Abstract
Neurons derived from the same progenitor may acquire different fates according to their birth timing/order. To reveal temporally guided cell fates, we must determine neuron types as well as their lineage relationships and times of birth. Recent advances in genetic lineage analysis and fate mapping are facilitating such studies. For example, high-resolution lineage analysis can identify each sequentially derived neuron of a lineage and has revealed abrupt temporal identity changes in diverse Drosophila neuronal lineages. In addition, fate mapping of mouse neurons made from the same pool of precursors shows production of specific neuron types in specific temporal patterns. The tools used in these analyses are helping to further our understanding of the genetics of neuronal temporal identity.
Introduction
During the course of neurogenesis, exceptionally diverse neuronal cell types, as characterized by various morphological, electrophysiological, and molecular features, are generated by a limited number of neural progenitors. These neural progenitors undergo spatial patterning, during which they respond to various inductive signals and express unique combinations of transcription factors [1, 2, 3, 4, 5]. Such organization allows the progenitors to produce characteristic sets of progeny in distinct regions of the developing nervous system, a process that has been highly conserved from invertebrates to vertebrates.
In Drosophila, neural progenitors, called neuroblasts (Nbs) repeatedly undergo asymmetric cell divisions to deposit intermediate precursors while regenerating the progenitors (Figure 1). In Drosophila, neural progenitors, called neuroblasts (Nbs) deposit intermediate precursors via repeated asymmetric divisions (Figure 1). In the embryonic ventral ganglion, DiI labeling of single Nbs and their progenies allowed the Technau and Doe labs to discover families of neurons and glia produced by each Nb [6, 7, 8]. Specific Nbs deposit neurons in an invariant sequence [9, 10]. Consequently, within a given neuronal lineage, the identity of each neuron might depend chiefly on its birth time/order via a process of temporal cell fate specification. These observations have led to the identification of a cascade of transcription factors that act in precursors to specify multiple neuron types in sequence [9, 11, 12]. Further investigations of timing mechanism(s) in Drosophila melanogaster have followed [13, 14, 15, 16, 17••].
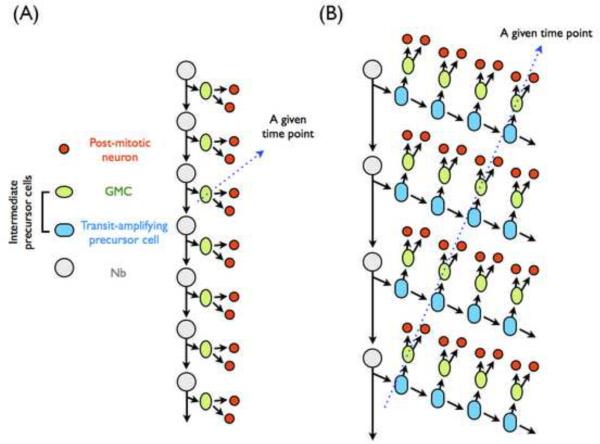
Figure 1.
Patterns of neural progenitor proliferation in Drosophila. (A) The classic mode of neural proliferation involves the generation of an intermediate precursor, GMC, from a neural progenitor. A GMC divides only once to yield two post-mitotic neurons. The serially derived GMCs may make post-mitotic neurons sequentially, such that only one GMC per lineage divides at any given developmental time (as indicated by a blue stroke). (B) Distinctive from classical GMC, specialized intermediate precursors, known as transit-amplifying precursors, are generated by those posterior Asense-negative Nbs in the Drosophila central brain. This type of intermediate precursors can undergo a limited number of cell divisions, not only regenerating itself but also giving rise to a GMC for each cell cycle. Consequently, multiple pairs of post-mitotic neurons can be generated at a given developmental time from precursors of distinct temporal origins (as indicated by a blue stroke).
In the vertebrate nervous system, birthdating of post-mitotic neurons by pulse labeling of proliferating cells provided initial evidence for such temporally guided neuron type specification [18, 19]. For example, cortical neurons exhibiting distinct properties reside in different laminas of the cortex [20]. Birthdating of post-mitotic neurons by transplantation and pulse labeling with [H3]thymidine or 5-bromo-2'-deoxyuridine (BrdU) has established the correlation between the laminar position of cortical neurons and their birth date [20, 21, 22, 23]. Specific cortical neurons are thus made at specific times of development. The orderly production of distinct neurons has also been observed in the developing retina, spinal cord, and olfactory bulb [2, 24, 25].
