis; Variation, geographic; Recurrence
Introduction
An estimated 795,000 persons have strokes in the USA each year, of which approximately 185,000 have recurrent stroke events [1]. Recurrent events are associated with higher mortality rates, higher levels of disability and increased costs as compared with initial stroke events [2].
Regional variation has been demonstrated for stroke mortality [3,4,5] and stroke hospitalization rates [6], with high rates seen in the Southeast of the USA, termed the ‘Stroke Belt’. However, little is known about national patterns of recurrent ischemic stroke for elderly patients. Variation in recurrent stroke may in part reflect differences in the use of secondary prevention therapies. We determined whether there were geographic patterns in recurrent ischemic stroke rates within the year following hospital discharge for an ischemic stroke among Medicare patients at the county level in the USA. We further examined whether these county level patterns persisted after adjustment for patient characteristics.
Patients and Methods
The study cohort was identified from national Medicare part A administrative records of the Centers for Medicare and Medicaid Services and included all fee-for-service Medicare beneficiaries aged ≥65 years who were hospitalized with an incident ischemic stroke (discharge diagnosis: International Classification of Diseases, 9th Revision, Clinical Modification, ICD-9-CM, codes 433, 434 and 436) from January 1, 2000 to December 31, 2002. Incident stroke patients were defined as not having had an ischemic stroke hospitalization within the year prior to the index hospital admission. The patients were grouped by county using the ZIP code of their residence.
Beneficiaries were excluded if they were transferred between acute care facilities, were discharged from a non-acute-care hospital within a single day against medical advice (as it is unlikely that these patients suffered a stroke), died during the index stroke hospitalization, had <12 months of continuous Medicare fee-for-service status before or after the index event, lived outside the USA or had an unknown county of residence.
Age, sex, race and the ZIP code of their residence were obtained from the Medicare Enrollment Database. Prior diagnoses and the Deyo score, an adaptation of the Charlson comorbidity score that is based on the presence or absence of 17 comorbid conditions and in which higher scores reflect a greater disease burden [7], were determined using ICD-9-CM diagnostic codes from both the index stroke admission and hospitalizations within the prior 12 months. We calculated the number of hospitalizations in the year prior to the index stroke (dichotomized as <2 and ≥2). For a summary of the patient and county characteristics see table 1.
Table 1.
Patient and county characteristics of US counties, 2000–2002
| Individual patient characteristics |
p | ||||
|---|---|---|---|---|---|
| total (n = 895,916) | recurrent stroke (n = 84,590) | stroke-free (n = 811,326) | County characteristics (n = 3,130) | ||
| Demographics | |||||
| Mean age 8 SD, years | 78.2 ± 7.6 | 76.9 ± 7.1 | 78.3 ± 7.6 | <0.0001 | 78.1 ± 7.6 |
| Female gender | 56.6 | 53.0 | 57 | <0.0001 | 55.4 |
| Race/ethnicity | <0.0001 | ||||
| White | 86.6 | 86.2 | 86.7 | 90.0 | |
| Black | 9.7 | 10.4 | 9.6 | 7.1 | |
| Hispanic | 1.2 | 1.3 | 1.2 | 0.9 | |
| Other race | 2.5 | 2.1 | 2.5 | 0.2 | |
| Medical history | |||||
| ≥2 prior hospitalizations1 | 14.1 | 41.9 | 11.2 | <0.0001 | 14.5 |
| Deyo score >3 | 26.1 | 56.8 | 22.9 | <0.0001 | 25.1 |
| Diabetes | 28.3 | 34.7 | 27.7 | <0.0001 | 27.7 |
| Hypertension | 64.2 | 64.9 | 64.1 | <0.0001 | 62.1 |
| Obesity | 1.8 | 1.6 | 1.8 | <0.0001 | 1.8 |
| Smoker | 8.9 | 9.6 | 8.8 | <0.0001 | 9.4 |
| Dementia | 7.6 | 8.2 | 7.6 | <0.0001 | 6.8 |
| CHF | 10.3 | 17.4 | 9.6 | <0.0001 | 10.5 |
| COPD | 18.9 | 24.9 | 18.2 | <0.0001 | 19.4 |
| Prior AMI | 10.2 | 15.3 | 9.7 | <0.0001 | 9.7 |
| Cancer | 2.5 | 4.1 | 2.4 | <0.0001 | 2.5 |
Values are percentages unless otherwise specified. CHF = Congestive heart failure; COPD = chronic obstructive pulmonary disease; AMI = acute myocardial infarction.
