Abstract
During earlier experiments, an SSR molecular marker (176 Soy HSP) showing high correlation (70%) with resistance/susceptibility to javanese root-knot nematode Meloidogyne javanica was identified in soybean. After being sequenced, results indicated that the SSR 176 Soy HSP marker was inserted in the promoter region of Gmhsp17.6-L gene. It was also detected in this region that resistant genotypes presented insertions between AT(31) and AT(33) in size and susceptible genotypes, AT(9). Gmhsp17.6-L gene coding region presented a perfect match in amino acid sequence in all soybean genotypes. A ribonuclease protection assay showed that Gmhsp17.6-L gene mRNA transcripts were present in all genotypes. A real-time relative quantification (qPCR) indicated in the resistant individuals higher mRNA transcripts levels, which presented in the sequencing more AT(n) insertions. These results suggest that the number of AT(n) insertions inside this promoter region could modulate up or down gene levels. Those findings can lead to the possibility of manipulating, between some limits, the mRNA transcripts levels using different sizes of AT(n) insertions.
1. Introduction
Diseases caused by nematodes are among the main factors that contribute to yield losses in soybean, especially in tropical and subtropical regions. It is estimated that 11% of the annual losses in world soybean production result from this parasitism [1]. In Brazil, nematode species of the genus Meloidogyne, especially M. javanica and M. incognita, represent a serious problem in many production areas, such as north of Rio Grande do Sul State, southeast and north of Parana State, south and north of São Paulo State, and south of Minas Gerais State. Also, nematode infections are increasing in soybean expansion areas in central Brazil. Unfortunately, management strategies such as crop rotation are not achieving efficient control of nematode populations. Likewise, a lack of effectively resistant cultivars, with broad adaptation across the country, is contributing to the growing problem [2].
Molecular markers allow us to evaluate resistance genes that show Mendelian inheritance and their use helps to identify genetic polymorphisms, eliminating aspects of phenotypic variation, such as environmental effects. With molecular markers, selections can be made at earlier stages of breeding, reducing costs from nematode population maintenance and inoculation of plants. Microsatellites, also called SSR (“simple sequence repeats”), correspond to DNA sequences with a few base pairs (2–6) in length, repeated in tandem, such as (AT)n, (ATT)n, (GCC)n, and so forth. The variation in the number of these repeated elements generates variability that can be used to identify polymorphisms in different genotypes [3].
Silva and coworkers [4], in previous genetic studies involving M. javanica resistance, found that the amplification or nonamplification of the microsatellite marker 176 Soy HSP had a high significant correlation with the number of galls observed on the roots of soybean plants, obtained from resulting crosses between resistant and susceptible genotypes. This marker belongs to the F linkage group and is located between the RFLP markers A186D and A757V. In another study, Fuganti and collaborators [5] confirmed that in this linkage group, in which other quantitative trait loci (QTLs) conditioning resistance to nematodes have already been reported [4, 6], the SSR loci 176 Soy HSP and Satt 114 showed significant correlation with number of galls observed on soybean roots inoculated with the pathogen. 176 Soy HSP and Satt 114 markers explained 46% and 43%, respectively, of phenotypic variation in number of galls per root and 70% of variation on average score on nematode infection.
Based on these previous results obtained by Fuganti and collaborators [5], the 176 Soy HSP microsatellite molecular marker was selected to be further studied in soybean plants, resistant and susceptible to M. javanica when inoculated and noninoculated with the pathogen. The resulting sequence for this molecular marker showed high similarity with a promoter region for a gene encoding a small heat-shock-protein (sHSP) found in soybean, Gmhsp17.6-L (Genbank accession no. M11317).
These proteins, although identified initially in response to heat stress, are now known to be induced by many types of environmental stress, including drought, freezing, and high salinity [7]. As molecular chaperones, they are involved in folding and refolding of proteins during their synthesis and transport, as well as the association of polypeptides with each other and other macromolecules to form oligomeric complexes. They comprise a diverse group of proteins mediating the correct assembly of polypeptides but are not themselves part of the functional assembled structures [8].
The genes encoding HSPs (hsps) are highly conserved and many of these genes and their products can be assigned to families on the basis of sequence homology and typical molecular weight: hsp110, hsp100, hsp90, hsp70, hsp60, hsp40, hsp10, and small/low molecular weight (smHSPs/LMW) hsp families [9]. In higher plants, six nuclear gene families encode an LMW protein which corresponds to their location within distinct cellular compartments, including the cytoplasm, plastids, rough ER, and mitochondria [10]. Additionally, specific smHSPs are expressed during various phases of plant development [11].