Elucidating how neurons acquire different fates based on birth order or time is essential for understanding how the complex brain develops. However, our lack of a complete description of the cell lineages prevents detailed mechanistic study on the temporal origin of the vertebrate neuronal diversity. This problem is further confounded by the fact that many neural progenitors yield intermediate precursors, which themselves divide multiple times before differentiating into post-mitotic neurons. Recent evidence shows self-renewing intermediate precursors in Drosophila as well. In the classical view, intermediate precursors called ganglion mother cells (GMCs), divide only once to make two post-mitotic neurons (Figure 1A). In contrast, the recently found transit-amplifying precursors undergo a limited run of asymmetric cell divisions to deposit multiple pairs of post-mitotic neurons (Figure 1B) [26, 27, 28••].
A comprehensive study on neuronal temporal identity and its underlying mechanisms depends on thorough lineage analysis to identify the offspring of a progenitor and determine the order in which they arise. Here we review how novel tools of genetic lineage analysis and fate mapping have facilitated study of neuronal temporal identity. An improved system of mosaic analysis with repressible cell markers, called twin-spot MARCM, permits high-resolution lineage analysis [29••]. It allows one to determine each sequentially derived post-mitotic neurons in a protracted Drosophila neuronal lineage. A similar system is available in mice, and its application to lineage analysis is an exciting area of technical development [30, 31]. Recent in-vivo studies of vertebrate neuronal temporal identity have primarily employed an inducible genetic marking technique to fate map specific precursors at different times of development [25, 32, 33••, 34•]. All these genetic tools further permit conditional manipulation of genes in the precursors making specific neuron types, and have helped elucidate the intrinsic mechanisms controlling neuronal temporal identity.
Technical advances: high-resolution cell lineage analysis in fly and temporally inducible genetic fate mapping in mouse
Classical lineage tracing in the Drosophila ventral ganglion depends on DiI labeling of an identifiable Nb, and determining neuronal birth order further requires pulse labeling with BrdU or analysis of temporally induced clones. These approaches are rather tedious and only work for birthdating of embryonic neurons that express known markers [8, 9]. Cell lineage analysis in the Drosophila brain, especially during post-embryonic development, was made easier by various genetic techniques that permit positive labeling of clones, including MARCM [35], G-TRACE [36] and Twin-Spot Generator [37]. However, high-resolution lineage analysis to determine the neurons derived from each sequentially born intermediate precursor remained challenging until the development of twin-spot MARCM (Figure 2A and 2B) [29••].
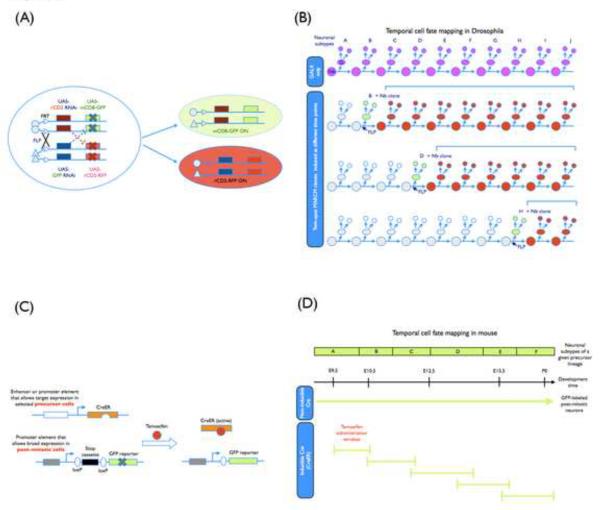
Figure 2.
