Abstract
Malaria is a major global health problem that kills 1-2 million people each year. Despite exhaustive research, naturally acquired immunity is poorly understood. Cry1A proteins are potent immunogens with adjuvant properties and are able to induce strong cellular and humoral responses. In fact, it has been shown that administration of Cry1Ac protoxin alone or with amoebic lysates induces protection against the lethal infection caused by the protozoa Naegleria fowleri. In this work, we studied whether Cry1Ac is able to activate the innate immune response to induce protection against Plasmodium berghei ANKA (lethal) and P. chabaudi AS (nonlethal) parasites in CBA/Ca mice. Treatment with Cry1Ac induced protection against both Plasmodium species in terms of reduced parasitaemia, longer survival time, modulation of pro- and anti-inflammatory cytokines, and increased levels of specific antibodies against Plasmodium. Understanding how to boost innate immunity to Plasmodium infection should lead to immunologically based intervention strategies.
1. Introduction
Each year, malaria infects approximately 500 million people and kills one to two million people, mainly children below the age of five years [1]. Despite decades of research on the subject, naturally acquired immunity to Plasmodium is still poorly understood [2–4]. There are reports about the immunosuppressive effects of Plasmodium infection in humans [5] and in animal models [6]. It is believed that the initial interaction of the parasitised red blood cells with the host immune system is one of the most important factors in determining the nature of the subsequent innate and acquired response, and in determining whether or not severe pathology, such as cerebral malaria, severe anaemia, or cachexia, results [7–9].
On the other hand, insecticidal proteinaceous crystals called Cry proteins are produced as protoxins by Bacillus thuringiensis (Bt) during sporulation. Upon ingestion, crystalline protoxins are solubilised and proteolytically activated by midgut proteases of susceptible insects. The activated toxin, which is not toxic to vertebrates, binds to specific receptors on the brush-border membrane surface of the midgut epithelium of the insect, inducing the formation of pores and eventually leading to insect mortality [10]. In particular, Cry1Ac is a pore-forming protein that is specifically toxic to lepidopteran insect larvae and acts by binding to the cell-surface receptor aminopeptidase N in the Manduca sexta midgut via the sugar N-acetyl-D-galactosamine (GalNAc) [11, 12].
Although most studies on Cry proteins have been performed with regard to their toxicity in insects, we have described that recombinant Cry1Ac protoxin from Bacillus thuringiensis is a potent mucosal and systemic immunogen with adjuvant properties [13, 14].
In addition, we have shown that recombinant Cry1A toxins possess the ability to induce serum and mucosal specific antibody responses as well as to modulate IgG subclasses due to their strong immunogenic properties [14, 15]. Furthermore, it has been demonstrated that Cry proteins from B. thuringiensis can induce strong cellular immune responses. In particular, we have described that these toxins are able to promote IFN-γ responses [16]. In malaria infections, an initial IFN-γ response, mainly produced by NK cells, is implicated in the activation of macrophages, which leads to parasite elimination [17, 18]. In a previous study, we found that administration of the immunogenic protein with adjuvant properties, Cry1Ac protoxin alone or with amoebic lysates, markedly increased protective immunity against experimental N. fowleri meningoencephalitis in mice [13]. In this work, we determined the ability of Cry1Ac protoxin to activate the innate immune response. So we tested whether the pretreatment with the protein alone improved the resistance of mice to Plasmodium chabaudi AS and P. berghei ANKA experimental infections.
2. Materials and Methods
2.1. Mice and Parasites
CBA/Ca mice were kindly donated by Dr. W Jarra (National Institute for Medical Research, London). The mice were bred, fed, and maintained in a specific, pathogen-free environment at the FES Zaragoza, Universidad Nacional Autónoma de México animal house facility in accordance with the institutional and national official guideline NOM-062-ZOO-1999 for use and care of laboratory animals.
P. chabaudi AS and P. berghei ANKA were donated by Dr. William Jarra (National Institute for Medical Research, London).
2.2. Infection and Treatment
Batches of 6 to 8 sex- and age-matched (6–8 weeks) CBA/Ca mice were treated weekly with Cry1Ac protoxin (5 μg/mouse i.p.) or with vehicle (PBS) during four weeks. One day after the last treatment, mice were inoculated intravenously with either 5 × 104 P. chabaudi AS- or 5 × 104 P. berghei ANKA-parasitised erythrocytes. On the days indicated, mice were sacrificed under ether anaesthesia. As controls, a parallel batch of noninfected mice was divided into two groups and treated with PBS or Cry1Ac at the same dose. Data presented are representative of two separate experiments.
2.3. Blood Sampling
Parasitaemias were evaluated daily by examination of Giemsa-stained blood smears. Numeration of the parasitaemia was performed under oil, using a Zeiss Standard 20 microscope (Carl Zeiss Ltd., Welwyn Garden City). Parasitaemias of 0.5% and above were determined by counting the number of parasitised erythrocytes present in a total of 200 red blood cells. Lower levels of parasitaemia were assessed by counting the number of parasitised erythrocytes present in 50 fields. The course of infection in each group is shown as the geometric mean of the percentage of parasitaemia.
