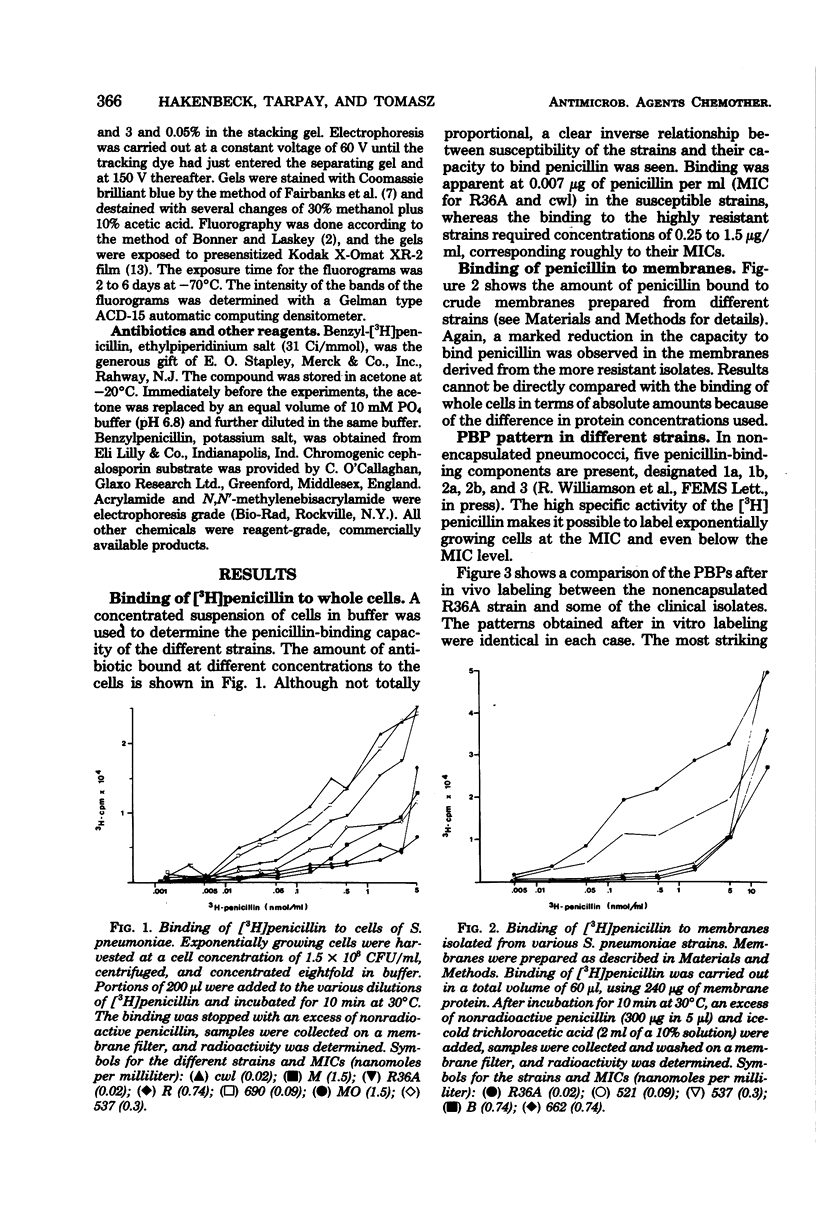
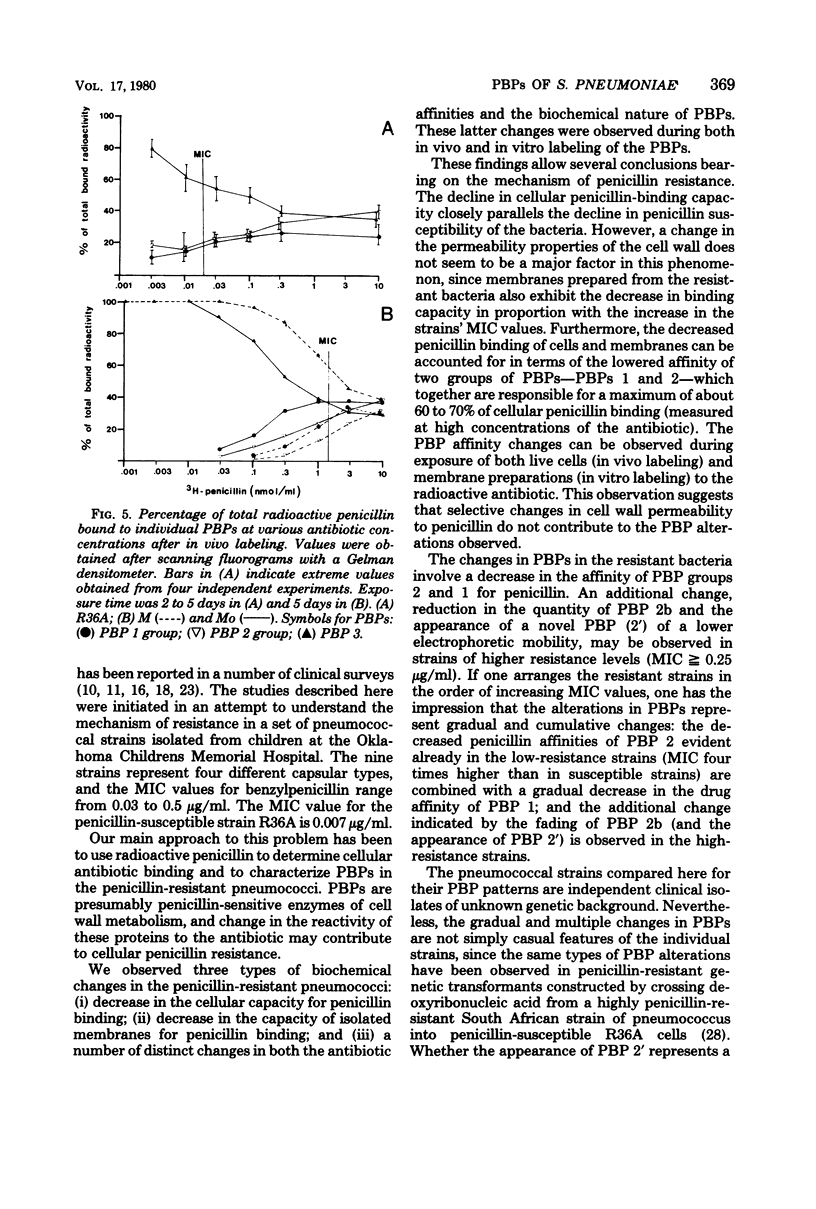
Abstract
Penicillin-binding properties and characteristics of penicillin-binding proteins (PBPs) were investigated in several clinical isolates of Streptococcus pneumoniae differing in their susceptibilities to penicillin (minimal inhibitory concentration [MIC], 0.03 to 0.5 microgram/ml) and compared with the penicillin-susceptible strain R36A (MIC, 0.07 microgram/ml). Several changes accompanied the development of resistance: the relative affinity to penicillin of whole cells, isolated membranes, and two major PBPs after in vivo or in vitro labeling decreased (with increasing resistance). Furthermore, one additional PBP (2') appeared in four of five relatively resistant strains with an MIC of 0.25 microgram/ml and higher. PBP 3 maintained the same high affinity toward penicillin in all strains under all labeling conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. I. Correlation between the penicillin sensitivity and combining activity of intact bacteria and cell-free extracts. J Exp Med. 1954 Mar;99(3):207–226. doi: 10.1084/jem.99.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. II. The reactivity with penicillin of resistant variants of streptococci, pneumococci, and staphylococci. J Exp Med. 1954 Jul 1;100(1):103–115. doi: 10.1084/jem.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Park J. T. Correlation between growth inhibition and the binding of various penicillins and cephalosporins to Staphylococcus aureus. J Bacteriol. 1969 Aug;99(2):459–462. doi: 10.1128/jb.99.2.459-462.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Giles A. F., Reynolds R. E. Bacillus megaterium resistance to cloxacillin accompanied by a compensatory change in penicillin binding proteins. Nature. 1979 Jul 12;280(5718):167–168. doi: 10.1038/280167a0. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansman D., Glasgow H., Sturt J., Devitt L., Douglas R. Increased resistance to penicillin of pneumococci isolated from man. N Engl J Med. 1971 Jan 28;284(4):175–177. doi: 10.1056/NEJM197101282840403. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maass E. A., Johnson M. J. PENICILLIN UPTAKE BY BACTERIAL CELLS. J Bacteriol. 1949 Apr;57(4):415–422. doi: 10.1128/jb.57.4.415-422.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace J. W., Janik D. S., Sauer R. L., Quilligan J. J., Jr Penicillin-resistant pneumococcal meningitis in an immunocompromised infant. J Pediatr. 1977 Sep;91(3):506–507. doi: 10.1016/s0022-3476(77)81339-5. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Naraqi S., Kirkpatrick G. P., Kabins S. Relapsing pneumococcal meningitis: isolation of an organism with decreased susceptibility to penicillin G. J Pediatr. 1974 Nov;85(5):671–673. doi: 10.1016/s0022-3476(74)80513-5. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Shepherd S. T., Chase H. A. Identification of the binding protein which may be the target of penicillin action in Bacillus megaterium. Nature. 1978 Feb 9;271(5645):568–570. doi: 10.1038/271568a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez W. J., Saz A. K. Differential binding of penicillin by membrane fractions from penicillin-susceptible and -resistant gonococci. Antimicrob Agents Chemother. 1978 Apr;13(4):589–597. doi: 10.1128/aac.13.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Cooper P. D., Roberts P. W. The site of action of penicillin. 1. Uptake of penicillin on bacteria. Biochem J. 1950 Feb;46(2):157–161. doi: 10.1042/bj0460157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks S., Tomasz A. Secretion of cell wall polymers into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob Agents Chemother. 1978 Feb;13(2):293–301. doi: 10.1128/aac.13.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]