Hospitalized ≥2 times within the prior year.
Recurrent ischemic stroke hospitalizations were identified from inpatient hospitalization claims for 12 months following the index admission. There is no standard definition of a recurrent ischemic stroke in clinical guidelines [8]. Ischemic stroke hospitalizations occurring within 7 days of the discharge date were classified as complications of the incident stroke and were not considered a recurrent event; prior cohort studies have excluded events within the first 24 h to 28 days [8,9,10]. We used the Death Master File known as the Social Security Death Index, which contains 95% of all deaths among individuals aged 65 years and older [11], to determine the patients’ survival or date of death.
Recurrent stroke rates were calculated as the number of recurrent ischemic stroke hospitalizations per county divided by the number of patient days at risk, censored for death. Patient characteristics were compared by recurrent stroke status using χ2 and t tests as appropriate. All p values were two-sided. The average patient age and overall percentage of Medicare patients with each characteristic were determined for each county.
Risk-adjusted analyses were conducted at the county level. A bayesian Poisson conditional autoregressive model [12] was used to calculate a risk-standardized rate of stroke recurrence for each county. Relative risk estimates and 95% CI were identified for all covariates (online suppl. text 1, for all online suppl. material, see www.karger.com/doi/10.1159/000274804). All bayesian modeling was done in the 64-bit version of WinBUGS 1.4.3. [13].
Results
Data from 895,916 fee-for-service Medicare beneficiaries discharged with an incident stroke from 2000 to 2002 were grouped into 3,130 counties. In this population, 9.4% had a recurrent ischemic stroke within 1 year. Patients with recurrent ischemic stroke were more often male, younger, Black, had more comorbidities, a Deyo score of three or greater, higher rates of diabetes, and were more likely to have two or more hospitalizations in the prior year (table 1). The patient characteristics were consistent at both the individual and county level.
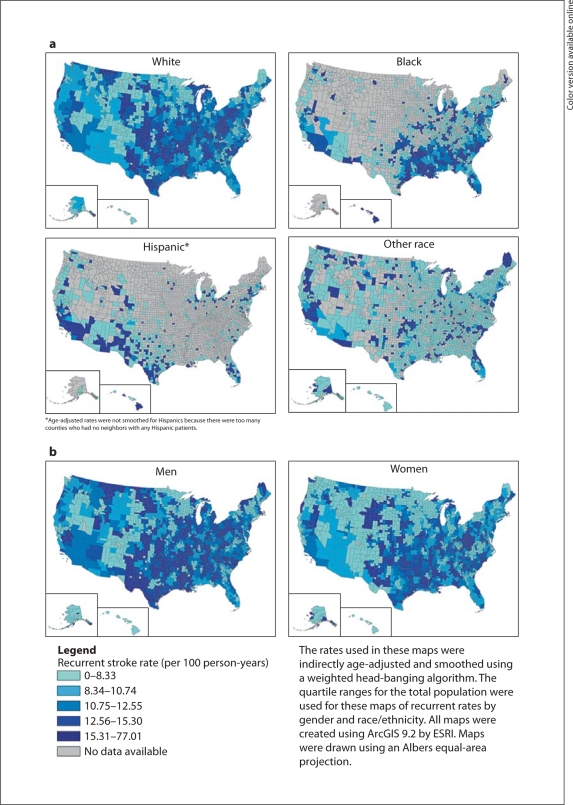
There was marked geographic variation in age-adjusted 1-year rates of recurrent stroke, with the highest rates in the southern regions (West South Central, East South Central and South Atlantic) of the USA (online suppl. fig. 1). High rates were also seen in Montana and Wyoming, with lower rates in the western and northeastern USA. These geographic patterns were similar for both Whites and Blacks (fig. 1a), and women and men (fig. 1b), but the absolute recurrence rates were higher among men (fig. 1b). The 1-year recurrence rates were lower in counties with a greater proportion of female patients and counties with a greater proportion of patients who were non-White, Black or Hispanic, and were higher for counties with a greater percentage of patients with significant comorbid conditions (table 2).