In soybean, the genes encoding the small molecular weight HSPs are the predominant class of HSPs synthesized. These proteins are regulated largely at the transcriptional level. Promoter elements, designated as heat-shock elements (HSEs), are located upstream of a TATA box and are responsible for the dramatic thermal induction of HS genes. And although the predominant mechanism of activation of these genes is mediated by binding of the HS transcription factors (HSFs) to the HSEs [12], the induction of hs promoters also involves independent cis elements, as AT-rich sequences, which present potential to influence transcriptional activity.
Some plant proteins bind these AT elements and the protein/DNA complexes when fractioned by electrophoresis are grouped as high-mobility complexes (HMCs) and low mobility complexes (LMCs) [13]. HMC proteins anchored to AT-rich sequences in the DNA facilitate macromolecular assembly by charge blocking [13]. This affinity for DNA targets the microenvironment in which HMCs exert their influence, controlling gene expression by facilitating assembly of the functional initiation complex for RNA polymerase(s) and excluding histones in order to facilitate HSF binding upon induction [13].
Thus, considering the biological function of these AT-rich elements in heat-shock promoters and the significant differences found in this region, between the genotypes tested, our objective was to analyze Gmhsp17.6-L (Genbank accession no. M11317) mRNA expression level, using the ribonuclease protection assay (RPA) and real-time quantitative PCR (RT-qPCR), in an attempt to elucidate how genetic differences the samples may influence in the resistance and/or susceptibility responses in plants to the javanese root-knot nematode, M. javanica.
2. Material and Methods
2.1. Plant Material
Soybean genotypes BRS133 (susceptible to M. javanica) and PI595099 (resistant) were used as parents and from a population derived from this cross, lines JF7002, JF7027, and JF7056 were selected from resistant population and lines 256-S, 259-S, and 266-S were chosen from susceptible one. These F10 lines were selected based in two evaluations, where soybean plants were infected with 3.000 eggs of M. javanica and nematodes were quantified 30 and 72 days later. The numbers of galls per plant were determined consisting in one evaluation. The other was performed scoring nematode infection from 0 (total absence of galls) to 10 (abundant) [14].
Genomic DNA was extracted from leaves of samples, following the protocol proposed by Keim et al. [15], quantified and checked for integrity.
2.2. SSR Molecular Marker Amplification, Cloning and Sequencing Fragment
Fuganti and coworkers [5], searching for molecular markers to assist genotype selection, identified the SSR marker 176 Soy HSP as highly linked to soybean-plant response to javanese root-knot nematode, M. javanica. Since this SSR marker explained 46% and 70%, respectively, of phenotypic variation in number of galls per root and average grade on pathogen infection, amplification product was cloned according to Ausubel et al. [16] and sequenced following the recommendations of Sambrook et al. [17].
The sequence obtained from the SSR marker 176 Soy HSP was analyzed for similarity with known sequences in the NCBI Blast [18]. It showed high similarity with a sequence region inside the small heat-shock protein (sHSP) promoter gene, found in soybean, Gmhsp17.6-L (Genbank accession no. M11317). Using the Gmhsp17.6-L gene sequence available in Genbank, new primers (176SoyHSP_F 5′TTT TTG TTT AAG TTA CTG TAC TGT3′ and 176SoyHSP_R 5′GCT AGT CTT CTA CAA CCT TCT A3′) were designed that allowed the amplification of PCR fragments in both genotypes, resistant and susceptible.
2.3. Sequencing CDS Region from Gmhsp17.6-L Gene in Genotypes Resistant and Susceptible to M. javanica
Since heat shock proteins have been known to be involved in responses to stress, including biotic stresses such as nematode infection, it seemed reasonable to assume that genetic differences in the promoter of Gmhsp17.6-L may have an impact on nematode resistance associated with this SSR. As a result, a set of primers (pSoyHSP_F 5′GGG CTG CAG GAA TTC TGA AAT TGG GTC TTT TTG3′and SoyHSPCL_R 5′CCC CCC GGG TTA ACC AGA GAT TTC TAT AGC CT3′), with sites for restriction enzymes to facilitate the cloning process, was designed to amplify, clone and sequence the entire Gmhsp17.6-L gene.
DNA from parental genotypes PI59099 and BRS133, and from all individuals from both populations (256-S, 259-S, 266-S, JF7002, JF7027 and JF7056), was used in PCR reactions, which were carried out in a Perkin Elmer 9600 thermocycler using the following reagent concentrations: 3.0 μL of DNA template (10 ng/μL), 2.0 μL of buffer reaction 10x (100 mM of Tris-HCl pH 8.3, 500 mM of KCl and 400 μL of MilliQ water), 1.0 μL of MgCl2 (50 mM), 1.0 μL of deoxyribonucleotide triphosphate (dNTP) (2.5 mM), 0.2 μL of Taq DNA polymerase (5 U/μL), and 1.0 μL of primers forward and reverse (5 μM) (Research Genetics Incorporation—Map Pairs), with MilliQ water added for a final volume of 20 μL. The cycling parameters used were as follows: 94°C/7 minutes and thirty cycles of 94°C/1 minute, 58°C/1, minute and 72°C/2 minutes. A final 7 minutes of extension at 72°C completed the program.