(A) A schematic representation of genetic elements used in the twin-spot MARCM system. With the adoption of two sets of reporters and corresponding silencers (miRNA-based suppressors against two reporter genes in the current design) that have been placed on opposing homologous chromosome arms and distal to the recombination site, the paired Nb and two-cell clones can be labeled differentially at the same time in a mosaic brain after FRT/FLP-mediated mitotic recombination. Symbol “X” indicates the suppression of reporter gene expression. (B) Temporal cell fate mapping with twin-spot MARCM analysis. For a given neuronal lineage, a selected GAL4 driver was used to label the sequentially produced neural subtypes (A–J subtypes shown in the figure). By including this GAL4 driver in twin-spot MARCM, the temporal identity of individual marked two-cell clones generated at different developmental time points can be unambiguously determined based on the size and composition of their sister Nb clones, allowing high-resolution birth order mapping. (C) A schematic representation of genetic elements used in the inducible genetic fate mapping. Two transgenes are involved. One encodes an inducible site-specific recombinase CreER, which is a fusion protein of Cre recombinase with a tamoxifen-responsive Estrogen Receptor ligand-binding domain. Only after tamoxifen administration, CreER activity is turned on, providing a temporal control over the recombination event. Moreover, CreER expression under the control of a distinct promoter (or enhancer) element is restricted to specific precursors. The other transgenic element encodes an inheritable reporter whose expression depends on Cre-mediated excision of a stop signal and persistently marks the progeny of CreER-positive precursors. (D) Temporal cell fate mapping with the inducible genetic fate mapping in mouse. For a given neuronal precursor lineage, the sequentially derived neural subtypes (A–F types labeled by a non-inducible Cre activity) can be selectively labeled by the CreER activity depending on the time window of tamoxifen administration. Their birth order could be subsequently deduced.
Twin-spot MARCM permits labeling of the two homozygous daughter cells derived from a heterozygous precursor in distinct colors in an otherwise unstained background. With minimal neuronal migration in the Drosophila brain, neurons are labeled in clones and the differentially marked sister clones reside in pair. The neuron(s) derived from an intermediate precursor would thus pair with a multicellular Nb clone that consists of neurons born afterwards. Analysis of the associated Nb clone reveals where (lineage origin) and when (birth order) the labeled neuron(s) has derived. This allows identification of single neurons and determination of their developmental origin at the same time. It can identify, in principle, each sequentially derived neuron in any protracted neuronal lineage to reveal birth time/order-dependent neuron type specification. Either the marked single neuron(s) or the associated Nb clone can be made homozygous for any mutation distal to the site of mitotic recombination [29••, 35]. The prospective cell fate or neuron type composition of the mutant clone can be determined from its wild-type sister clone. Twin-spot MARCM offers high-resolution cell lineage analysis and greatly facilitates genetic mosaic analysis of neuronal temporal identity.
In vertebrates, lineage tracing by retroviral library injection to mark clonally related cells has nicely demonstrated that multiple neuron types could derive from a common progenitor. Pulse labeling of dividing precursors has further revealed different neuron types are made in distinct temporal patterns. However, it was challenging to target specific precursors for detailed lineage analysis until the development of recombinase-based genetic fate mapping [32, 33••, 38], which permits genetic fate mapping of specific precursors at specific times of development. This involves targeted expression of a chimeric recombinase whose activity can be temporally controlled (Figure 2C and 2D) [33••] and requires a promoter that can drive the expression of the recombinase continuously and specifically in the pool of the intermediate precursors of one's interest. Transient activation of the recombinase mediates excision of a premature stop from the reporter gene in the intermediate precursors that exist during the time of recombinase activation. This turns on reporter gene expression selectively in the progeny derived from the intermediate precursors present at a particular time of development [33••]. Thus, we can mark neurons born at different times, supposedly from the same precursors, for analysis of neuronal temporal identity. The recombinase can simultaneously excise a pre-engineered endogenous gene to conditionally knock out gene function in the precursors making specific neurons [39]. Such temporally inducible genetic fate mapping techniques have extended the study of neuronal temporal identity by molecular genetics into the complex mouse brain.
Insights from cell lineage analysis of Drosophila brain
Cell lineage analysis in the Drosophila brain shows that each Nb is programmed to make specific neurons in an invariant sequence [40, 41, 42, 43•, 44•]. Notably, timing and duration of specific neuronal subtype generation differ from lineage to lineage, suggesting individual Nbs alters its temporal identity in distinct tempos. For example, the Nbs that yield neurons constituting the olfactory learning and memory center, the mushroom bodies (MBs), produce only two types of neurons through larval development [40, 43•]. In contrast, the Nbs of antennal lobe (AL) projection neurons (PNs) make many more neuron types in a shorter period of time [41, 45]. Twin-spot MARCM analysis of a group of six neurons that innervate a subset of midline neuropils of the central complex (CX) has further revealed that these CX neurons are individually unique in neurite trajectory and that their common progenitor makes them in an invariant contiguous order [29••]. These observations indicate that some Nbs may “track” every division through the protracted process of neurogenesis from embryos to larvae. It would be interesting to determine the cell number of each AL PN type by twin-spot MARCM to see if Drosophila Nbs really count cell divisions and not only produce multiple neuron types in an invariant sequence but also deposit a fixed number of neurons for each neuron type.