Fig. 1.
Smoothed, age-adjusted 1-year recurrent ischemic stroke rates by US county, 20002003, by race/ethnicity (a) and gender (b).
Table 2.
Association of selected patient characteristics with recurrent stroke
| Relative risk | 95% CI | |
|---|---|---|
| Demographics | ||
| Age (per 10 years) | 0.80 | 0.70–1.00 |
| Female gender | 0.72 | 0.58–0.89 |
| Race/ethnicity | ||
| White | 1.00 | |
| Black | 0.97 | 0.87–1.08 |
| Hispanic | 0.71 | 0.49–1.04 |
| Other race | 0.54 | 0.42–0.68 |
| Medical history | ||
| ≥2 prior hospitalizations | 12.64 | 9.24–17.29 |
| Deyo score >3 | 2.28 | 1.81–2.90 |
| Diabetes | 0.87 | 0.39–1.11 |
| Hypertension | 0.78 | 0.66–0.94 |
| Obesity | 0.88 | 0.41–1.84 |
| Smoker | 0.83 | 0.66–1.04 |
| Dementia | 0.77 | 0.53–1.11 |
| CHF | 0.92 | 0.64–1.33 |
| COPD | 1.00 | 0.81–1.25 |
| Prior AMI | 1.04 | 0.75–1.46 |
| Cancer | 0.36 | 0.17–0.78 |
WinBUGS model results; the index year of hospitalization was included in the model. CHF = Congestive heart failure; COPD = chronic obstructive pulmonary disease; AMI = acute myocardial infarction.
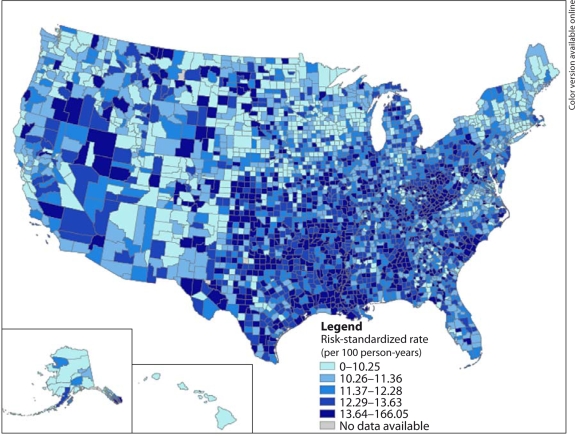
After adjustment for patient characteristics, higher risk-standardized rates are seen in parts of the southeastern, Atlantic and central USA, while lower rates were present in the western and northeastern regions (fig. 2). The residual variation in county-specific recurrent ischemic stroke rates (online suppl. fig. 2) suggests that additional unmeasured factors could explain some of the geographic variation.
Fig. 2.
Risk-standardized 1-year recurrent ischemic stroke rates by US county, 20002003.
Discussion
Nationwide, we found that 9.4% of elderly ischemic stroke patients experience a recurrent ischemic stroke within 1 year; however, there is marked geographic variation in county recurrence rates across the country, with lower rates seen in the northeastern and western regions and high rates in the southern and central USA. These regional differences persisted even after controlling for patient characteristics and vascular risk factors.