After electrophoresis, the amplification product was checked using agarose gel (0.7%) electrophoresis prepared with TBE (Tris base, boric acid, EDTA) buffer 1x and stained with ethydium bromide. All resulting fragments were cloned according to Ausubel et al. [16], sequenced following the recommendations of Sambrook et al. [17], and analyzed for similarity with known sequences in the NCBI Blast [18].
2.4. Ribonuclease Protection Assay (RPA)
Due to the significant sequence differences detected among resistant and susceptible soybean genotypes, an RPA was performed with the objective of detecting whether these differences caused inactivation of Gmhsp17.6-L expression in some genotypes. Thus, total RNA was extracted using Trizol reagent (Life Technologies) from resistant PI595099 and susceptible BRS133 parental genotypes. Half of the samples were treated with M. javanica eggs (designated “inoculated”) and the other half was not (designated “noninoculated”).
As no difference was observed in the CDS region of the resistant and susceptible genotypes (data not shown), the Gmhsp17.6-L coding sequence was used as a probe template, flanked in one strand by the T7 bacteriophage promoter and in the other, by the SP6 promoter, as described in the pGEM-T Easy Vector System I (Promega Corp.).
MAXIscript In Vitro Transcription Kit (Catalog no. AM1308-AM1326, Ambion, Inc.) was used, as described in the instruction manual, to produce complete antisense transcript runoffs (RPA2_F 5′GAC ATC ATC AAA CAA GAG AA3′and RPA2_R 5′TCT CTC CGC TAA TCT GAA3′) from Gmhsp17.6-L CDS inserted in the pGEM-T Easy Vector. After labeling with 32P-CTP (cytosine triphosphate), only full-length probes were purified from a denaturing gel (5% polyacrylamide/8 M urea/1x TBE) according to the manufacturer's protocol. A positive control, provided with the kit, was used, consisting of DNA from a 250 bp Kpn l-Xba l fragment of the mouse β-TRI-actin gene, subcloned from pAL41 (Alonso et al., 1986).
RPAs were performed with HybSpeed RPA-Hygh-Speed Hybridization Ribonuclease Protection Assay kit (Catalog #1412, Ambion, Inc.), following manual procedures. Hybridization was conducted between antisense probes from Gmhsp17.6-L gene and total RNA obtained from treated plants (T01: susceptible BRS133 inoculated with nematodes, T02: susceptible BRS133 noninoculated with nematodes, T03: resistant PI595099 inoculated with nematodes, and T04: resistant PI595099 noninoculated with nematodes).
Two digestion control tubes were prepared, with yeast RNA. After hybridization, ribonuclease was added to the mixture in all experimental samples and to one tube of yeast RNA control tube. To the remaining yeast-RNA control tube, only RNase digestion buffer (without RNase) was added. Inactivation/precipitation process was then carried out and protected fragments were separated in a denaturing gel (5% polyacrylamide/8 M urea/1x TBE) and after electrophoresis were detected by exposure to X-ray film.
2.5. Quantitative Real-Time PCR (RT-qPCR)
Using the Gmhsp17.6-L gene sequence available from Genbank, specific primers (SoyHSP_PSC_F 5′GCT GTG TGT CAT TGT CAT CGA A3′and SoyHSP_PSC_R 5′CAC GGT CTA TTT CTT GCC TAC ATC3′) for RT-qPCR were designed with Primers Express Software v2.0 package (Applied Biosystems). These primers amplified an 80 bp fragment, from the gene sequence, after the stop codon.
The biological material used comprised the parental genotypes PI595099 and BRS133, three susceptible lines (256-S, 259-S, and 266-S), and three resistant (JF7002, JF7027, and JF7056) lines from the population. After 8 days in a growth chamber, seedlings were transferred to a greenhouse and inoculated with approximately 660 J2 juvenile Meloidogyne javanica nematodes per plant. After 1, 3, and 6 days of infection, roots from inoculated and non-inoculated treatments were collected, frozen in liquid nitrogen, and stored at −80°C.
Using Trizol reagent (Life Technologies), total RNA from samples was extracted and quantified. RT-qPCR was conducted by reverse-transcribing 1.5 μg of total RNA with random primers and M-MLV RT (Invitrogen) according to the manufacturer's instructions. To optimize the cDNA concentration, an efficiency curve was constructed, with different template concentrations.