In sum, neighboring Nbs may produce multiple neuron types in distinct tempos. This suggests involvement of lineage-autonomous mechanisms in the birth order-dependent neuron type specification [17••, 42]. Most larval-born neurons, regardless of the birth order, undergo differential morphogenesis during metamorphosis, further arguing pre-determination of the distinct sibling fates [46]. These phenomena support the model that Drosophila Nbs are intrinsically programmed to express distinct temporal codes to confer distinct fates on the neurons born during different temporal identity windows.
In addition, molecular asymmetries during the neuron-producing mitoses can provide different fates to sister neurons [47, 48]. Such binary sibling fate decision underlies the production of PNs and local interneurons simultaneously in the Drosophila lateral AL neuronal lineage [49]. Notably, the same precursors make many distinct PNs while yielding few types of local interneurons [45]. This suggests the presence of dual sets of temporal codes that alter in distinct tempos through the production of the sequentially derived intermediate precursors. Finally, Drosophila PAN lineages, like vertebrate neural progenitors, produce post-mitotic neurons through transit amplifying precursors [26, 27, 28••]. Studying neuronal temporal identity in the PAN lineages may shed more light on the mechanisms of temporal patterning of neuron fates in higher brains.
Insights from genetic fate mapping of mouse neurons
Temporally inducible genetic fate mapping of mouse neurons made from the same pool of precursors has been recently applied to demonstrate the production of specific neuron types proceeds in specific temporal signatures [25, 34•, 39, 50]. For example, ten distinct subtypes of cortical interneurons that derive from Olig2-positive precursors arise at different embryonic time points [34•]. Depending on the time point of generation, each type of Olig2-positive cortical interneuron has its unique physiological property. Another example is the generation of olfactory bulb (OB) interneurons. At least seven identifiable subtypes of OB interneurons are produced from Dlx1/2-expressing precursors [25]. Tyrosine hydroxylase (TH)-positive interneurons, Blanes cells, and calbindin interneurons are largely derived during embryonic stage. In contrast, calretinin (CR) cell and CR-positive granule cell productions are low during embryogenesis and increase postnatally. Parvalbumin interneurons are produced just around the birth. Interestingly, the generation of 5T4-positive granule cells does not change significantly during neurogenesis. In both examples, some neuron types are preferentially born during early development, others predominantly derive during late development, while certain neuron types can be made throughout the development. Moreover, significant overlapping exists in the orderly production of different neuron types, and multiple neuron types can derive from the same pool of precursors at the same time.
Current genetic fate mapping in mice deals with pools of possibly heterogeneous precursors, which is in great contrast with the analysis of one Nb at one time in Drosophila. The overlapping production of discrete mouse neurons could simply result from presence of distinct precursors that make different neuron types at the same time. Besides, the control of recombinase activity by acute delivery of chemicals may not provide enough temporal resolution to target neurons born in a narrow window. Nonetheless, distinct mechanisms could govern the stage-specific production of different neuron types in fly versus mouse. For instance, both intrinsic mechanisms and extrinsic cues have been implicated in the proper specification of neuronal temporal cell fates in mice [2, 24, 51, 52]. In contrast, Drosophila Nbs are probably intrinsically programmed to make multiple neuron types in invariant sequences (see below).
Molecular mechanisms of neuronal temporal cell fate specification
Mosaic analysis of gene functions utilizing the above genetic tools is expected to expedite the discovery of genes controlling neuronal temporal cell fates. At present, we have not moved far beyond the Hb→Kr→Pdm→Cas cascade of temporal factors governing neuronal temporal identity. They are expressed in a non-overlapping sequence in diverse lineages of the Drosophila embryonic ventral ganglion [42]. Hb and Kr act in neural precursors to define the first and second temporal identity in both Nb 7–1 and Nb 3–1 lineages [11, 16]. Intriguingly, Pdm and Cas determine the third and fourth temporal identity in the Nb 7–1 lineage, but regulate the expression of the preceding temporal factor in the Nb 3–1 lineage [12, 16]. Loss of Pdm or Cas delays, but not abolishes, the production of next neuron types, and ectopic Pdm or Cas is not sufficient to specify the third or fourth temporal cell fate in the Nb3–1 lineage [12, 16]. In addition, dynamic expression of Svp promotes the transition from Hb to Kr during early lineage development and may govern another temporal factor expression later in development [14, 15, 53]. So, a temporal factor may directly dictate a temporal cell fate or acts to regulate the expression of temporal fate determinants, and its mechanism of action may even vary from one lineage to another (Figure 3).