2.4. Recombinant Cry1Ac Escherichia coli JM103 (pOS9300)
The recombinant Cry1Ac E. coli JM103 (pOS9300) strain was kindly donated by Dr. Dean, from Ohio State University. The bacteria were grown in Luria-Bertani medium containing 50 μg/mL of ampicillin, and Cry1Ac production was induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) [19]. Recombinant Cry1Ac was purified from the IPTG-induced E. coli JM103 (pOS9300) cultures as follows. Cell pellets harvested by centrifugation were suspended in 50 mM Tris-HCl, 50 mM EDTA (pH 8) (TE buffer) and sonicated (Fisher Sonic Dismembrator Model 300) three times for 5 minutes on ice. Inclusion bodies were collected by centrifugation at 10 000 × g for 10 minutes, and pellets were washed twice with TE buffer, twice with 0.5 M NaCl, and once with 0.5 M/NaCl-1% Triton X-100, once with 0.5 M NaCl, once with cold distilled water and were finally solubilised in CBP buffer (0.1 M Na2CO3, 1% 2-mercaptoethanol [pH 9.6]). Particulate material was discarded by centrifugation at 10 000 × g for 10 min, and the purified solubilised protoxin was stored at 4°C and examined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein concentration was determined using the Bradford method [20]. The presence of endotoxin contamination in the Cry1Ac protoxin preparations was tested using the E-toxate, Part 1 kit (Sigma), which has a sensitivity limit of 0.05–0.1 endotoxin units (EU)/mL, following the manufacturer's instructions. Endotoxin levels in the purified Cry1Ac protoxin preparations were below 0.1 EU/mL. These preparations were further treated with an excess of a polymyxin B resin (Bio-Rad, Hercules CA, USA) to remove any possible remnants of endotoxin.
2.5. Cytokine mRNA Expression
Groups of CBA/Ca mice were treated weekly with Cry1Ac protoxin or with PBS for four weeks. Twenty-four hours after the last inoculation, mice were infected with either P. chabaudi AS or P. berghei ANKA. On the days indicated, three mice of each group were sacrificed under ether anaesthesia, and spleen mRNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). DNA was digested with DNase I (Invitrogen) according to the manufacturer's instructions, and RNA was quantified spectrophotometrically at 260 nm. Next, 1.5 μg of RNA were retrotranscribed using 1.5 μg of oligo dT (Invitrogen), 0.5 mM dNTPs (Pharmacia, Uppsala, Sweden), 40 U RNase inhibitor, and 200 U MMLV-RT (Invitrogen). Next, 1 μL of the resulting cDNA was used to amplify IFN-γ and TGF-β by PCR. Each sample was amplified in duplicate using a previously described method [21].
Each set of primers as well as the cDNA concentration was optimized for a number of cycles to obtain amplicons in the linear phase of amplification. The following gene-specific primer sequences were used: (IFN-γ) forward: 5′ TGC ATC TTG GCT TTG CAG CTC TTC CTC ATG GC 3′, reverse 5′ TGG ACC TGT GGG TTG TTG ACC TCA TTG GC 3′; (TGF-β) forward 5′ GAC CGC AAC AAC GCC ATC TA 3′ reverse 5′ GGC GTA TCA GTG GGG GTC AG 3′; (β-actin) forward 5′ GTG GGC CGC TCT AGG CAC CAA 3′, reverse 5′ CTC TTT GAT GTC ACG CAC GAT TTC 3′. PCR reactions were performed in a total volume of 20 μL. Amplification was carried out in 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 0.1 mg/mL gelatin, 200 mM of each dNTP, 2 mM MgCl2, 100 nM of each primer, 0.5 U Ampli Taq polymerase (Applied Biosystems, Branchburg, NJ, USA), and 15 ng of cDNA. The β-actin gene and either IFN-γ or TGF-β were then simultaneously amplified in a single tube. After 27–29 cycles, the PCR products were separated on 5% polyacrylamide gels and stained with ethidium bromide. Each band was analysed by densitometry, and the results are shown as the relation of the absorbance of the corresponding cytokine to that of β-actin in the same sample.
2.6. Cytokine Serum Measurement
On days indicated, mice from both the P chabaudi AS- and the P. berghei ANKA-infected groups were sacrificed under ether anaesthesia. Immediately, blood from the heart was extracted and then centrifuged at 2000 × g at 4°C for 15 min. The serum was removed and aliquoted into two tubes and snap frozen at −70°C until used.
The levels of the cytokines interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-5 (IL-5), interferon-γ (IFN-γ), and tumour necrosis factor- (TNF-α) in the serum samples were measured using a cytometric bead array performed according to the manufacturer's instructions (BD Mouse Th1/Th2 Cytokine CBA Kit Biosciences-Pharmingen, Heidelberg, Germany) with the following modifications. We performed all steps in microtubes, and we started the standard curve at a concentration of 0.625 pg/mL. The sensitivity achieved with these minor adaptations to the protocol was 0.9 ± 0.05 pg/mL while the variation inter-assay was approximately 5%.
2.7. Measurement of Protective Antibodies
Antibody-specific responses were evaluated using a previously described method [21].
A lysate of pRBC was used as the capture antigen; it was prepared as follows. P. chabaudi AS- or P. berghei ANKA-infected mice (25% parasitaemia) were bled into PBS-heparin at 4°C to provide parasitised erythrocytes. The blood was passed through a CF11 cellulose powder (Whatman, Maidstone, UK) column to remove leukocytes and then washed three times with PBS by centrifugation at 750 × g for 15 min at 4°C. The final cell pellet was resuspended to 5 mL in PBS, and 3 μL of 10% (w/v) saponin in PBS was added to lyse the erythrocyte membranes. After centrifugation at 18,000 × g for 5 min at 4°C, the supernatant was removed, the pellets were resuspended to 3 mL in PBS, and the cells were lysed by ultrasonication for 3 seconds with 21% amplitude (Ultrasonic Processor Model GE750, USA). The protein concentration of the lysates was determined using a Bio-Rad commercial reagent, and the lysate was diluted in carbonate-buffered solution to give a coating concentration of 10 μg/mL. A volume of 100 μL/well was applied to flat-bottomed 96-well ELISA plates (Corning USA). First, the plates were washed with 0.05% (v/v) Tween 20 in PBS, and then the excess binding sites were blocked using a solution of 3% skim milk in PBS for 2 hours at 37°C. The plates were then incubated with test sera in duplicate for 1 h at 37°C and diluted to 1/20 in PBS. Plates were washed extensively before detection of parasite-specific Abs using goat antimouse horseradish peroxidase-conjugated monoclonal Abs (mAb) specific to IgG1, IgG2a, IgG2b, IgG3, total IgG, or IgM (Zymed, San Francisco California, USA) diluted in 0.02% skim milk, 0.05% Tween 20 in PBS to previously calibrated dilutions, which were applied to plates for 1 h at 37°C. Plates were washed before incubation with a streptavidin peroxidase solution (diluted 1 : 3000 in 0.05% Tween 20 in PBS) before the final wash. Plates were developed with ortho phenylenediamine at 0.4 mg/mL in citrate buffer (pH 5) with 0.03% of hydrogen peroxide as a substrate and incubated in the dark at room temperature for 20 min. Absorbance was determined at 492 nm by measurement of optical density (OD) using a Stat-Fax 2100 microplate reader (Awareness Technology Inc, USA). No standard of known concentration for each Ig isotype was available. Hence results were expressed directly as OD 492 nm values and compared to an internal standard of normal CBA mouse serum obtained from eight- to ten-week-old naive female mice. This internal standard provided a background value of nonspecific responsiveness to the lysate used.