RT-qPCR reactions were performed with an ABI PRISM 7300 Real Time PCR system (Applied Biosystems) in 96-well reaction plates. All reactions were run in technical triplicates, consisting of 8.0 μL of MilliQ water, 0.5 μL ROX active reference dye, 12.5 μL of Platinum SyBR q-PCR SuperMix-UDG (Invitrogen), 1 μL of forward and reverse primers, and 2 μL of cDNA, which consisted of bulked samples from all three collection days, from inoculated and non-inoculated plants.
Ribosomal 18S rRNA gene (Genbank accession no. XO2623.1) was used as an endogenous control [19]. The cycling parameters used were as follows: 50°C/2 minutes, and 95°C/2 minutes, and forty-five cycles of 95°C/15 seconds, 62°C/30 seconds, and 72°C/30 seconds. Following the final amplification cycle, a melting curve was acquired by heating to 95°C/15 seconds, cooling to 60°C/30 seconds, and slowly heating to 95°C/15 seconds.
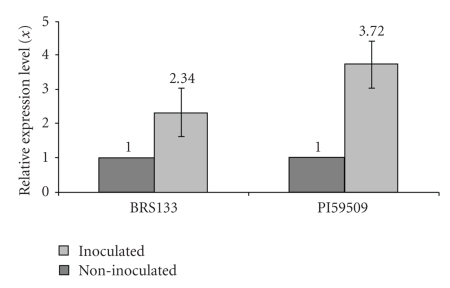
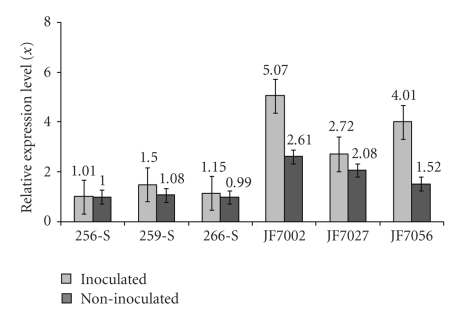
All analyses were done using the SDS software package (Applied Biosystems) using the ΔΔCt method. For parental RT-qPCR analysis (Figure 7), genotype PI595099 non-inoculated was chosen as a calibrator (value 1X). For population analyses (Figure 8), susceptible line 256-S non-inoculated was selected as a calibrator (value 1X), as this sample showed the smallest AT(n) insertions inside the Gmhsp 17.6-L promoter region. The calibrator sample serves as the basis for comparative gene expression among all the samples.
Figure 7.
Relative quantification of Gmhsp176-L mRNA expression levels (x), in parental PI595099 and BRS133, in inoculated and non-inoculated treatments. Data were obtained by real-time PCR with primers SoyHsp PSC-F and SoyHsp PSC-R, designed using Primer Express Software v20 package and the entire Gmhsp176-L gene sequence available in Genbank (Accession no. M11317). All analyses were carried out using SDS software package. Analyses were done using resistant parental genotype PI595099 as calibrator (value 1X).
Figure 8.
Relative quantification of Gmhsp176-L mRNA expression levels (x), in resistant (JF7002, JF7027 and JF7056) and susceptible (256-S, 259-S and 266-S) offsprings, in inoculated and non-inoculated treatments. Data were obtained by real-time PCR with primers SoyHsp PSC-F and SoyHsp PSC-R, designed using Primer Express Software v20 package and the entire Gmhsp176-L gene sequence available in Genbank (Accession no. M11317). All analyses were carried out using SDS software package. As a calibrator, value 1X, the susceptible individual 256-S was chosen, which presented in the promoter alignment smallest AT(n) repetition.
3. Results
3.1. SSR Marker 176 Soy HSP PCR Amplification and Sequencing of GmHSP17.6-L Gene
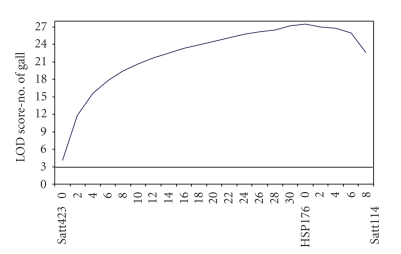
The QTL analysis revealed the presence of at least one gene located next to the 176 Soy HSP marker, with an LOD (likelihood of odds) score of 27.5 (Figure 1). The LOD value measures the probability that data were generated due to the presence of a quantitative trait loci (QTL).
Figure 1.
QTL analysis using MAPMAKER EXP and MAPMAKER QTL software indicating LOD-score values to SSR molecular markers, Satt114, Satt423 and 176 Soy HSP, from soybean F linkage group.