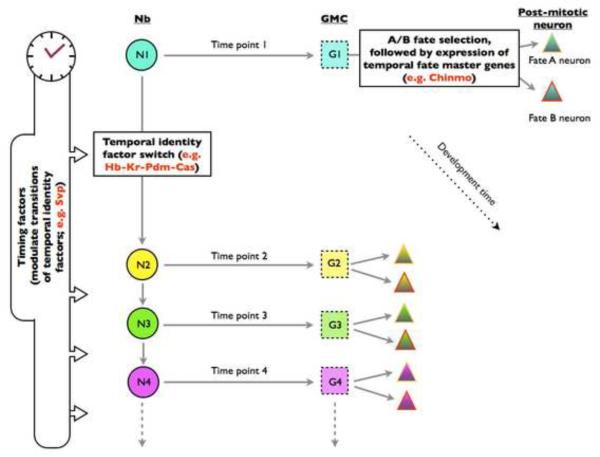
Figure 3.
A model of birth time/order-dependent neuron type specification in Drosophila CNS. As neurogenesis proceeds, a distinct set of temporal identity factors (as indicated with different colors), which sequentially express in the Nb (circle) and its GMC progeny (square), determines their individual temporal identities at specific time points. On top of the temporal identity factors, certain timing factors are used to regulate their temporal expression profiles, controlling the switch of temporal identity windows. Upon birth of post-mitotic neurons (triangle), temporal fate master genes start to express, dictating the terminal cell fates. The molecular asymmetries during neuron-producing mitoses may confer different fates on sister neurons born from the same GMCs. Together, multiple layers of temporal modulations underlie the production of many distinct neurons from a common progenitor.
While lineage analysis of Drosophila post-embryonic neurogenesis reveals more and more temporal cell fates, we observe no evidence for reuse of those embryonic temporal identity factors. Novel temporal factors remain to be identified. Interestingly, Chinmo, a BTB-zinc finger nuclear protein, may govern multiple neuronal temporal cell fates in diverse lineages [43•]. Levels of Chinmo proteins in newborn post-mitotic cells dictate multiple temporal cell fates in the MB lineages. It also governs multiple temporal fates in the rapidly changing anterodorsal PN lineage [43•; Kao & Lee, unpublished results]. However, loss of Chinmo selectively affects the third temporal identity among six contiguously derived CX neurons [29••]. In addition, Chinmo, unlike Hb, Kr, Pdm, or Cas, acts in post-mitotic neurons to specify their temporal cell fates [43•]. These observations implicate Chinmo as a temporal fate master gene. Identifying the temporal factors that regulate chinmo expression should shed new light on the temporal patterning of neural development.
Could similar intrinsic machinery operate in vertebrates? Two winged helix transcription factors, Foxg1 [54, 55] and Foxn4 [56], have been implicated in regulating cortical and retinal progenitors' abilities to sequentially produce different types of progeny, respectively. Foxg1 is not expressed in cortical progenitors until the first cortical layer of neurons are born, and has been shown to suppress early temporal identity during late cortical neurogenesis [54]. In contrast, Foxn4 is selectively expressed in young retinoblasts to promote the production of early neuron types in the retinal lineages [56]. Further, the mouse homolog of Drosophila Hb, Ikaros, acts to confer early temporal competence to mouse retinal progenitor cells [57••]. Ikaros expression in retinal progenitors is necessary and sufficient for the generation of early-born retinal cell types, suggesting a conserved mechanism of temporal cell fate specification in diverse organisms. However, it remains undetermined if these vertebrate temporal factors govern neuronal temporal cell fates in distinct lineages. Besides, the environment may regulate the temporal identity of neural progenitors. Both instructive (e.g. BDNF signaling during cerebral cortex neurogensis) [58] and negative feedback (e.g. SHH expressed in retinal ganglion cells) [59] environmental signals have been implicated in governing neuronal temporal cell fates in mice [2, 24, 52]. Dynamic changes in the extrinsic cues as well as the competence of the progenitors may jointly govern the sequential derivation of distinct neuron types at correct times of development.