2.8. Statistical Analysis
Statistical analysis was performed with the Stat Graphs software (version 5.1). Differences between groups were tested for statistical significance by nonparametric analysis of variance (Kruskal-Wallis). A P value <.05 was considered significant. All data are expressed as the mean ± S.D. Each experiment was performed in duplicate.
3. Results
3.1. Cry1Ac Treatment Decreases Parasitaemia in CBA/Ca Mice Infected with Plasmodium chabaudi AS or P. berghei ANKA
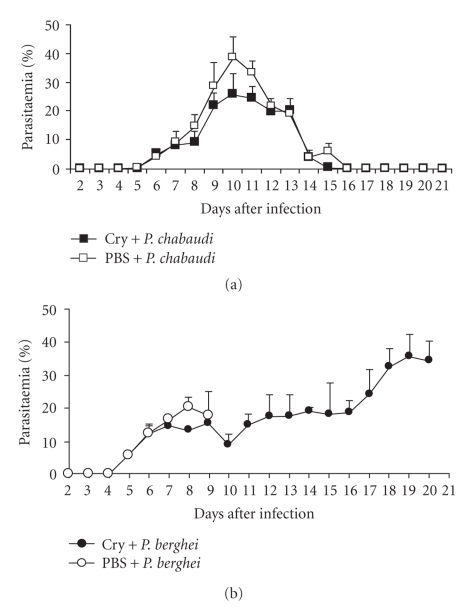
Groups of CBA/Ca mice were injected once weekly for four weeks with Cry1Ac protoxin or PBS as described in the Materials and Methods. One day after the last injection, mice were intravenously infected either with P. chabaudi AS or with P. berghei ANKA. Mice treated with Cry1Ac protoxin prior to P. chabaudi AS infection developed a moderate parasitaemia that increased from day 6 postinfection (PI) to reach a peak of 27% at day 10 PI. Parasitaemia resolved spontaneously and was cleared by day 15 PI. In contrast, control mice treated with vehicle (PBS) developed higher parasitaemias from day 6 to 15 PI (significantly (P < .05) from days 8 to 11 PI) compared to mice treated with Cry1Ac protoxin. Parasitaemia reached a peak of 40% at day 10, and the parasite was completely cleared at day 16 PI, one day later than in the group of mice treated with Cry1Ac (Figure 1(a)).
Figure 1.
Effect of Cry1Ac on parasitaemia in CBA/Ca mice. Groups of eight mice were treated with Cry1Ac protoxin once a week for four weeks. One day after the last injection, mice were infected with P. chabaudi AS (a) or P. berghei ANKA (b). Groups of control mice were treated with PBS. Data are representative of two separate experiments.
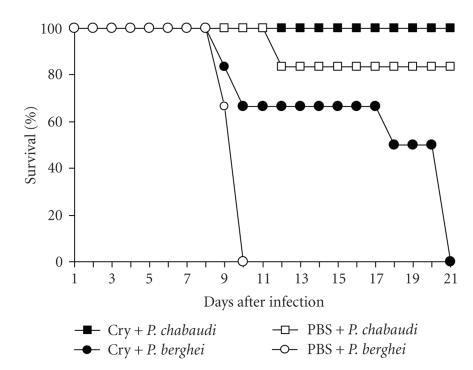
In contrast, infection with P. berghei ANKA was lethal. In control CBA/Ca mice treated with vehicle (PBS), parasitaemia increased from day 5 PI to reach a peak of 23% at day 8 PI. There was a slight decrease in parasitaemia at day 9, and then mice started to die on day 10 PI with parasitaemias around 20%, confirming the reported lethality of this strain [22, 23]. Infected mice previously treated with the protoxin Cry1Ac developed lower levels of parasitaemia than PBS-treated mice from days 7 to 9 PI; the number of parasites in their blood decreased on day 10 PI and then started to rise slowly. Half of this group of mice survived until day 20 PI with parasitaemias of approximately 40% (Figures 1(b) and 2).
Figure 2.
Effect of Cry1Ac on the survival of Plasmodium-infected mice. Cry1Ac pretreated mice (n = 8) were infected with P. chabaudi AS or P. berghei ANKA. Survival was recorded until day 21 PI, at which time all of the P. berghei ANKA-infected mice had died. Data are representative of two separate experiments.
3.2. Cry1Ac Protoxin Increases Survival in Plasmodium-Infected Mice
Despite the fact that Plasmodium chabaudi AS is not considered to be lethal, infection with this parasite could be fatal for 10% to 20% of CBA/Ca mice. Interestingly, mice treated with Cry1Ac and infected with P. chabaudi AS had a survival rate of 100% compared to mice treated with PBS, which had a survival rate of 80%. On the other hand, mice treated with Cry1Ac and infected with the lethal parasite P. berghei ANKA showed an increased survival of 12 days compared to control mice treated with PBS, which died at day 9 PI (Figure 2).