The sequenced fragment obtained from the 176 Soy HSP microsatellite marker, amplification of which occurred only in susceptible individuals (Figure 2), consisted of 95 bp, including the sequences of the SSR primers. A search for similarity with other known sequences revealed that this fragment showed 100% homology to the promoter region of a small heat-shock protein found in soybean and deposited in Genbank as Gmhsp17.6-L (accession no. M11317) (Figure 3).
Figure 2.
Agarose gel showing the fragment amplification with the 176 Soy HSP microsatellite marker, for resistant parental PI595099, susceptible parental BRS133, and for individuals from resistant (Samples JF7002, JF7027, JF7056, JF7057 and JF7183) and susceptible (Samples 254-S, 256-S, 259-S, 266-S and 277-S) population. No template control. Ladder 50 bp. Arrow shows amplified band in the susceptible parental, which also appears in the susceptible population.
Figure 3.
Homology between the DNA fragment sequenced from the 176 Soy HSP molecular marker and the promoter region of the Glycine max small heat shock protein, Gmhsp176-L (Genbank accession no. M11317) gene.
Using the available gene sequence, new primers were designed with the objective of generating bands and also sequencing the gene sequence in all resistant and susceptible samples (Figure 4). All resulting fragments were cloned and sequenced; all tested individuals aligned and showed high similarity with the Gmhsp17.6-L gene sequence, including promoter (Figure 5) and CDS regions (data not shown). Amino acid sequence alignments were performed and no mismatches were found among the parental genotypes. Individuals of both populations when compared to and with the Gmhsp17.6-L sequence available from Genbank (data not shown) also did not show amino acid sequence difference.
Figure 4.
Agarose gel showing the entire Gmhsp176-L gene amplificatiom from parental resistant, PI 595099, parental susceptible, BRS133, and offspring susceptible 256-S, 259-S and 266-S, and resistants JF7002, JF7027 and JF756. Ladder 100 bp. PCR was carried out using a set of primers, designed with the entire gene sequence available in Genbank (Accession no. M11317).
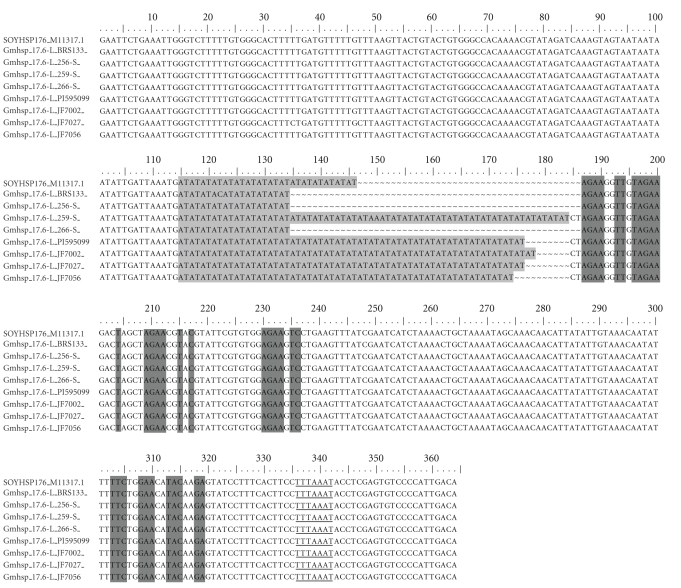
Figure 5.
Gmhsp176-L promoter region alignment from the resistant parental, PI595099, susceptible parental, BRS133, and fragments from susceptible offsprings 256-S, 259-S (heterozygous individual) and 266-S and from resistant offsprings JF7002, JF7027 and JF7056. SOYHSP176_11317-Gmhsp176-L gene sequence, available at the Genbank. PCR amplification was conducted with primers, which delimitated promoter region. In light gray, AT(n) insertion, in dark gray some heat shock elements, with consensus core sequences 5′AGAAnnTTCT3′, 5′cTTCtaGAAgcTTCtaGAAg3′, and 5′CTnGAAnnTTCnAG3′ and underlined probable TATA box.
However, in the promoter region, differences between genotypes were found, especially concerning AT(n) repetitions. Thus, sequences obtained from resistant samples presented higher numbers of AT(n) repetitions, including parental (PI595099-AT(32)) and population individuals (JF7002-AT(33), JF7027-AT(32), and JF7056-AT(31)) when compared with sequences from susceptible individuals, parental (BRS133-AT(09)) and population individuals (256-S and 266-S-AT(09)), and also to the sequence from Gmhsp17.6-L gene (AT(15)), deposited in Genbank. Although, initially evaluated in the greenhouse as susceptible, individual 259-S presented slower mobility PCR fragments consistent with resistant lines and, consequently, showed more AT(n) repetitions inside Gmhsp17.6-L promoter region, similar to the resistant samples.