Conclusion
Neurons are born with certain identity based on their developmental origin. The genetic tools that mark neurons transiently derived from specific precursors unambiguously demonstrate birth time/order-dependent neuron type specification. Furthermore, such tools permit targeted manipulation of genes, guaranteeing identification of many more genes controlling neuronal temporal identity. Determining the requirement and sufficiency as well as where and when they act to govern neuronal temporal identity should continue to provide mechanistic insights into how a neural progenitor can keep track of time to deposit specific neurons at specific times of development.
Acknowledgements
We are grateful to C. T. Zugates, T. Awasaki and the anonymous reviewer for their thoughtful comments on this manuscript. We thank National Institutes of Health and Howard Hughes Medical Institute for grant support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and annotations
- 1.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 3.Urbach R, Technau GM. Early steps in building the insect brain: neuroblast formation and segmental patterning in the developing brain of different insect species. Arthropod Structure&Development. 2003;32:103–123. doi: 10.1016/S1467-8039(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 4.Skeath J, Thor S. Genetic control of Drosophila nerve cord development. Curr Opin Neurobiol. 2003;13:8–15. doi: 10.1016/s0959-4388(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 5.Technau GM, Berger C, Urbach R. Generation of cell diversity and segmental pattern in the embryonic central nervous system of Drosophila. Dev Dyn. 2006;235:861–869. doi: 10.1002/dvdy.20566. [DOI] [PubMed] [Google Scholar]
- 6.Bossing T, Udolph G, Doe CQ, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol. 1996;179:41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- 8.Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126:4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- 9.Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 10.Karcavich R, Doe CQ. Drosophila neuroblast 7–3 cell lineage: a model system for studying programmed cell death, Notch/Numb signaling, and the sequential specification of ganglion mother cell identity. J Comp Neurol. 2005;481:240–251. doi: 10.1002/cne.20371. [DOI] [PubMed] [Google Scholar]
- 11.Pearson BJ, Doe CQ. Regulation of neuroblast competence in Drosophila. Nature. 2003;425:624–628. doi: 10.1038/nature01910. [DOI] [PubMed] [Google Scholar]
- 12.Grosskortenhaus R, Robinson K, Doe CQ. Pdm and Castor specify late-born motor neuron identity in the NB7–1 lineage. Genes Dev. 2006;20:2618–2627. doi: 10.1101/gad.1445306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosskortenhaus R, Pearson B, Marusich A, Doe CW. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev Cell. 2005;8:193–202. doi: 10.1016/j.devcel.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Kanai MI, Okabe M, Hiromi Y. seven-up controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell. 2005;8:203–213. doi: 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Mettler U, Vogler G, Urban J. Timing of identity: spatiotemporal regulation of hunchback in neuroblast lineages of Drosophila by Seven-up and Prospero. Development. 2006;133:429–437. doi: 10.1242/dev.02229. [DOI] [PubMed] [Google Scholar]
- 16.Tran KD, Doe CQ. Pdm and Castor close successive temporal identity windows in the NB3–1 lineage. Development. 2008;135:3491–3499. doi: 10.1242/dev.024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Jacob J, Maurange C, Gould A. Temporal control of neuronal diversity: common regulatory principles in insets and vertebrates? Development. 2008;135:3481–3489. doi: 10.1242/dev.016931. [DOI] [PubMed] [Google Scholar]; This review paper summaries recent progresses of temporal cell fate determinants in both insect and vertebrate systems. By deliberately describing the functional roles of known temporal identity factors, they propose general regulatory principles underlying birth-order-dependent neural specification in both model systems.