3.3. Cry1Ac Protoxin Modulates Cytokine mRNA Expression in Splenocytes from Malaria-Infected Mice
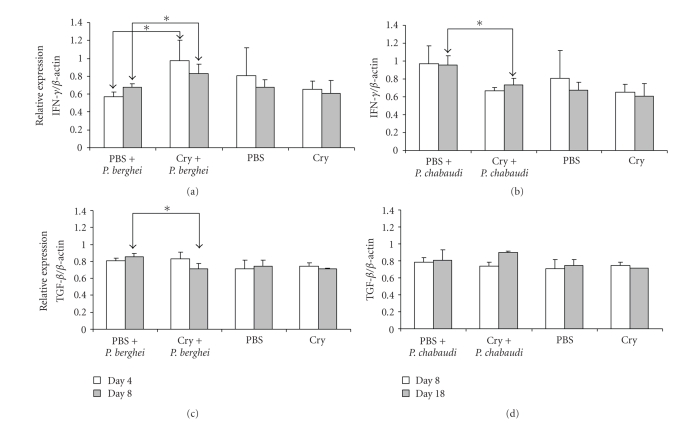
RT-PCR analysis was performed to determine the levels of cytokine mRNA expression in splenocytes from infected and uninfected mice. Cells were obtained at days 4 and 8 PI with P. berghei ANKA and at days 8 and 18 PI with P. chabaudi AS. The levels of cytokine mRNA expression were normalised to β-actin mRNA levels, which was used as an internal standard. Moderate, constitutive mRNA expression of IFN-γ and TGF-β was detected in control mice receiving the vehicle, while in uninfected mice treated with Cry1Ac, similar levels of these cytokines were recorded (Figure 3).
Figure 3.
Effect of Cry1Ac on the mRNA expression of IFN-γ (a and b) and TGF-β (c and d) in mice infected with P. chabaudi AS or P. berghei ANKA. Mice were treated with Cry1Ac protoxin or PBS as a control. One day after the last injection, mice were infected with P. berghei ANKA or P. chabaudi AS. On days 4 and 8 PI (a and c) or days 8 and 18 PI (b and d), three mice from each group were killed, and their splenic mRNA was extracted and retrotranscribed. The cDNA obtained was used to amplify the β-actin gene and either IFN-γ or TGF-β by PCR in the same tube. PCR products were separated on 5% polyacrylamide gels. Bands were analysed by densitometry, and the results are shown as the absorbance of the corresponding cytokine divided by the absorbance of β-actin. Data are representative of two separate experiments. Asterisks indicate statistically significant differences between the indicated groups.
In P. berghei ANKA-infected mice that previously had been administered Cry1Ac protoxin, increased levels of IFN-γ mRNA expression were detected at days 4 and 8 PI compared to infected mice receiving just the vehicle or uninfected mice. At day 4 PI, similar levels of TGF-β were found in both infected groups (slightly higher than those in uninfected mice). In contrast, at day 8 PI, the levels of TGF-β mRNA were lower in mice pretreated with Cry1Ac protoxin than in those receiving just the vehicle (Figures 3(a) and 3(c)).
In control mice infected with P. chabaudi AS, the levels of IFN-γ mRNA at days 8 and 18 PI were higher than those in mice pretreated with Cry1Ac protoxin, which exhibited IFN-γ levels akin to those in uninfected mice. On the other hand, the mRNA levels of TGF-β recorded in P. chabaudi AS-infected mice did not change significantly either by infection or by pretreatment with Cry1Ac protoxin.
3.4. Cry1Ac Modifies Cytokine Levels in Sera from Plasmodium-Infected Mice
The levels of cytokines (IFN-γ and IL-4) in serum samples were measured with a cytometric bead array. Samples were obtained at days 4 and 8 or 8 and 18 postinfection with P. berghei ANKA and P. chabaudi AS, respectively.
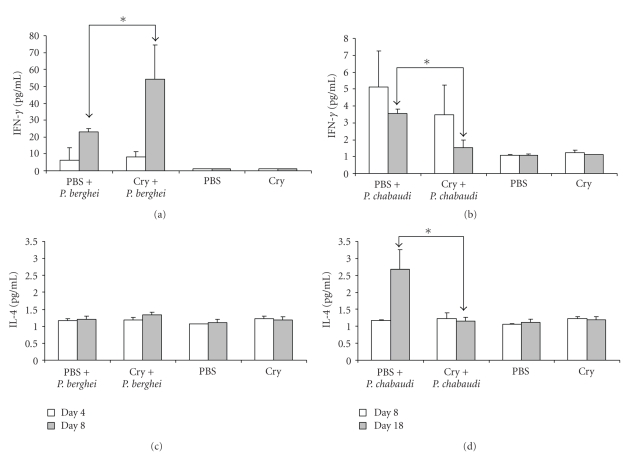
The levels of IFN-γ were significantly increased at day 8 PI with P. berghei ANKA in mice pretreated with Cry1Ac, confirming the RT-PCR results. At day 4 postchallenge, the IFN-γ mRNA levels recorded were low and akin to infected control mice, although they are greater than the levels in uninfected control mice. The levels of IL-4 were also low and did not vary significantly as a result of infection with P. berghei ANKA or administration of Cry1Ac protein (Figure 4). In contrast, following infection with P. chabaudi AS, the levels of IFN-γ were higher in control mice than in those pretreated with Cry1Ac protoxin. The IFN-γ levels induced after P. chabaudi AS infection were considerably lower compared to those elicited following P. berghei ANKA infection, but they were still higher than those present in uninfected mice.
Figure 4.
Effect of Cry1Ac on serum cytokines in Plasmodium-infected mice.Mice were treated with Cry1Ac protoxin or PBS once weekly for four weeks. One day after the last injection, mice were infected with P. berghei ANKA or P. chabaudi AS. On days 4 and 8 PI (a and c) or days 8 and 18 PI (b and d), three mice from each group were killed, their sera was separated from peripheral blood and the levels of the cytokines IFN-γ (a and b) and IL-4 (c and d) were measured by cytometric bead array. Data are representative of two separate experiments. Asterisks indicate statistically significant differences between the indicated groups.