3.2. Detection of Gmhsp17.6-L mRNA Transcripts Using Ribonuclease Protection Assay Technique
The RPA performed to check for a possible Gmhsp17.6-L gene inactivation due to AT(n) insertion inside the promoter region, showed that both resistant (PI595099) and susceptible (BRS133) parents, in inoculated and non-inoculated treatments, expressed the small heat-shock transcript, encoded by Gmhsp17.6-L gene (Figure 6). In Lines 01 and 02 susceptible parental BRS133 in inoculated and non-inoculated treatments, respectively, is shown while resistant parental PI595099, in inoculated and non-inoculated treatments, is presented in Lines 03 and 04, respectively.
Figure 6.

Ribonuclease protection assay results showing the expression of Gmhsp176-L mRNA transcripts. Figure shows susceptible parental BRS133 in inoculated and non-inoculated treatments, respectively and resistant parental PI595099, in inoculated and non-inoculated treatments, respectively. Also, a positive control provided with the RPA kit, a 250 pb Kpn l-Xba l fragment of the mouse β-actin gene subcloned from pAL41 is showed and probes control, a positive digestion control and a probe-negative digestion control. Black box indicates the bands and white arrow the positive control band.
3.3. Gmhsp17.6-L mRNA-Transcript Level Quantification Using Quantitative Real-Time PCR (RT-qPCR) Methodology
Graphics resulting from RT-qPCR analysis showed Gmhsp17.6-L expression levels in resistant (PI595099) and susceptible (BRS133) parents (Figure 7) and in individuals from both populations (256-S, 259-S, 266-S, JF7002, JF7027, and JF7056) (Figure 8), inoculated and non-inoculated with J2 nematodes. All results indicated that resistant samples, with longer AT(n) insertions when inoculated with the pathogen, presented a higher Gmhsp17.6-L gene expression level when compared to susceptible samples. However, in population analysis, JF7002 (AT(33)), JF7027 (AT(32)), and JF7056 (AT(31)) samples indicated even higher levels of Gmhsp17.6-L expression, not only when inoculated, but also in the non-inoculated plants, suggesting that these individuals have higher levels of heat-shock transcripts in unstressed conditions and that this level is raised further when plants are submitted to nematode infection.
4. Discussion
The presence of a gene located next to the 176 Soy HSP marker, in soybean's F linkage group, indicates that this region can play an important role in nematode resistance, as already described in the literature; various authors have shown significant correlations between molecular markers and phenotypic responses related to nematode infection, such as infection score and number of galls per plant [4–6].
After being sequenced, this SSR marker showed high homology with the Gmhsp17.6-L promoter region. Although this molecular marker at first was amplified only in susceptible samples, a set of different primers was able to amplify it in resistant samples as well; why the amplification does not occur in resistant plants, even though homology for the SSR primers exists, is a matter for further discussion and study. A possible explanation for the nonamplification of 176 Soy HSP marker is that the superior number of AT repetitions found, in the promoter region of resistant plants, might form a hairpin structure, for example, making difficult the amplification process by the Taq DNA polymerase, consequently inhibiting the formation of amplicons.
The gene alignment (Figure 5) from fragments from susceptible and resistant plants showed that resistant individuals have a larger microsatellite insertion inside the Gmhsp17.6-L promoter. Thus, resistant individuals presented JF7002-AT(33), JF7027-AT(32), and JF7056-AT(31), whereas susceptible individuals presented AT(09). The whole coding region alignment also proved that the main difference among all genotypes tested occurs only in the promoter region as no differences were observed in nucleotide sequence or in the amino acid sequence alignment (data not shown).
Based on these preliminary results, it was first postulated that the microsatellite insertion present in the Gmhsp17.6-L promoter region could be switching off the Gmhsp17.6-L gene in susceptible plants since the 176 Soy HSP marker was amplified only in these individuals. However, our findings reveal that both resistant and susceptible plants have the microsatellite insertion. Therefore, the number of AT repetitions inserted in the promoter region may be influencing soybean response to the nematode by controlling gene expression, inactivating, activating, or even altering the level of the protein controlled by this promoter.
The RPA was performed to test the hypothesis that the length of the AT insertion in the promoter region altered Gmhsp17.6-L mRNA transcripts expression. Results demonstrated that all genotypes in all treatments expressed the heat-shock protein mRNA transcripts (Figure 6). This result actually should have been expected as the literature indicates that plant heat-shock proteins function as molecular chaperones, protecting cells from protein denaturation resulting from environmental stress (Zhu et al. [20]). Thus, our next hypothesis was that the insertion inside the promoter could be affecting gene expression. To examine this possibility, a relative quantification real-time PCR was performed, as no measurable quantitative differences were detected among samples using the RPA technique.