- 18.Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99:691–709. [PMC free article] [PubMed] [Google Scholar]
- 19.Berry M, Rogers AW, Eayrs JT. Pattern of cell migration during cortical histogenesis. Nature. 1964;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- 20.Butt SJB, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 21.McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell SK. The generation of neuronal diversity in the central nervous system. Annu Rev Neurosci. 1991;14:269–300. doi: 10.1146/annurev.ne.14.030191.001413. [DOI] [PubMed] [Google Scholar]
- 23.Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 25.Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bello BC, Izergina N, Caussinus E, Reichert H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural development. 2008;3:5. doi: 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the discovery of posterior Asense-negative neuroblast lineages, in which multiple transit amplifying intermediate progenitors are generated. Moreover, the authors also provide evidence that maturation of transit amplifying intermediate progenitor requires the activity of Brat and Numb factors.
- 29••.Yu HH, Chen CH, Shi L, Huang Y, Lee T. Twin-spot MARCM to reveal the development origin and identity of neurons. Nature Neurosci. 2009;12:947–953. doi: 10.1038/nn.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this technical paper, authors describe the development of Twin-spot MARCM and how one can use this technique for high-resolution lineage analysis and temporal cell fate studies.
- 30.Zong H, Espinosa JS, Su HH, Muzudar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa JS, Luo L. Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J Neurosci. 2008;28:2301–2312. doi: 10.1523/JNEUROSCI.5157-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zervas M, Millet S, Ahn S, Joyner AL. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004;43:345–357. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 33••.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]; This paper summaries the development and applications of the CreER-inducible genetic fate mapping technique, which allows the labeling of target cells with more temporal specificity.
- 34•.Miyoshi G, Butt SJB, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; By taking a inducible genetic cell fate mapping strategy, authors have demonstrated at least 10 subtypes of cortical interneurons are generated from Olig2-expressing precursors sequentially in a time-dependent manner.
- 35.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 36.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do MT, Yackle K, Cespedes A, Hartenstein V, Call GB, Banerjee U. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin R, Sustar A, Bonvin M, Binari R, Rodriguez ADV, Villalta C, Heffern E, Grunwald D, Bakal C, Desplan C, Schubiger G, Wu CT, Perrimon N. The twin spot generator for differential Drosophila lineage analysis. Nat Methods. 2009;6:600–602. doi: 10.1038/nmeth.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter NL, Awatramani RB, Farley FW, Dymecki SM. Ligand-activated Flpe for temporally regulated gene modifications. Genesis. 2005;41:99–109. doi: 10.1002/gene.20101. [DOI] [PubMed] [Google Scholar]
- 39.Butt SJB, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2–1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee T, Luo L. Development of Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 41.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 42.Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- 43•.Zhu S, Lin S, Kao CF, Awasaki T, Chiang AS, Lee T. Gradients of Drosophila Chinmo BTB-Zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]; Chinmo, which is identified from a MARCM-based mutant screen, has been demonstrated to be a potential temporal identity gene required for proper specification of multiple neuronal cell fates in at least two different neuronal lineages.
- 44•.Baek M, Mann RS. Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. J Neurosci. 2009;29:6904–6916. doi: 10.1523/JNEUROSCI.1585-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Through a MARCM-based lineage analysis, the authors provide detailed morphological descriptions and birth-order maps for most Drosophila leg motor neurons.
- 45.Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- 46.Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- 47.Cayouette M, Raff M. Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5:1265–1269. doi: 10.1038/nn1202-1265. [DOI] [PubMed] [Google Scholar]
- 48.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Lin S, Lai SL, Yu HH, Chihara T, Luo L, Lee T. Lineage-specific effects of Notch/Numb signaling in post-embryonic development of Drosophila brain. doi: 10.1242/dev.041699. manuscript submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim EJ, Battiste J, Nakagawa Y, Johnson JE. Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci. 2008;38:595–606. doi: 10.1016/j.mcn.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- 52.Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 53.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppress early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 55.Hanashima C, Fernandes M, Hebert JM, Fishell G. The role of Foxg1 and dorsal midline signaling in the generation of Cajal-Retzius subtypes. J Neurosci. 2007;27:11103–11111. doi: 10.1523/JNEUROSCI.1066-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 57••.Elliot J, Jolicoeur C, Ramamurthy V, Cayouette M. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron. 2008;60:26–39. doi: 10.1016/j.neuron.2008.08.008. [DOI] [PubMed] [Google Scholar]; This paper has provided convincing evidence showing that that Ikaros, a mouse homolog of Drosophila Hb, functions as a potent regulator of early temporal competence.
- 58.Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26:13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Dakubo G, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132:5103–5113. doi: 10.1242/dev.02096. [DOI] [PubMed] [Google Scholar]