The national rate of 1-year recurrent ischemic stroke hospitalization found in this analysis is comparable to the recurrent stroke rates found in other cohort studies (ranging from 5 to 15%) [14,15,16,17,18,19]. At a national level, we found that counties with older populations, more women and larger populations of individuals who were non-White, Black or Hispanic had lower recurrent ischemic stroke rates. An increased prevalence of hypertension was associated with a lower risk of recurrent stroke, potentially due to better control among diagnosed cases [20]. Consistent with prior work, we also found that counties with a greater comorbidity burden had higher ischemic recurrence rates [15,21,22,23].
The geographic patterns identified in this study demonstrate that stroke recurrence rates vary dramatically across the country and corroborate prior work on regional differences in stroke mortality and hospitalizations. Consistent with previous studies of first-ever stroke [5,24], we have identified high stroke rates in the southeastern USA. However, the reasons why these areas have a high stroke incidence and recurrence remains unclear. In addition, recurrent stroke rates were also high in areas of the Northwest, locations which have not previously been identified as areas of high stroke incidence. The causes of these geographic disparities remain largely unknown [25,26]. High stroke incidence rates and the increased risk of recurrent stroke in the Southeast do not appear to be due to regional differences in population demographics (such as race/ethnicity) or risk factors [24,27,28,29]. Future research is needed to determine if other potential factors such as genetic variation, environmental factors, healthcare services and infectious disease exposure might be associated with the regional patterns observed in stroke outcomes.
This study has some limitations. The selection of index events, outcomes and comorbidities are based on discharge diagnoses defined by ICD-9-CM codes. Ischemic stroke codes (ICD-9-CM: 433, 434 and 436) have been validated in numerous studies [30,31] and have a high sensitivity for primary discharge codes [32,33]. Acute myocardial infarction also has a similarly high sensitivity and specificity [34]; however, the determination of other comorbidities such as smoking and obesity are less robust using administrative data [35]. Although the present analysis included only recurrent ischemic events, prior studies have found that the majority of recurrences (65–80%) are ischemic and are of the same type as the incident event [18,19]. The administrative data upon which these analyses are based do not include cause-specific death. In addition, we cannot account for the estimated 10% of strokes that did not result in a hospitalization [36]. We were, therefore, unable to determine whether these rates vary by county. Finally, our results for this elderly population may not be generalizable to Medicare patients treated at a health maintenance organization; however, more than 80% of elderly patients in this country are covered by fee-for-service Medicare.
Recurrent ischemic stroke is a common event and a major cause of disability and death. We found significant regional variation in the risk of recurrent ischemic stroke across the USA. These patterns persisted after adjustment for patient demographics and comorbid conditions, with the highest rates observed in the southern and central USA. Future research is needed to understand factors that may contribute to these geographic patterns and target programs that may reduce the burden of ischemic stroke among the elderly.
Acknowledgements
The project described was supported by grant No. R01NS043322 from the National Institute of Neurological Disorders and Stroke and a dissertation award from the Agency for Healthcare Research and Quality (1R36HS016959-01A1).