Results from RT-qPCR showed that when comparing the Gmhsp17.6-L expression pattern between resistant and susceptible samples—parental genotypes and populations—higher levels of Gmhsp17.6-L heat-shock protein transcripts appeared in resistant samples inoculated with the pathogen (Figures 7 and 8). These same individuals presented longer AT(n) insertions inside the promoter, which could be targeted to differential mRNA transcript expression.
Also, it was observed that when susceptible-population sample 256-S, which presented the smallest AT(n) insertion on the promoter region, was used as calibrator (value 1X), all resistant individuals presented higher gene-transcript levels, even when non-inoculated, which implies an evolutionary mechanism in nematode resistance; even in the absence of the pathogen, resistant genotypes express higher level of Gmhsp17.6-L mRNA transcripts. Similarly, the highest level of expression of gene Gmhsp17.6-L was detected in resistant individual JF7002, which also showed the longest AT(33) repetition, although, in general, all resistant samples showed high Gmhsp17.6-L transcript levels.
However, analysis of the heterozygous susceptible individual 259-S, which had an AT(n) insertion even longer than those presented by resistant individuals, showed mRNA transcripts level on RT-qPCR as low as susceptible samples. These data suggest a length limit for AT(n) insertion, apart from which there is no more Gmhsp17.6-L gene induction or activation. However, new studies on this heterozygous individual need to be done in an attempting to elucidate how AT(n) acts in this specific case.
Expression of HSPs is controlled by HSFs (heat-shock factors), which is the central mechanism controlling response to heat stress. HSFs exist as inactive proteins mainly in the cytoplasm. Stress causes activation with oligomerization and, eventually, recompartmentation to the nucleus, where they bind to target sequences (HSEs) inducing the expression of genes responsible for heat stress response [12].
In addition to HSEs (heat-shock elements), a number of sequence motifs have quantitative effects on the expression of certain heat-shock genes. In plants, there is evidence for involvement of CCAAT-box elements, GAGA-sequences, AT-rich sequences, and scaffold-attachment regions [21–23].
Specifically, independent cis elements, AT-rich sequences, potentially influence transcriptional activity. In general, plant proteins that bind to these elements are grouped in two categories based on relative mobility of protein/DNA complexes when fractionated by electrophoresis: high-mobility complexes (HMCs) and low-mobility complexes (LMCs) [13].
In particular, HMC proteins anchored to AT-rich sequences in the DNA facilitate macromolecular assembly by charge blocking. A conserved amino acid is involved in the binding of this class of proteins to double-stranded AT-rich elements. The putative DNA-binding domain consists of a glycine-arginine-proline sequence flanked in the N-terminal direction by several basic amino acids [24]. Based on this proposed model, HMCs binding to AT-element sequences of genes may facilitate the integration of transcription factors into the preinitiation complex of the TATA proximal domain. These proteins also control gene expression by facilitating assembly of the functional initiation complex for RNA polymerase(s) and excluding histones in order to facilitate HSFs binding upon induction [13].
In this context, and when resistant individuals inoculated with the pathogen showed longer AT(n) insertions inside the promoter region, when compared with non-inoculated individuals, we hypothesized that the higher levels of Gmhsp17.6-L gene mRNA transcripts presented by these samples may result from more-effective integration of proteins with transcriptions factors forming preinitiation complexes. We hypothesized that when a longer binding sequence AT(n) is presented, combined with histone exclusion and binding of more induced HSFs, higher levels of gene transcription result. Based on these data, new studies are being planned in an attempting to prove, in vivo, that the higher number of AT(n) repetition inside the promoter region leads to higher gene transcription and protein expression.
Acknowledgments
The authors thank all of the researchers who, in various ways, contributed to the completion of this work. Also, they acknowledge CAPES and IFS (International Foundation for Science Grant no. C/2941-1) for financial support and EMBRAPA Soybean for laboratory facilities.