In control P. chabaudi AS-infected mice, the levels of IL-4 increased at day 18 PI, while in mice pretreated with Cry1Ac, the levels of this cytokine did not change (Figure 4). Levels of IL-2, IL-5 and TNF-α were not modified by any of these treatments (results not shown).
3.5. Protoxin Cry1Ac Increases the Levels of Specific Antibodies for P. berghei ANKA and P. chabaudi AS in Infected Mice
Specific anti-P. berghei ANKA antibodies were induced in sera from mice infected with the parasite (at days 4 and 8 PI), while sera from control uninfected mice, which were untreated or received PBS or Cry1Ac alone, did not have detectable anti-P.berghei ANKA antibodies (Figure 5).
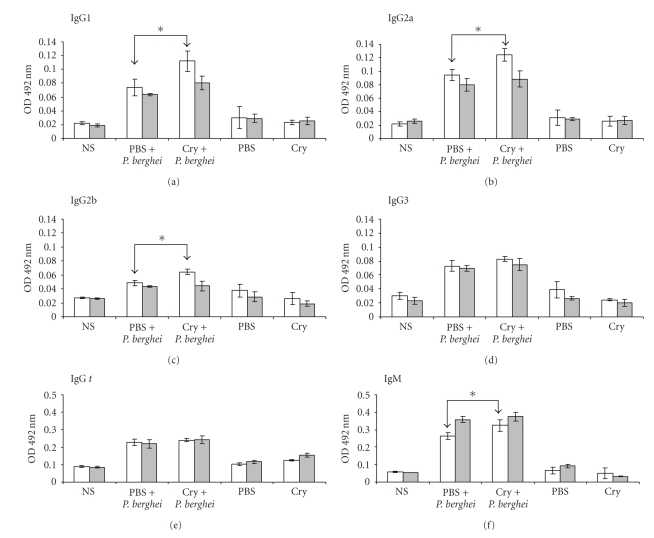
Figure 5.
Parasite-specific antibody responses in mice treated with Cry1Ac and infected with P. berghei ANKA. Mice were treated with Cry1Ac protoxin or PBS as described previously and were infected with P. berghei ANKA. At days 4 (white bars) and 8 (grey bars) PI, the sera of three mice from each group were collected and measured by ELISA for antibody levels. The results are expressed as the mean OD value ± SD (n = 3). Control absorbance values were provided by normal mouse serum (NS) obtained from age- and sex-matched CBA/Ca mice. Data are representative of two separate experiments. Asterisks indicate statistically significant differences between the indicated groups.
The treatment with protoxin Cry1Ac before infection increased the levels of IgG1, IgG2a, IgG2b, and IgM in P. berghei ANKA-infected mice. Interestingly, this increase was only detected on day 4 PI compared to mice receiving the vehicle alone. At day 8 PI, similar levels of IgG and IgM responses were induced in both experimental groups (Figure 5).
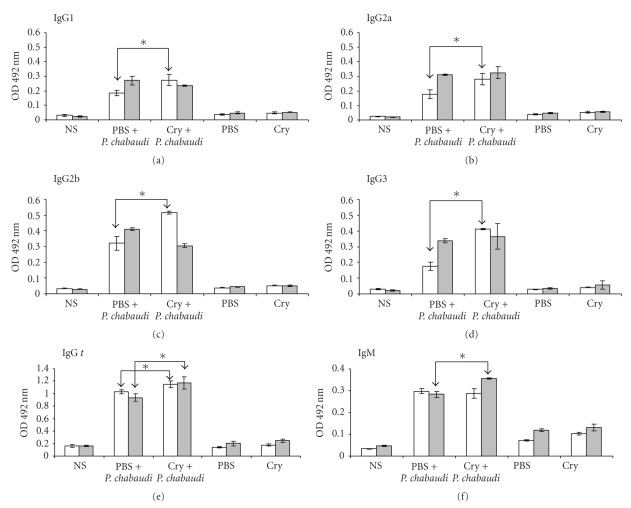
In infected mice pretreated with Cry1Ac, the specific anti-P. chabaudi AS IgG response was significantly higher on days 8 and 18 than that elicited in infected mice pretreated with the vehicle (Figure 6). Regarding the analyses of the different IgG subclasses, specific responses at day 4 PI of the four IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3) were also significantly higher in the group receiving Cry1Ac before the infection compared to the group receiving the vehicle alone (Figure 5). However, at day 8 PI, the IgG2b responses detected were higher in the vehicle group with respect to the Cry1Ac group, while the IgG responses of the rest of the isotypes recorded were similar between the two groups.
Figure 6.
Parasite-specific antibody responses in mice treated with Cry1Ac and infected with P. chabaudi AS. Mice were treated with Cry1Ac protoxin or PBS as described previously and were infected with P. chabaudi AS. At days 8 (white bars) and 18 (grey bars) PI, the sera of three mice from each group were collected and measured by ELISA for antibody levels. The results are expressed as the mean OD value ± SD (n = 3). Control absorbance values were provided by normal mouse serum (NS) obtained from age- and sex-matched CBA/Ca mice. Data are representative of two separate experiments. Asterisks indicate statistically significant differences between the indicated groups.
The IgM-specific response at day 8 PI was significantly higher in the group that received Cry1Ac than in the group treated with vehicle.
The specific antibody responses recorded in mice infected with P. berghei ANKA were lower in relation to those elicited in mice infected with P. chabaudi AS. This result was expected since the latter group of mice had a longer period of antigenic stimulation. In sera from uninfected mice receiving either the vehicle (PBS) or Cry1Ac, specific anti-P. berghei ANKA antibodies were not detected.
4. Discussion
Our results demonstrate that administration of the Cry1Ac protoxin from B. thuringiensis induces protection against the malaria parasite when it is administered in CBA/Ca mice before infection with P. chabaudi AS and induces a longer survival time in P. berghei ANKA-infected mice (Figures 1 and 2). Protection was shown by lower levels of parasitaemia (first peak) in groups of mice infected with either P. chabaudi AS or P. berghei ANKA compared to control mice. In addition, Cry1Ac protoxin modulated the mRNA expression of proinflammatory cytokines, such as IFN-γ and TGF-β, and increased the levels of IgG and IgM in both P. berghei ANKA- and P. chabaudi AS-infected mice.