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Samsa GP, Bian J, Lipscomb J, et al. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–349. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 3.Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in Blacks and Whites in the United States. Stroke. 1997;28:1639–1647. doi: 10.1161/01.str.28.8.1639. [DOI] [PubMed] [Google Scholar]
- 4.Lanska DJ, Kryscio R. Geographic distribution of hospitalization rates, case fatality, and mortality from stroke in the United States. Neurology. 1994;44:1541–1550. doi: 10.1212/wnl.44.8.1541. [DOI] [PubMed] [Google Scholar]
- 5.El-Saed A, Kuller LH, Newman AB, et al. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke. 2006;37:1975–1979. doi: 10.1161/01.STR.0000231453.98473.67. [DOI] [PubMed] [Google Scholar]
- 6.Casper M, Nwaise I, Croft J, et al. Atlas of Stroke Hospitalizations among Medicare Beneficiaries. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 7.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 8.Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke. 2004;35:1925–1929. doi: 10.1161/01.STR.0000133129.58126.67. [DOI] [PubMed] [Google Scholar]
- 9.Sarti C, Stegmayr B, Tolonen H, et al. Are changes in mortality from stroke caused by changes in stroke event rates or case fatality? Results from the WHO MONICA Project. Stroke. 2003;34:1833–1840. doi: 10.1161/01.STR.0000081224.15480.52. [DOI] [PubMed] [Google Scholar]
- 10.Bonita R, Anderson CS, Broad JB, et al. Stroke incidence and case fatality in Australasia: a comparison of the Auckland and Perth population-based stroke registers. Stroke. 1994;25:552–557. doi: 10.1161/01.str.25.3.552. [DOI] [PubMed] [Google Scholar]
- 11.Hill ME, Rosenwaike I. The Social Security Administration's Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001;64:45–51. [PubMed] [Google Scholar]
- 12.Besag J, York J, Mollie A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–59. [Google Scholar]
- 13.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 14.Petty GW, Brown RD, Jr, Whisnant JP, et al. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Shi T, Zamanillo MC, et al. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44:626–634. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis. 2001;12:171–180. doi: 10.1159/000047700. [DOI] [PubMed] [Google Scholar]
- 17.Lisabeth LD, Smith MA, Brown DL, et al. Ethnic differences in stroke recurrence. Ann Neurol. 2006;60:469–475. doi: 10.1002/ana.20943. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto N, Whisnant JP, Kurland LT, et al. Natural history of stroke in Rochester, Minnesota, 1955 through 1969: an extension of a previous study, 1945 through 1954. Stroke. 1973;4:20–29. doi: 10.1161/01.str.4.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Wolf PA, Kannel WB, et al. Survival and recurrence following stroke. The Framingham Study. Stroke. 1982;13:290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 20.Ostchega Y, Dillon CF, Hughes JP, et al. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc. 2007;55:1056–1065. doi: 10.1111/j.1532-5415.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 21.Moroney JT, Bagiella E, Paik MC, et al. Risk factors for early recurrence after ischemic stroke: the role of stroke syndrome and subtype. Stroke. 1998;29:2118–2124. doi: 10.1161/01.str.29.10.2118. [DOI] [PubMed] [Google Scholar]
- 22.Hillen T, Coshall C, Tilling K, et al. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003;34:1457–1463. doi: 10.1161/01.STR.0000072985.24967.7F. [DOI] [PubMed] [Google Scholar]
- 23.Ruland S, Richardson D, Hung E, et al. Predictors of recurrent stroke in African Americans. Neurology. 2006;67:567–571. doi: 10.1212/01.wnl.0000232738.02278.28. [DOI] [PubMed] [Google Scholar]
- 24.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Follow-Up Study. Am J Epidemiol. 1996;144:665–673. doi: 10.1093/oxfordjournals.aje.a008979. [DOI] [PubMed] [Google Scholar]
- 25.Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1999;317:160–167. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 27.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the Reasons for Geographic and Racial Differences in Stroke Study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 28.Voeks JH, McClure LA, Go RC, et al. Regional differences in diabetes as a possible contributor to the geographic disparity in stroke mortality: the Reasons for Geographic and Racial Differences in Stroke Study. Stroke. 2008;39:1675–1680. doi: 10.1161/STROKEAHA.107.507053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cushman M, Cantrell RA, McClure LA, et al. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 31.Leibson CL, Naessens JM, Brown RD, et al. Accuracy of hospital discharge abstracts for identifying stroke. Stroke. 1994;25:2348–2355. doi: 10.1161/01.str.25.12.2348. [DOI] [PubMed] [Google Scholar]
- 32.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benesch C, Witter DM, Jr, Wilder AL, et al. Inaccuracy of the International Classification of Diseases (ICD-9-CM) in identifying the diagnosis of ischemic cerebrovascular disease. Neurology. 1997;49:660–664. doi: 10.1212/wnl.49.3.660. [DOI] [PubMed] [Google Scholar]
- 34.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol. 2001;1:2. doi: 10.1186/1471-2377-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]