References
- 1.Barker KR. Introduction and synopsis of advancements in nematology. In: Barker KR, Pederson GA, Windham GL, editors. Plant and Nematode Interactions. Madison, Wis, USA: American Society of Agronomy; 1998. pp. 1–20. [Google Scholar]
- 2.Silva JFV. Problemas fitossanitários da soja no Brasil, com ênfase em nematóides. In: Proceedings of the 21st Congresso Brasileiro de Nematologia; 1998; Maringá, Brazil. UEM; pp. 16–20. [Google Scholar]
- 3.Ferreira ME, Grattapaglia D. Introdução ao Uso de Marcadores Moleculares em Análise Genética. 3rd edition. Brasília, Brazil: Embrapa-Cenargen; 1998. [Google Scholar]
- 4.Silva JFV, Ferraz LCBC, Arias CAA, Abdelnoor RV. Identificação de marcadores moleculares de microssatélite associados à resistência de genótipos de soja a Meloidogyne javanica. Nematologia Brasileira. 2001;25(1):79–83. [Google Scholar]
- 5.Fuganti R, Beneventi MA, Silva JFV, et al. Identification of microsatellite molecular markers to assisted selection of soybean genotypes resistant to Meloidogyne javanica. Nematologia Brasileira. 2004;28(2):125–130. [Google Scholar]
- 6.Tamulonis JP, Luzzi BM, Hussey RS, Parrott WA, Boerma HR. DNA markers associated with resistance to Javanese root-knot nematode in soybean. Crop Science. 1997;37(3):783–788. [Google Scholar]
- 7.Joshi CP, Nguyen HT. Differential display-mediated rapid identification of different members of a multigene family, HSP16.9 in wheat. Plant Molecular Biology. 1996;31(3):575–584. doi: 10.1007/BF00042230. [DOI] [PubMed] [Google Scholar]
- 8.Ellis RJ, Hemmingsen SM. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends in Biochemical Sciences. 1989;14(8):339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- 9.Gething MJ, editor. Guidebook to Molecular Chaperones and Protein-Folding Catalysts. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 10.Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. Journal of Experimental Botany. 1996;47(296):325–338. [Google Scholar]
- 11.Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Molecular Biology. 1996;32(1-2):191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- 12.Baniwal SK, Bharti K, Chan KY, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. Journal of Biosciences. 2004;29(4):471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- 13.Barros MD, Czarnecka E, Gurley W. Anatomy of a soybean heat shock element. In: Verma DPS, editor. Control of Plant Gene Expression. New York, NY, USA: CRC Press; 1993. [Google Scholar]
- 14.Luzzi BM, Boerma HR, Hussey RS. Resistance to three species of root-knot nematode in soybean. Crop Science. 1987;27:259–262. [Google Scholar]
- 15.Keim P, Olson TC, Shoemaker RC. A rapid protocol for isolating soybean DNA. Soybean Genetics Newsletter. 1988;15:150–152. [Google Scholar]
- 16.Ausubel F, Brent R, Kingston RE, et al. Short Protocols in Molecular Biology. New York, NY, USA: John Wiley & Sons; 1995. [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd edition. New York, NY, USA: Cold Spring Harbor; 1989. [Google Scholar]
- 18.Altschul SF, Madden TL, Schaffer AA, Zhang J, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia P, Taylor WR, Greenberg AH, Wright JA. Comparison of glyceraldehyde-3-phosphate dehydrogenase and 28S-ribosomal RNA gene expression as RNA loading controls for northern blot analysis of cell lines of varying malignant potential. Analytical Biochemistry. 1994;216(1):223–226. doi: 10.1006/abio.1994.1028. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JK, Shi J, Bressan RA, Hasegawa PM. Expression of an Atriplex nummularia gene encoding a protein homologous to the bacterial molecular chaperone DNA. Journal of the Plant Cell . 1993;5(3):341–349. doi: 10.1105/tpc.5.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czarnecka E, Key JL, Gurley WB. Regulatory domains of the Gmhsp17.5-E heat shock promoter of soybean: a mutation analysis. Molecular and Cellular Biology. 1989;9(8):3457–3463. doi: 10.1128/mcb.9.8.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieping M, Schöffl F. Synergistic effect of upstream sequences, CCAAT box elements, and HSE sequences for enhanced expression of chimaeric heat shock genes in transgenic tobacco. Molecular and General Genetics. 1992;231(2):226–232. doi: 10.1007/BF00279795. [DOI] [PubMed] [Google Scholar]
- 23.Schöffl F, Schröder G, Kliem M, Rieping M. A SAR sequence containing 395 bp DNA fragment mediates enhanced, gene-dosage-correlated expression of a chimaeric heat shock gene in transgenic tobacco plants. Transgenic Research. 1993;2(2):93–100. doi: 10.1007/BF01969382. [DOI] [PubMed] [Google Scholar]
- 24.Ashley CT, Pendleton CG, Jennings WW, Saxena A, Glover CVC. Isolation and sequencing of cDNA clones encoding Drosophila chromosomal protein D1. A repeating motif in proteins which recognize at DNA. The Journal of Biological Chemistry. 1989;264(14):8394–8401. [PubMed] [Google Scholar]