Due to the different courses of parasitaemia between CBA/Ca mice infected with the lethal P. berghei ANKA, which killed all of the mice around day 9 PI, and the non-lethal P. chabaudi AS, the samples were analysed at different days (4 and 8 versus 8 and 18, resp.) to get the best comparison between both infections.
We evaluated the effect of Cry1Ac protoxin against Plasmodium infection because we have previously described that this protein may be a valuable tool for the improvement of mucosal vaccines; when Cry1Ac protoxin is coadministered as an adjuvant, it increases protective immunity against experimental Naegleria fowleri meningoencephalitis, an acute fulminant infection initiated at the nasal mucosa, in mice [13]. Interestingly, intranasal administration of Cry1Ac alone also had protective effects against N. fowleri infection, as this treatment increased survival, as immunization with amoebal lysates alone did, suggesting that Cry1Ac protoxin may boost innate immunity.
In addition, it has been reported that Cry1Ac protoxin enhances the respiratory burst of human monocytes and neutrophils [24]. Accordingly, our unpublished results suggest that Cry1Ac activates mouse macrophages, inducing the expression of the costimulatory molecules B7-1 and B7-2 and the production of some proinflammatory cytokines (IFN-γ and MCP-1), but further studies are required to elucidate the mechanisms involved.
Despite the fact that Cry proteins are not toxic to vertebrates and Cry1Ac is known to form pores exclusively in the midgut epithelial cells of lepidopteran insect, the existence of an unknown receptor in mammals has been suggested, because Cry1Ac protoxin binds to brush border membrane vesicles prepared from mouse small intestine in vitro [14]. The nature of the molecules interacting with Cry proteins in mammalian enterocytes seems to be different than the receptor glycoproteins described in insects, such as the 120-kDa aminopeptidase N [11] and the 210-kDa cadherin-like glycoprotein (Bt-R1) [25] because binding to its receptor was not inhibited by GalNAc, mannose, or biotin.
On the other hand, it has been shown that IFN-γ is able to activate macrophages, which are responsible for elimination of the the malaria parasite [26–28]. Our results show that treatment with Cry1Ac protoxin decreased parasitaemia in mice infected with P. berghei ANKA at day 8 PI. At that time, downregulation of TGF-β expression and an increase in both mRNA expression and serum levels of IFN-γ were found (Figures 3 and 4). This finding is consistent with a previous report showing that protective immunity was associated with a decrease in TGF-β and a concomitant increase in IFN-γ production in P. yoelii 17XL-infected mice [29]. In addition, it has been shown that upregulation of IFN-γ in P. berghei ANKA-infected mice leads to the activation of macrophage, which increases parasite elimination [30]. However, mice infected with P. berghei ANKA in our study were not fully protected, and all of them died at day 21 PI. A possible explanation for this finding is that the effect of Cry1Ac protoxin is limited; it could promote a decrease in parasitaemia in mice at day 8 PI, which, in turn, diminishes the antigenic stimulation and downregulates the immunopathology. As the parasite was not completely eliminated, it started to proliferate again, and by day 11, PI Cry1Ac protoxin no longer had an effect.
On the other hand, clearance of P. chabaudi in mice depends first on their ability to mount an early proinflammatory cytokine response and second on their ability to downregulate the inflammatory response before the onset of the immunopathology [31]. In our study, mice treated with protoxin Cry1Ac and infected with P. chabaudi AS more efficiently eliminated parasitaemia, despite the fact that this group demonstrated a decrease in IFN-γ mRNA expression that correlated with decreased serum levels of this cytokine compared to mice treated with PBS (Figures 3 and 4). This fact could be related to the upregulation of TGF-β, which has been shown to play a double role in malaria infections; it is able to lead pro- and anti-inflammatory responses that may downregulate the production of IFN-γ [29]. It is also possible that the improved parasite elimination in the mice treated with Cry1Ac could be related to higher levels of IgG and IgM antibodies, which could be associated with better parasite elimination due to an increase in phagocytosis [32–36].
Treatment with protoxin Cry1Ac before Plasmodium infection induced a stronger antibody response in CBA/Ca mice to both Plasmodium berghei ANKA and P. chabaudi AS infections compared to control mice treated with PBS (Figures 5 and 6). Currently, the reason for this increase is not clear; however, this finding could be explained by a cross-reaction resulting from molecular similarities between epitopes of Plasmodium and the Cry1Ac protoxin. We have performed ELISA assays in which the Cry1Ac protoxin or P. berghei ANKA antigen was bound to the plate and several dilutions of P. berghei ANKA immune mice serum were tested. In these assays, immune sera to P. berghei ANKA or P. chabaudi AS recognised Cry1Ac protoxin and developed higher OD values compared to normal mouse serum (data not shown), strongly suggesting that P. berghei ANKA and Cry1Ac share common antigens. However, further study is required to characterise the precise antigens involved.
Another possible mechanism, in which the pretreatment with Cry1Ac may increase survival in Plasmodium-infected mice and may increase both the levels of Plasmodium-specific antibodies elicited after the challenge and the levels of IFN-γ, may involve the activation of innate immune cells, such as antigen presenting cells, that permit a faster establishment of adaptive immune responses. In agreement with this proposal, our unpublished data indicate that Cry1Ac protoxin activates mouse macrophages, inducing the expression of the costimulatory molecules B7-1 and B7-2 and the production of proinflammatory cytokines.
The isotypes of protective antibodies in Plasmodium infections are under debate. In general, it is accepted that IgG1 and IgG2a are protective [37–40]. However, in some reports, IgG2b or IgG3 are also mentioned as being protective [41, 42]. In mice, it is well known that IFN-γ, the principal Th1 effector cytokine, regulates the production of the opsonising or cytophilic isotype IgG2a [43], which is in accordance with our results in P. berghei-infected mice, and that IL-4 is central to the synthesis of IgG1, while TGF-β is involved in the synthesis of IgG2b [38]. In our study, we found that pretreatment with Cry1Ac protoxin significantly increases the IgG subclasses assessed and IgM in P. chabaudi AS- or P. berghei ANKA-infected mice, which could be associated with parasite clearance because it has been shown that antibody responses play a critical role in immune protection against Plasmodium in asexual blood stages. This role has been demonstrated by passive transfer experiments using sera or purified immunoglobulins from adults residing in areas with hyperendemic malaria [44, 45]. However, the mechanisms by which malaria-specific antibodies interfere with the development and/or multiplication of the asexual stages of human Plasmodia are still unclear. It has been postulated that antibodies inhibit parasite growth in cooperation either with monocytes or neutrophils via antibody-dependent cellular inhibition [46, 47] or by immunophagocytosis through Fc receptors expressed on the cell surface after binding their parasite target [48]. In addition, a correlation between immune protection and the ability of serum to mediate opsonisation of infected erythrocytes has been described [49]. Further studies are required to clarify the mechanisms involved in boosting the innate immunity against malaria.
Like most adjuvants Cry1Ac protoxin might exert its activity by activating innate immune cells. The data presented in this study suggest that pretreatment with this protein could lead to design prophylactic strategies to improve resistance against malaria infections. However, further studies are required to clarify the mechanisms involved in boosting the innate immunity against Plasmodium infection.
Acknowledgments
The authors thank Armando Cervantes for his help with the statistical analysis. This work was supported by the following Grants: PAPIIT IN214007, IN220310, IN221807, and CONACYT 080920.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bull PC, Marsh K. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends in Microbiology. 2002;10(2):55–58. doi: 10.1016/s0966-842x(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 3.Doolan DL, Dobaño C, Baird JK. Acquired immunity to Malaria. Clinical Microbiology Reviews. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clinical and Experimental Immunology. 2003;133(2):145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A. Immunosuppression in children with malaria. The Lancet. 1972;1(7743):169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 6.Whitmore DB. Suppression of the immune response to heterologous erythrocytes in mice infected with Plasmodium berghei berghei. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1972;66(1):5–6. doi: 10.1016/0035-9203(72)90007-7. [DOI] [PubMed] [Google Scholar]
- 7.Urban BC, Ing R, Stevenson MM. Early interactions between blood-stage Plasmodium parasites and the immune system. Current Topics in Microbiology and Immunology. 2005;297:25–70. doi: 10.1007/3-540-29967-x_2. [DOI] [PubMed] [Google Scholar]
- 8.Grech K, Watt K, Read AF. Host-parasite interactions for virulence and resistance in a malaria model system. Journal of Evolutionary Biology. 2006;19(5):1620–1630. doi: 10.1111/j.1420-9101.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- 9.Wykes MN, Liu XQ, Beattie L, et al. Plasmodium strain determines dendritic cell function essential for survival from malaria. PLoS Pathogens. 2007;3(7, article e96) doi: 10.1371/journal.ppat.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofte H, Whiteley HR. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiological Reviews. 1989;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight PJK, Crickmore N, Ellar DJ. The receptor for Bacillus thuringiensis CrylA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Molecular Microbiology. 1994;11(3):429–436. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnepf E, Crickmore N, Van Rie J, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews. 1998;62(3):775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas-Hernandez S, Rodriguez-Monroy MA, Lopez-Revilla R, Resendiz-Albor AA, Moreno-Fierros L. Intranasal coadministration of the Cry1Ac protoxin with amoebal lysates increases protection against Naegleria fowleri meningoencephalitis. Infection and Immunity. 2004;72(8):4368–4375. doi: 10.1128/IAI.72.8.4368-4375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vázquez-Padrón RI, Moreno-Fierros L, Neri-Bazán L, de la Riva GA, López-Revilla R. Intragastric and intraperitoneal administration of Cry1Ac protoxin from Bacillus thuringiensis induces systemic and mucosal antibody responses in mice. Life Sciences. 1999;64(21):1897–1912. doi: 10.1016/s0024-3205(99)00136-8. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero GG, Moreno-Fierros L. Carrier potential properties of Bacillus thuringiensis Cry1A toxins for a diphtheria toxin epitope. Scandinavian Journal of Immunology. 2007;66(6):610–618. doi: 10.1111/j.1365-3083.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero GG, Russell WM, Moreno-Fierros L. Analysis of the cellular immune response induced by Bacillus thuringiensis Cry1A toxins in mice: effect of the hydrophobic motif from diphtheria toxin. Molecular Immunology. 2007;44(6):1209–1217. doi: 10.1016/j.molimm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Roetynck S, Baratin M, Johansson S, Lemmers C, Vivier E, Ugolini S. Natural killer cells and malaria. Immunological Reviews. 2006;214(1):251–263. doi: 10.1111/j.1600-065X.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. Journal of Immunology. 2002;169(6):2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 19.Ge AZ, Pfister RM, Dean DH. Hyperexpression of a Bacillus thuringiensis delta-endotoxin-encoding gene in Escherichia coli: properties of the product. Gene. 1990;93(1):49–54. doi: 10.1016/0378-1119(90)90134-d. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Legorreta-Herrera M, Ventura-Ayala ML, Licona-Chavez RN, Soto-Cruz I, Hernandez-Clemente FF. Early treatment during a primary malaria infection modifies the development of cross immunity. Parasite Immunology. 2004;26(1):7–17. doi: 10.1111/j.0141-9838.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- 22.Campino S, Bagot S, Bergman M-L, et al. Genetic control of parasite clearance leads to resistance to Plasmodium berghei ANKA infection and confers immunity. Genes and Immunity. 2005;6(5):416–421. doi: 10.1038/sj.gene.6364219. [DOI] [PubMed] [Google Scholar]
- 23.Shibui A, Hozumi N, Shiraishi C, et al. CD4+ T cell response in early erythrocytic stage malaria: Plasmodium berghei infection in BALB/c and C57BL/6 mice. Parasitology Research. 2009;105(1):281–286. doi: 10.1007/s00436-009-1435-8. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Orozco AR, Rico Rosillo G, Lopez-Revilla R. The effect of Cry1Ac on human monocytes and neutrophil activation. Allergy and Clinical Immunology International. 2005;17(2):64–65. [Google Scholar]
- 25.Nagamatsu Y, Koike T, Sasaki K, Yoshimoto A, Furukawa Y. The cadherin-like protein is essential to specificity determination and cytotoxic action of the Bacillus thuringiensis insecticidal CryIAa toxin. FEBS Letters. 1999;460(2):385–390. doi: 10.1016/s0014-5793(99)01327-7. [DOI] [PubMed] [Google Scholar]
- 26.Ockenhouse CF, Schulman S, Shear HL. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by γ-interferon-activated, monocyte-derived macrophages. Journal of Immunology. 1984;133(3):1601–1608. [PubMed] [Google Scholar]
- 27.Yoneto T, Yoshimoto T, Wang C-R, et al. Gamma interferon production is critical for protective immunity to infection with blood-stage Plasmodium berghei XAT but neither NO production nor NK cell activation is critical. Infection and Immunity. 1999;67(5):2349–2356. doi: 10.1128/iai.67.5.2349-2356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shear HL, Srinivasan R, Nolan T, Ng C. Role of IFN-γ in lethal and nonlethal malaria in susceptible and resistant murine hosts. Journal of Immunology. 1989;143(6):2038–2044. [PubMed] [Google Scholar]
- 29.Omer FM, de Souza JB, Riley EM. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. Journal of Immunology. 2003;171(10):5430–5436. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- 30.Couper KN, Blount DG, Hafalla JCR, Van Rooijen N, de Souza JB, Riley EM. Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia. Infection and Immunity. 2007;75(12):5806–5818. doi: 10.1128/IAI.01005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson MM, Riley EM. Innate immunity to malaria. Nature Reviews Immunology. 2004;4(3):169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 32.Pleass RJ, Ogun SA, McGuinness DH, Van De Winkel JGJ, Holder AA, Woof JM. Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood. 2003;102(13):4424–4430. doi: 10.1182/blood-2003-02-0583. [DOI] [PubMed] [Google Scholar]
- 33.Tebo AE, Kremsner PG, Luty AJF. Fcγ receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clinical and Experimental Immunology. 2002;130(2):300–306. doi: 10.1046/j.1365-2249.2002.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mota MM, Brown KN, Holder AA, Jarra W. Acute Plasmodium chabaudi chabaudi malaria infection induces antibodies which bind to the surfaces of parasitized erythrocytes and promote their phagocytosis by macrophages in vitro. Infection and Immunity. 1998;66(9):4080–4086. doi: 10.1128/iai.66.9.4080-4086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Y, Kowalewski R, Kim S, Elkon KB. The role of IgM antibodies in the recognition and clearance of apoptotic cells. Molecular Immunology. 2005;42(7):781–787. doi: 10.1016/j.molimm.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 36.Shear HL, Nussenzweig RS, Bianco C. Immune phagocytosis in murine malaria. Journal of Experimental Medicine. 1979;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akanmori BD, Waki S, Suzuki M. Immunoglobulin G(2a) isotype may have a protective role in Plasmodium berghei NH65 infection in immunised mice. Parasitology Research. 1994;80(8):638–641. doi: 10.1007/BF00932945. [DOI] [PubMed] [Google Scholar]
- 38.Waki S, Uehara S, Kanbe K, Nariuch H, Suzuki M. Interferon-gamma and the induction of protective IgG2a antibodies in non-lethal Plasmodium berghei infections of mice. Parasite Immunology. 1995;17(10):503–508. doi: 10.1111/j.1365-3024.1995.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 39.Langhorne J, Kim KJ, Asofsky R. Distribution of immunoglobulin isotypes in the nonspecific B-cell response induced by infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cellular Immunology. 1985;90(1):251–257. doi: 10.1016/0008-8749(85)90187-x. [DOI] [PubMed] [Google Scholar]
- 40.Cavinato RA, Bastos KRB, Sardinha LR, Elias RM, Alvarez JM, D’Império Lima MR. Susceptibility of the different developmental stages of the asexual (schizogonic) erythrocyte cycle of Plasmodium chabaudi chabaudi to hyperimmune serum, immunoglobulin (Ig)G1, IgG2a and F(ab′)2 fragments. Parasite Immunology. 2001;23(11):587–597. doi: 10.1046/j.1365-3024.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 41.Kedzierski L, Black CG, Stowers AW, Goschnick MW, Kaslow DC, Coppel RL. Comparison of the protective efficacy of yeast-derived and Escherichia coli-derived recombinant merozoite surface protein 4/5 against lethal challenge by Plasmodium yoelii. Vaccine. 2001;19(32):4661–4668. doi: 10.1016/s0264-410x(01)00244-4. [DOI] [PubMed] [Google Scholar]
- 42.Garraud O, Mahanty S, Perraut R. Malaria-specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends in Immunology. 2003;24(1):30–35. doi: 10.1016/s1471-4906(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 43.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192(4804):733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 45.Sabchareon A, Burnouf T, Ouattara D, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. American Journal of Tropical Medicine and Hygiene. 1991;45(3):297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 46.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. Journal of Experimental Medicine. 1990;172(6):1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. Journal of Experimental Medicine. 1995;182(2):409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrante A, Kumaratilake L, Rzepczyk CM, Dayer J-M. Killing of Plasmodium falciparum by cytokine activated effector cells (neutrophils and macrophages) Immunology Letters. 1990;25(1–3):179–187. doi: 10.1016/0165-2478(90)90112-4. [DOI] [PubMed] [Google Scholar]
- 49.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Research in Immunology. 1990;141(6):529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]